Volatile Organic Analysis Internship Report at APPL, Inc. - Clovis, CA
VerifiedAdded on 2023/06/10
|13
|2738
|424
Report
AI Summary
This internship report details experience gained at Agriculture & Priority Pollutant Laboratories Inc. (APPL, Inc.) within the Volatile Organic Analysis Department. The internship focused on sample testing and reporting, utilizing methods such as Gas Chromatography/Mass Spectrometry (GC/MS) for analyzing volatile organic compounds (VOCs) in soil and water samples. Key aspects covered include sample preservation techniques, methods like EPA 8260 and 524 for VOC determination, quality control procedures involving internal standards and calibration, and data analysis. The report highlights the importance of monitoring VOCs to prevent environmental and health hazards, emphasizing the role of APPL Inc. in providing accurate environmental sample analysis and data reporting.

INTERNSHIP REPORT
Agriculture & Priority and pollutant lab, Inc. (APPL, Inc)
VOLATILE ORGANIC ANALYSIS DEPARTMENT
Submitted by: Name
Agriculture & Priority and pollutant lab, Inc. (APPL, Inc)
VOLATILE ORGANIC ANALYSIS DEPARTMENT
Submitted by: Name
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contents
Contents......................................................................................................................................................2
1.0 Company brief.......................................................................................................................................3
2.0 Volatile Organic Compounds and Gas Chromatography (Mass Selective Detector)..............................4
3.0 Sample Preservation, Containers, Handing and Storage.......................................................................5
Soil samples.............................................................................................................................................5
Water sample..........................................................................................................................................6
4.0 Methods................................................................................................................................................6
5.0 Quality Control......................................................................................................................................8
5.1 Internal Standards.............................................................................................................................8
5.2 Tuning................................................................................................................................................8
5.3 Initial Demonstration of Capability....................................................................................................8
5.4 Verification of Calibration Curve........................................................................................................8
5.5 A method blank.................................................................................................................................9
6.0 Materials................................................................................................................................................9
7.0 Data analysis........................................................................................................................................11
8.0 Calculations.........................................................................................................................................12
9.0 Learning during Internship..................................................................................................................12
Contents......................................................................................................................................................2
1.0 Company brief.......................................................................................................................................3
2.0 Volatile Organic Compounds and Gas Chromatography (Mass Selective Detector)..............................4
3.0 Sample Preservation, Containers, Handing and Storage.......................................................................5
Soil samples.............................................................................................................................................5
Water sample..........................................................................................................................................6
4.0 Methods................................................................................................................................................6
5.0 Quality Control......................................................................................................................................8
5.1 Internal Standards.............................................................................................................................8
5.2 Tuning................................................................................................................................................8
5.3 Initial Demonstration of Capability....................................................................................................8
5.4 Verification of Calibration Curve........................................................................................................8
5.5 A method blank.................................................................................................................................9
6.0 Materials................................................................................................................................................9
7.0 Data analysis........................................................................................................................................11
8.0 Calculations.........................................................................................................................................12
9.0 Learning during Internship..................................................................................................................12

1.0 Company brief
In the period of my internship at APPL Lab, I served in the Volatile Organic department as an
intern where I successfully managed to gain extensive experience in sample testing and reporting
on the various quantities. I realized during the time of my stay at the Agriculture and Priority
Pollutant Laboratories Inc., APPL that this testing laboratory company is one of the most
amazing and interesting facilities that is located at the Central Valley of Clovis California. The
company, for a very long time been known owing to its undisputable ability to analyse
environmental samples extracted from various solid wastes methods, waste water methods as
well as methods of drinking water.
The laboratory adopts the latest and most popular electronic formats in the reporting of the
findings from the various analyses. This enhances accuracy and convenience in the presentation
of the findings of the analysis. One of the critical data deliverable is data programming which
has been adopted by APPL Inc. in a bid to attain the various dimensions of the needs of the
clients. The company is part of the U.S. Small Business Administration and registered as a small
business owned by a woman.
