Understanding Acid-Base Balance, pH Imbalance, and Related Disorders
VerifiedAdded on 2022/10/06
|4
|1128
|12
Homework Assignment
AI Summary
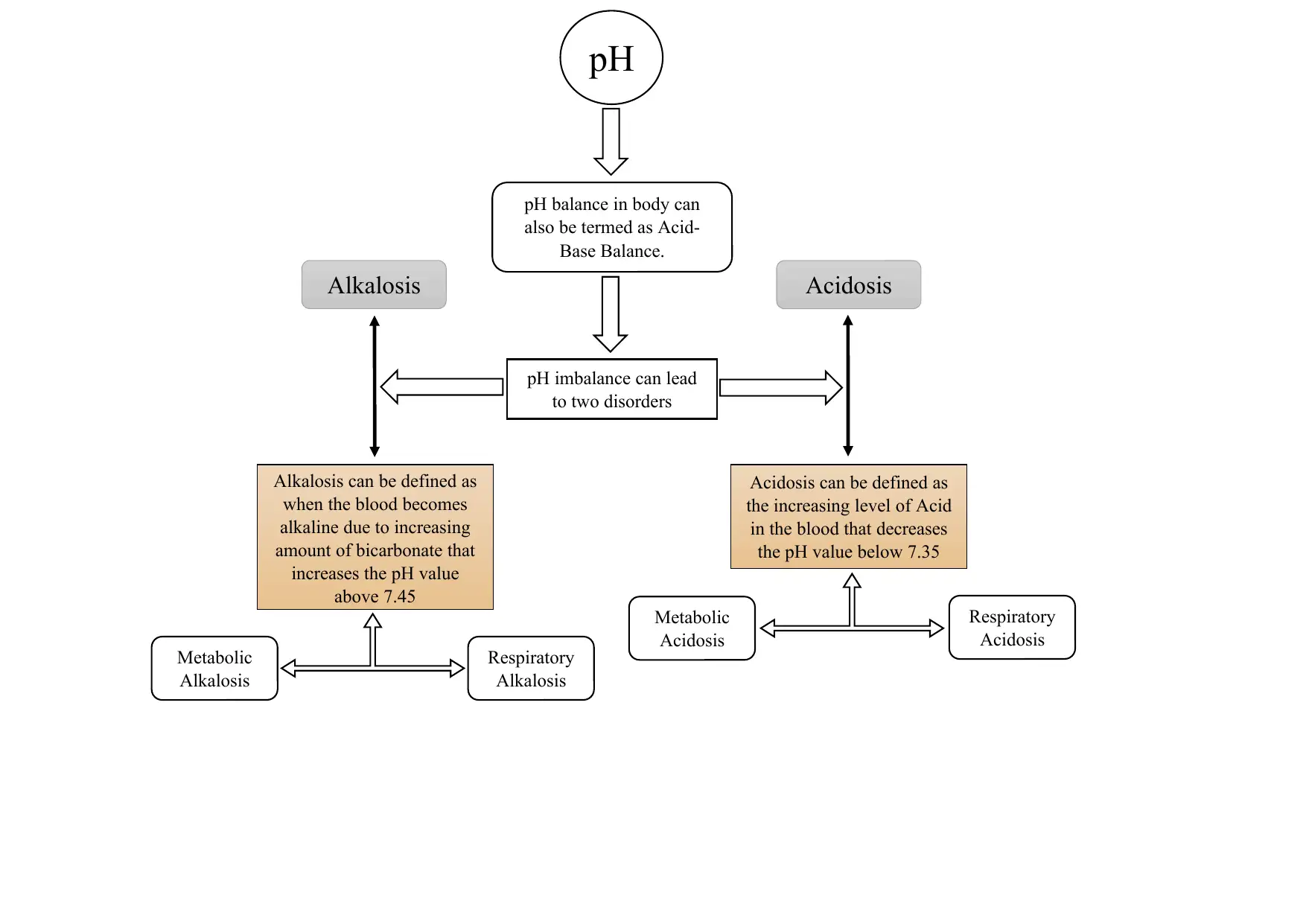
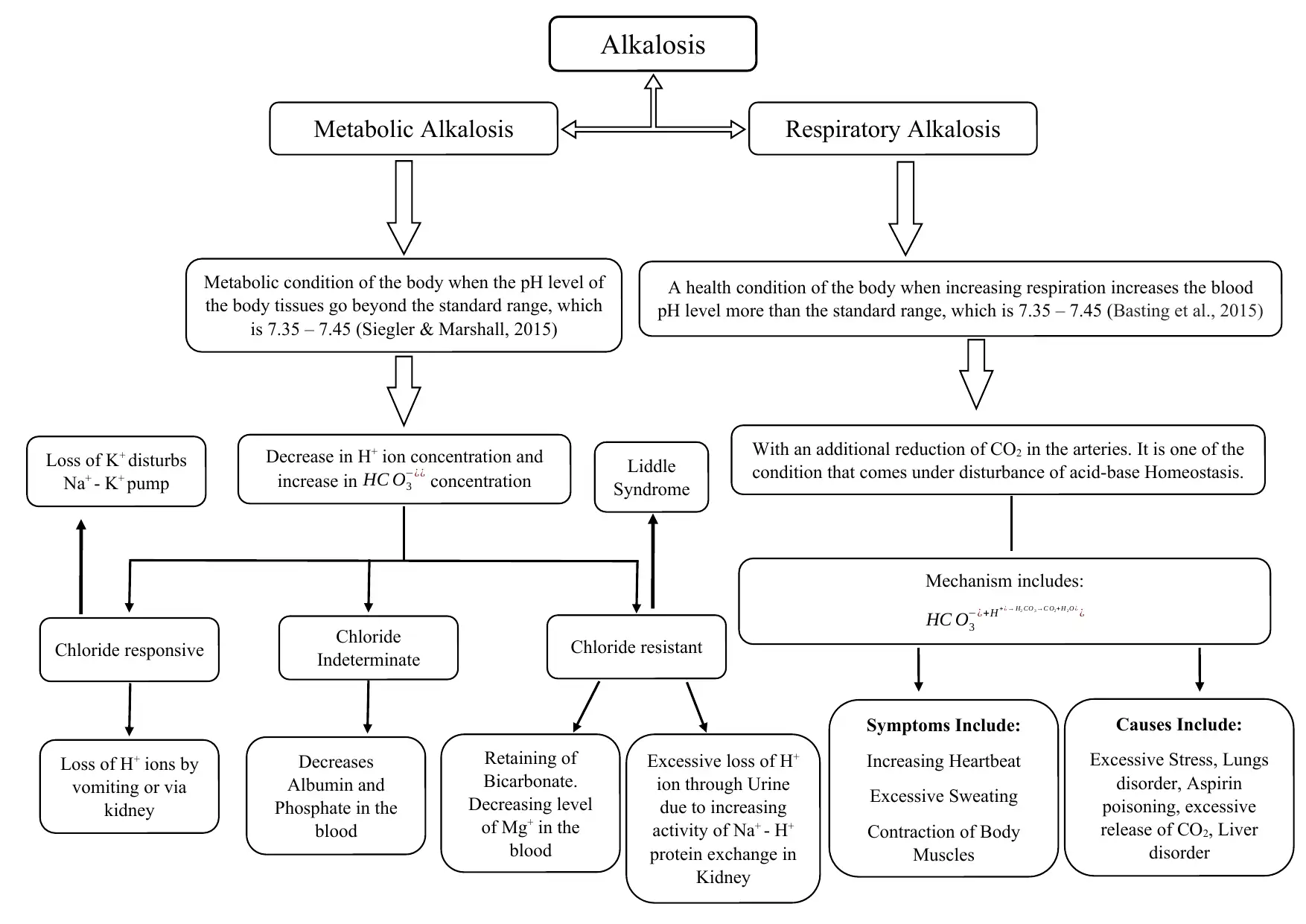
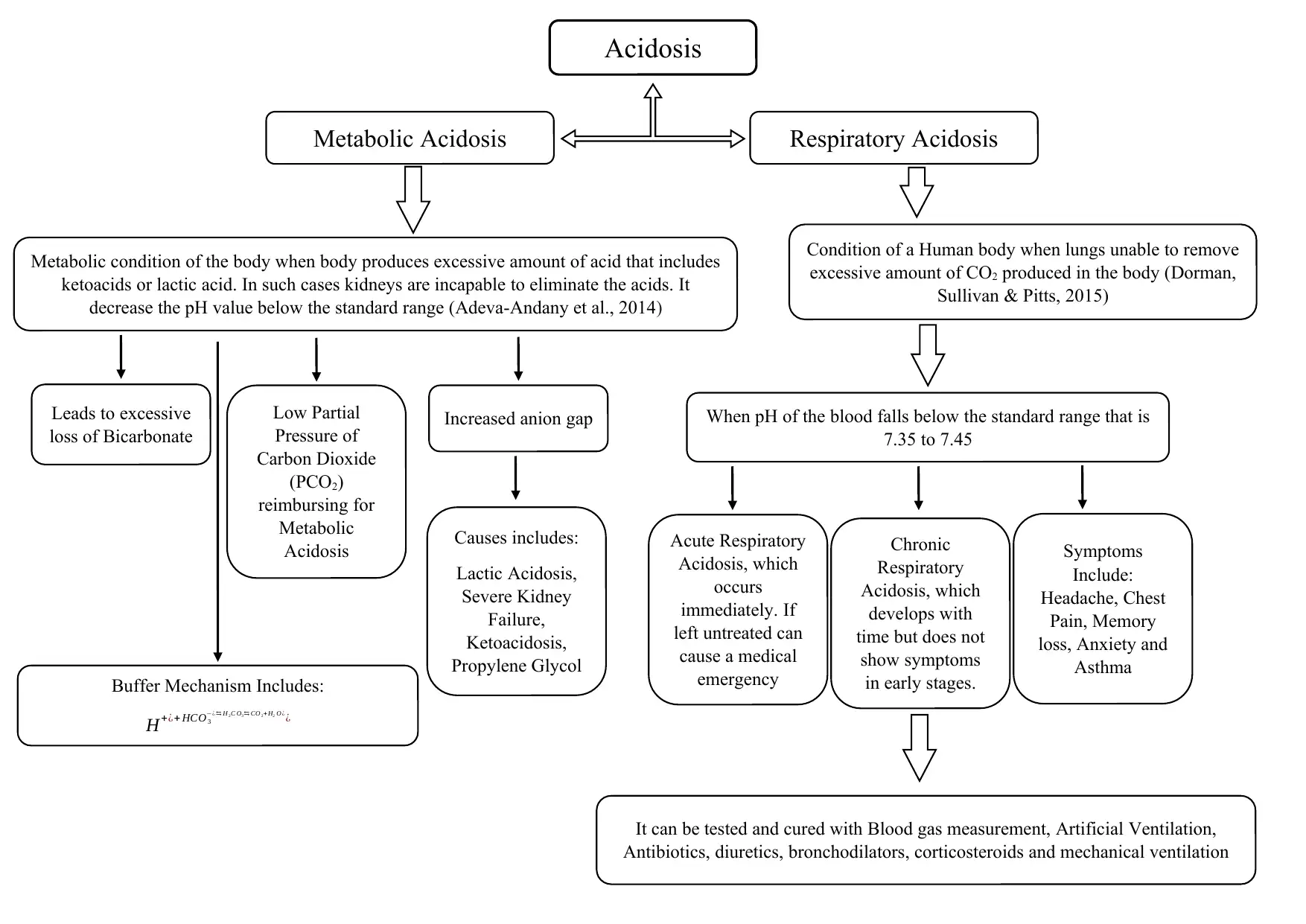
This assignment provides a comprehensive overview of acid-base balance, a critical concept in human physiology, and the disorders that can arise from imbalances in blood pH. It defines alkalosis and acidosis, detailing the conditions that occur when the blood becomes too alkaline or acidic, respectively. The document explores respiratory and metabolic disorders, including respiratory acidosis and alkalosis, and metabolic acidosis and alkalosis, outlining their causes, symptoms, and mechanisms. It explains how these conditions impact the body's pH balance, referencing relevant literature to support the information. The assignment also touches on the buffer mechanisms, including increased anion gap and the role of partial pressure of carbon dioxide (PCO2), to help the body maintain homeostasis. It further discusses specific conditions like Liddle syndrome and the role of chloride in metabolic alkalosis. Finally, the assignment mentions the various methods of testing and treatment, such as blood gas measurement, artificial ventilation, and medication.
1 out of 4








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)