Since it was started 19282, the company has managed to successfully work on and deliver
various projects with varying degrees of scope and as such it has been able to gain a wide and
elaborate experience in the handling of various reporting and testing. Still, the projects handled
the company are not just from the localized regions around the region in which the company is
located but instead from diverse location giving the company an opportunity to handle various
issues. The company concentrates on the analysis of samples of soil and water. Furthermore, the
company has managed to run a full service laboratory and is capable of concentrating on the
In the period of my internship at APPL Lab, I served in the Volatile Organic department as an
intern where I successfully managed to gain extensive experience in sample testing and reporting
on the various quantities. I realized during the time of my stay at the Agriculture and Priority
Pollutant Laboratories Inc., APPL that this testing laboratory company is one of the most
amazing and interesting facilities that is located at the Central Valley of Clovis California. The
company, for a very long time been known owing to its undisputable ability to analyse
environmental samples extracted from various solid wastes methods, waste water methods as
well as methods of drinking water.
The laboratory adopts the latest and most popular electronic formats in the reporting of the
findings from the various analyses. This enhances accuracy and convenience in the presentation
of the findings of the analysis. One of the critical data deliverable is data programming which
has been adopted by APPL Inc. in a bid to attain the various dimensions of the needs of the
clients. The company is part of the U.S. Small Business Administration and registered as a small
business owned by a woman.
Since it was started 19282, the company has managed to successfully work on and deliver
various projects with varying degrees of scope and as such it has been able to gain a wide and
elaborate experience in the handling of various reporting and testing. Still, the projects handled
the company are not just from the localized regions around the region in which the company is
located but instead from diverse location giving the company an opportunity to handle various
issues. The company concentrates on the analysis of samples of soil and water. Furthermore, the
company has managed to run a full service laboratory and is capable of concentrating on the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

issues affecting the environment even as it analyzed the soil and water samples hence has the
ability to attend to the over 3000 chemical concerns during the analysis of the various samples.
One of the main strengths or achievements of the company is its ability to conduct testing on air
or biological contamination. While at the company, I took part in various procedures in the
analysis of samples as well as the generation of suffice reporting in the Volatile Organic
department.
2.0 Volatile Organic Compounds and Gas Chromatography (Mass
Selective Detector)
Volatile organic compounds refer to those organic chemicals with high vapour pressure
recordings at their normal temperature and the room temperature. Volatile organic compounds
can maintain relatively low boiling points at extremely high vapour pressure and this facilitates
vaporization or sublimation of mist of the particles from solid to liquid states. Various countries
among the Canada, the United States of America and the European Union have each established
their own definitions of what volatile organic compounds are. European Union defines volatile
organic compounds as those compounds that have an initial boiling point lower than or equal to
250⁰C when the measurements are taken at standard conditions of atmospheric pressure.
There are various sources from which volatile organic compounds are generated and hence they
are found in various places include soil, air and water. Whole some of the volatile organic
compounds are naturally occurring; there is other than are artificially manufactured. Whether
natural or artificially made, any volatile organic compound poses a threat to the health of the
human being and to the surrounding at large. This thus leads to the need to a test for the volatile
organic compounds in both the soil and water. The availability and concentration of these
ability to attend to the over 3000 chemical concerns during the analysis of the various samples.
One of the main strengths or achievements of the company is its ability to conduct testing on air
or biological contamination. While at the company, I took part in various procedures in the
analysis of samples as well as the generation of suffice reporting in the Volatile Organic
department.
2.0 Volatile Organic Compounds and Gas Chromatography (Mass
Selective Detector)
Volatile organic compounds refer to those organic chemicals with high vapour pressure
recordings at their normal temperature and the room temperature. Volatile organic compounds
can maintain relatively low boiling points at extremely high vapour pressure and this facilitates
vaporization or sublimation of mist of the particles from solid to liquid states. Various countries
among the Canada, the United States of America and the European Union have each established
their own definitions of what volatile organic compounds are. European Union defines volatile
organic compounds as those compounds that have an initial boiling point lower than or equal to
250⁰C when the measurements are taken at standard conditions of atmospheric pressure.
There are various sources from which volatile organic compounds are generated and hence they
are found in various places include soil, air and water. Whole some of the volatile organic
compounds are naturally occurring; there is other than are artificially manufactured. Whether
natural or artificially made, any volatile organic compound poses a threat to the health of the
human being and to the surrounding at large. This thus leads to the need to a test for the volatile
organic compounds in both the soil and water. The availability and concentration of these
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

chemicals inside the soil and water need to be monitored in order to prevent any hazards to
human health and to the environment.
Gas Spectrometry or Mass Selective Detector is one of the most reliable analytical techniques
that are used in the testing of various samples. This analytical technique brings together the
features of both mass spectrometry and gas chromatography in the acknowledgement of the wide
range of elements that are available in a sample during testing. Gas spectrometry/ Mass Selective
Detector makes use of the retention times of the relative gas chromatography and the pattern of
elution of the various constituents that are found in the mixture to be tested in the detection of the
volatile organic compounds in soils and water.
When conducting the Gas Spectrometry/ Mass Selective Detector, I also took part in the Agilent
and Teledyne tekmar atomx. Agilent and teledyne tekmar atomx refers to combined patterns of
spectral fragmentation that aid in the identification of the features of the substance being tested.
This method is very helpful in the separation of the various individual components, detection of
the ions as well as the transfer of the various constituents to the ionizing chamber during mass
analysis.
3.0 Sample Preservation, Containers, Handing and Storage
It was possible receive most of the samples that were to be used in the lab in volatile organic
analysis vials.
Soil samples: The soil samples were utilized in arriving in brass sleeves or the jars as well as
in the volatile organic analysis. The samples that were coming in brass sleeves and the jars were
removed from the fridge and then thawed out for about half an hour. About 5.0 g of the soil
sample was the weighed out in a tare volatile organic analysis trial and 5 ml of Trap Water or
human health and to the environment.
Gas Spectrometry or Mass Selective Detector is one of the most reliable analytical techniques
that are used in the testing of various samples. This analytical technique brings together the
features of both mass spectrometry and gas chromatography in the acknowledgement of the wide
range of elements that are available in a sample during testing. Gas spectrometry/ Mass Selective
Detector makes use of the retention times of the relative gas chromatography and the pattern of
elution of the various constituents that are found in the mixture to be tested in the detection of the
volatile organic compounds in soils and water.
When conducting the Gas Spectrometry/ Mass Selective Detector, I also took part in the Agilent
and Teledyne tekmar atomx. Agilent and teledyne tekmar atomx refers to combined patterns of
spectral fragmentation that aid in the identification of the features of the substance being tested.
This method is very helpful in the separation of the various individual components, detection of
the ions as well as the transfer of the various constituents to the ionizing chamber during mass
analysis.
3.0 Sample Preservation, Containers, Handing and Storage
It was possible receive most of the samples that were to be used in the lab in volatile organic
analysis vials.
Soil samples: The soil samples were utilized in arriving in brass sleeves or the jars as well as
in the volatile organic analysis. The samples that were coming in brass sleeves and the jars were
removed from the fridge and then thawed out for about half an hour. About 5.0 g of the soil
sample was the weighed out in a tare volatile organic analysis trial and 5 ml of Trap Water or

Purge or MeOH was added to the vials. During the process of addition, care was taken to ensure
that there was no contact between the tips of the pipette and the soil and the vials immediately
closed after adding the substances.
Further, we took part in the preparations of the prewighted volatile organic analysis goals that
were to be used in the Terra Core Sampling. The tare balance was used in the preparation of
these samples in which 5 ml of Trap Water and Purge and about 1 g of sodium bisulphate was
quantitatively transferred alongside a stir bar to the cap and via instantly. 5 ml of Purge and Trap
MeOH was used for the case of preparation of vials of mid-level concentration. The vial that
contained the solution was thereafter without out and the weight, lot number, time and the
preservative that were used taken note of using the VOA Label program. The labels were printed
and suck on each of the corresponding vials.
Water sample: volatile organic analysis vials that contained HCl were taken to the client. For
those samples that were preserved, the analysis had to be started within not more than 14 days
from the day of sampling. Travel blanks were prepared that went along with the volatile samples
that were being taken to the laboratory for the purposes of determination of the available
contaminants during the process of shipment.
4.0 Methods
There were two main methods that were ideal in testing the samples that were used in this
experiment during my time in the laboratory: EPA 860 method and 524 drinking water method.
EPA 8260 method was critical in the determination of the volatile organic compounds that are
found in the water. The method was more specifically important in finding the matrices of the
various solid wastes that are found in the water. EP 8260 method is in most cases applicable in
that there was no contact between the tips of the pipette and the soil and the vials immediately
closed after adding the substances.
Further, we took part in the preparations of the prewighted volatile organic analysis goals that
were to be used in the Terra Core Sampling. The tare balance was used in the preparation of
these samples in which 5 ml of Trap Water and Purge and about 1 g of sodium bisulphate was
quantitatively transferred alongside a stir bar to the cap and via instantly. 5 ml of Purge and Trap
MeOH was used for the case of preparation of vials of mid-level concentration. The vial that
contained the solution was thereafter without out and the weight, lot number, time and the
preservative that were used taken note of using the VOA Label program. The labels were printed
and suck on each of the corresponding vials.
Water sample: volatile organic analysis vials that contained HCl were taken to the client. For
those samples that were preserved, the analysis had to be started within not more than 14 days
from the day of sampling. Travel blanks were prepared that went along with the volatile samples
that were being taken to the laboratory for the purposes of determination of the available
contaminants during the process of shipment.
4.0 Methods
There were two main methods that were ideal in testing the samples that were used in this
experiment during my time in the laboratory: EPA 860 method and 524 drinking water method.
EPA 8260 method was critical in the determination of the volatile organic compounds that are
found in the water. The method was more specifically important in finding the matrices of the
various solid wastes that are found in the water. EP 8260 method is in most cases applicable in
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

samples whose boiling are lower than 200⁰C and is not influenced by the water content of such
samples. In this method, the initial step is a direct injection procures which is composed of an
aqueous sample dose that is made up of very high levels of concentrated analyte. The step is then
followed by a purge and trap of the aqueous solution. It is recommended that the method be
carried out under temperature of 40⁰C in order to attain higher efficiencies. The last bit of the
steps in this method is vacuum distillation which is aimed at introducing the various volatile
organics into the aqueous system. The automated static headspace technique is also usable in this
method in aiding the addition of volatile organics into the system.
Another yet important method that was carried out in this laboratory is 524 drinking water
methods. This method involves the extraction of the volatile organic compounds and the
surrogates that have low solubility in a sample matrix through building up an inert gas through
the used aqueous sample. The purged constituents are captured in a tube that contains the
suitable materials as the solvent. Upon the completion of the purging process, the sorbent tube
undergoes heating and back flushing suing helium. This is done in order to desorb the
components of the trapped sample into the capillary gas chromatography. Programming of the
column temperature is done in order to enhance the separation of the various method analyte
which then undergo detection by mass spectrometry.
The measured retention times and mass spectra of the compound eluting from the gas
chromatography column are used in the identification of such compounds. The measure spectra
and retention times are compared against the reference retention times and mass spectra as
recorded in the database. These reference spectra and retention times are often obtaining through
taking measurements of the calibration standards under similar conditions that are used for
samples. Procedural standard calibration was used in the quantification of the analyte. The
samples. In this method, the initial step is a direct injection procures which is composed of an
aqueous sample dose that is made up of very high levels of concentrated analyte. The step is then
followed by a purge and trap of the aqueous solution. It is recommended that the method be
carried out under temperature of 40⁰C in order to attain higher efficiencies. The last bit of the
steps in this method is vacuum distillation which is aimed at introducing the various volatile
organics into the aqueous system. The automated static headspace technique is also usable in this
method in aiding the addition of volatile organics into the system.
Another yet important method that was carried out in this laboratory is 524 drinking water
methods. This method involves the extraction of the volatile organic compounds and the
surrogates that have low solubility in a sample matrix through building up an inert gas through
the used aqueous sample. The purged constituents are captured in a tube that contains the
suitable materials as the solvent. Upon the completion of the purging process, the sorbent tube
undergoes heating and back flushing suing helium. This is done in order to desorb the
components of the trapped sample into the capillary gas chromatography. Programming of the
column temperature is done in order to enhance the separation of the various method analyte
which then undergo detection by mass spectrometry.
The measured retention times and mass spectra of the compound eluting from the gas
chromatography column are used in the identification of such compounds. The measure spectra
and retention times are compared against the reference retention times and mass spectra as
recorded in the database. These reference spectra and retention times are often obtaining through
taking measurements of the calibration standards under similar conditions that are used for
samples. Procedural standard calibration was used in the quantification of the analyte. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

concentration of the components that has positively been identified is measured by comparing
the response of the mass spectrometry of the quantification ion that has been generated by the
compound under question with that of the mass spectrometry response of the quantification ion
that has been generated by a compound which was used as an internal standard. The surrogate
analyte are measured using the same procedure as the internal standard calibration owing to the
fact that their concentration is known in all the samples.
5.0 Quality Control
5.1 Internal Standards: Each of the field samples and quality control is added internal
standards. A retention time criteria of +/- 30 seconds deviation from the midpoint retention time
that is standard in the ICAL is adopted. An area response criteria of +/- 50% IS is also provided
of the ICAL midpoint standard.
5.2 Tuning: Tuning of the instrument is done before ICAAL and at the start of every period
that lasts 12 ours. A standard solution that contains 25 ug/ml of BFB in methanol is made and
introduced into the instruments for use in tuning. The criteria of the BFB tuning and criteria for
GC/MS calibration verification must be met before the commencement of analysis of the
samples.
5.3 Initial Demonstration of Capability: The SOP# QC006 is used in the completion of
the Demonstration of Capability, DOC.
5.4 Verification of Calibration Curve: The same standard that is used in ICAL applies to
the Calibration Curve Verification standard. A close CCV is needed for the case of DoD 5.0
projects. It is a requirement that all the analyte and surrogates that have been reported lie within
the response of the mass spectrometry of the quantification ion that has been generated by the
compound under question with that of the mass spectrometry response of the quantification ion
that has been generated by a compound which was used as an internal standard. The surrogate
analyte are measured using the same procedure as the internal standard calibration owing to the
fact that their concentration is known in all the samples.
5.0 Quality Control
5.1 Internal Standards: Each of the field samples and quality control is added internal
standards. A retention time criteria of +/- 30 seconds deviation from the midpoint retention time
that is standard in the ICAL is adopted. An area response criteria of +/- 50% IS is also provided
of the ICAL midpoint standard.
5.2 Tuning: Tuning of the instrument is done before ICAAL and at the start of every period
that lasts 12 ours. A standard solution that contains 25 ug/ml of BFB in methanol is made and
introduced into the instruments for use in tuning. The criteria of the BFB tuning and criteria for
GC/MS calibration verification must be met before the commencement of analysis of the
samples.
5.3 Initial Demonstration of Capability: The SOP# QC006 is used in the completion of
the Demonstration of Capability, DOC.
5.4 Verification of Calibration Curve: The same standard that is used in ICAL applies to
the Calibration Curve Verification standard. A close CCV is needed for the case of DoD 5.0
projects. It is a requirement that all the analyte and surrogates that have been reported lie within

+/- 50% of the true value and in cases where the CCV fails to meet the outlined criteria; a
notification is sent to the client who then provides or declines directions on further progress.
5.5 A method blank which is extracted and analyzed within 12 hour batches for each
analysis. The initial calibration of each of the samples to be tested is checked and verified once
after every 12 hours during the process if analysis which also involves the introduction
technique.
6.0 Materials
A number of materials are required to aid in attaining the EPA 8260 method including the
Purge and trap that was usable for the solid and aqueous samples
automated static headspace device that was mainly used for solid samples
azeotrophic distillation apparatus
vacuum distillation apparatus
desorption devices
an air sampling loop
injection port liners
methanol
stock solutions
gas chromatographic columns
hexadecane
hydrochloric acid and
Polyethylene glycol
For the 524 drinking water method, the materials that were required included:
notification is sent to the client who then provides or declines directions on further progress.
5.5 A method blank which is extracted and analyzed within 12 hour batches for each
analysis. The initial calibration of each of the samples to be tested is checked and verified once
after every 12 hours during the process if analysis which also involves the introduction
technique.
6.0 Materials
A number of materials are required to aid in attaining the EPA 8260 method including the
Purge and trap that was usable for the solid and aqueous samples
automated static headspace device that was mainly used for solid samples
azeotrophic distillation apparatus
vacuum distillation apparatus
desorption devices
an air sampling loop
injection port liners
methanol
stock solutions
gas chromatographic columns
hexadecane
hydrochloric acid and
Polyethylene glycol
For the 524 drinking water method, the materials that were required included:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Sample
Containers
Syringe valves
Purge and trap systems
GC/MSD system
Among the main reagents included
Water
Vinyl chloride
Sodium thiosulfate
Hydrochloric acid
Reagent water
Ascorbic acid
This process or method involved the preparing of reagent blank in which the syringe was filled
using reagent aster and then adjusted to the required level as the bubbles were being driven out.
The recommended volume of the fortification solution that is contained in the internal standard
and surrogates in then introduced through the Lure Lok valve into the reagent water. The reagent
blank is then taken to the purging device where it undergoes further testing. The samples are then
gathered and properly stirred to keep their ground conditions with a LRB set handed over along
each of the samples to ascertain that the control reagent blank has the ability to check the
situation and the conditions of the sample under test.
Spectra and chromatogram curves are used in the presentation of the concentration of the various
reagents against the time elapsed.
Containers
Syringe valves
Purge and trap systems
GC/MSD system
Among the main reagents included
Water
Vinyl chloride
Sodium thiosulfate
Hydrochloric acid
Reagent water
Ascorbic acid
This process or method involved the preparing of reagent blank in which the syringe was filled
using reagent aster and then adjusted to the required level as the bubbles were being driven out.
The recommended volume of the fortification solution that is contained in the internal standard
and surrogates in then introduced through the Lure Lok valve into the reagent water. The reagent
blank is then taken to the purging device where it undergoes further testing. The samples are then
gathered and properly stirred to keep their ground conditions with a LRB set handed over along
each of the samples to ascertain that the control reagent blank has the ability to check the
situation and the conditions of the sample under test.
Spectra and chromatogram curves are used in the presentation of the concentration of the various
reagents against the time elapsed.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
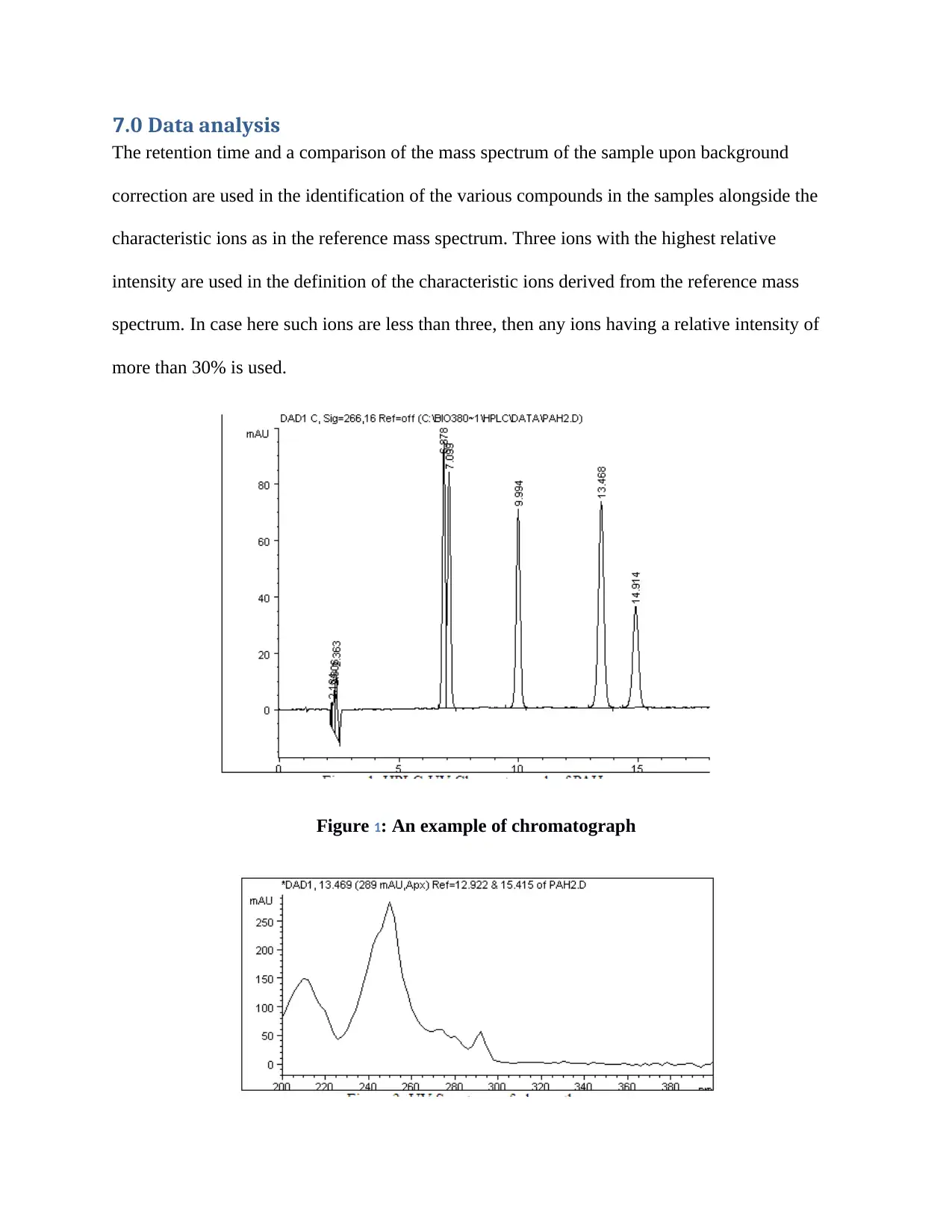
7.0 Data analysis
The retention time and a comparison of the mass spectrum of the sample upon background
correction are used in the identification of the various compounds in the samples alongside the
characteristic ions as in the reference mass spectrum. Three ions with the highest relative
intensity are used in the definition of the characteristic ions derived from the reference mass
spectrum. In case here such ions are less than three, then any ions having a relative intensity of
more than 30% is used.
Figure 1: An example of chromatograph
The retention time and a comparison of the mass spectrum of the sample upon background
correction are used in the identification of the various compounds in the samples alongside the
characteristic ions as in the reference mass spectrum. Three ions with the highest relative
intensity are used in the definition of the characteristic ions derived from the reference mass
spectrum. In case here such ions are less than three, then any ions having a relative intensity of
more than 30% is used.
Figure 1: An example of chromatograph

Figure 2: An example of spectra
8.0 Calculations
There are various standard components of Relative retention Time:
RRT= RTc/RTIS
Where:
RRT = Relative retention time for every target analyte
RTc=Retention time of a target analyte
RTIS= Retention time of the internal standard
Calculations including RRT and concentration of each identified analyte in the sample were done
with the aid of computers by programing them into the instrument.
9.0 Learning during Internship
I found an opportunity to engage in different methods and techniques that are used in testing at
APPL lnc Laboratory. The internship offered me an opportunity to learn how the different
laboratory software and important and usable in data reporting and instrumentation, preparation
of samples for analysis and well as extraction of samples of water and soil. The internship period
at APPL lnc Lab was such an amazing moment that offered me various challenging opportunities
and learning platforms. Ranging from the avalanche of instruments and the diverse team of
experts and professional in the company, I was surrounded by immense resources that saw me
get the best out of the company. I not only gained more knowledge on volatile organic analysis
8.0 Calculations
There are various standard components of Relative retention Time:
RRT= RTc/RTIS
Where:
RRT = Relative retention time for every target analyte
RTc=Retention time of a target analyte
RTIS= Retention time of the internal standard
Calculations including RRT and concentration of each identified analyte in the sample were done
with the aid of computers by programing them into the instrument.
9.0 Learning during Internship
I found an opportunity to engage in different methods and techniques that are used in testing at
APPL lnc Laboratory. The internship offered me an opportunity to learn how the different
laboratory software and important and usable in data reporting and instrumentation, preparation
of samples for analysis and well as extraction of samples of water and soil. The internship period
at APPL lnc Lab was such an amazing moment that offered me various challenging opportunities
and learning platforms. Ranging from the avalanche of instruments and the diverse team of
experts and professional in the company, I was surrounded by immense resources that saw me
get the best out of the company. I not only gained more knowledge on volatile organic analysis
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

