Medical Case Study: Evaluating Respiratory & Metabolic Acidosis
VerifiedAdded on 2023/06/14
|5
|1452
|118
Case Study
AI Summary
This case study presents a 25-year-old female patient experiencing fatigue, shortness of breath, and loss of consciousness, revealing a history of arrhythmia and seizures. Diagnostic tests indicate a blood pH of 7, high carbonic acid levels, low bicarbonate, and elevated anion gap, pointing towards physiological acidosis. The patient's symptoms and lab results suggest both respiratory and metabolic acidosis, potentially linked to her occupation in coal production, aspirin use, and diarrhea. The analysis explores the pathophysiology of acid-base imbalances, the compensatory mechanisms of the kidneys and lungs, and the potential for renal failure if the condition worsens. High levels of hydrogen ion was also found in urine samples due to retention of bicarbonate ions by the kidney. The study references various articles related to the diagnosis and treatment of acid-base disorders. Desklib provides access to similar case studies and solved assignments for students.
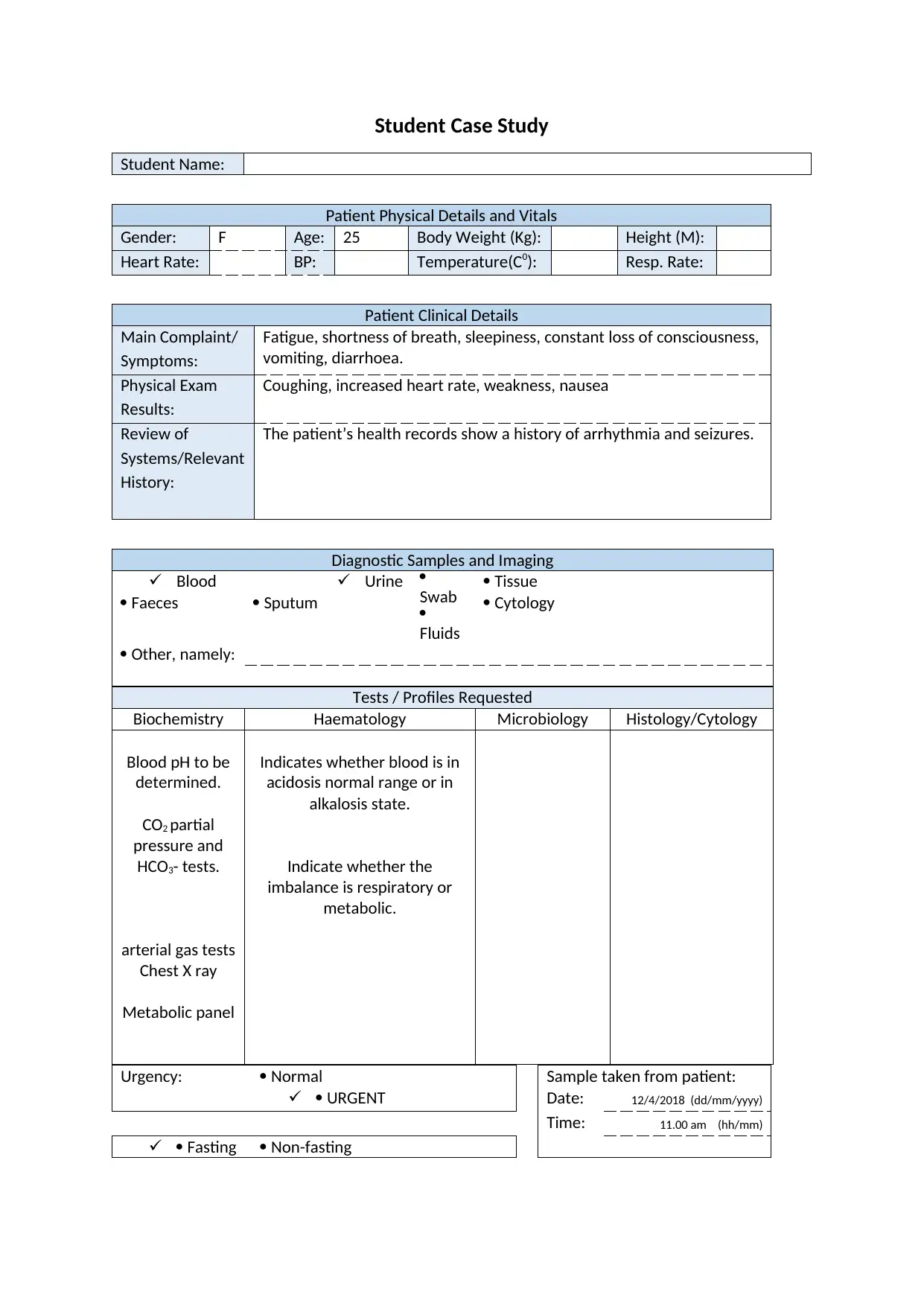
Student Case Study
Student Name:
Patient Physical Details and Vitals
Gender: F Age: 25 Body Weight (Kg): Height (M):
Heart Rate: BP: Temperature(C0): Resp. Rate:
Patient Clinical Details
Main Complaint/
Symptoms:
Fatigue, shortness of breath, sleepiness, constant loss of consciousness,
vomiting, diarrhoea.
Physical Exam
Results:
Coughing, increased heart rate, weakness, nausea
Review of
Systems/Relevant
History:
The patient’s health records show a history of arrhythmia and seizures.
Diagnostic Samples and Imaging
Blood
Faeces
Urine
Sputum
Swab
Fluids
Tissue
Cytology
Other, namely:
Tests / Profiles Requested
Biochemistry Haematology Microbiology Histology/Cytology
Blood pH to be
determined.
CO2 partial
pressure and
HCO3- tests.
arterial gas tests
Chest X ray
Metabolic panel
Indicates whether blood is in
acidosis normal range or in
alkalosis state.
Indicate whether the
imbalance is respiratory or
metabolic.
Urgency: Normal Sample taken from patient:
URGENT Date: 12/4/2018 (dd/mm/yyyy)
Time: 11.00 am (hh/mm)
Fasting Non-fasting
Student Name:
Patient Physical Details and Vitals
Gender: F Age: 25 Body Weight (Kg): Height (M):
Heart Rate: BP: Temperature(C0): Resp. Rate:
Patient Clinical Details
Main Complaint/
Symptoms:
Fatigue, shortness of breath, sleepiness, constant loss of consciousness,
vomiting, diarrhoea.
Physical Exam
Results:
Coughing, increased heart rate, weakness, nausea
Review of
Systems/Relevant
History:
The patient’s health records show a history of arrhythmia and seizures.
Diagnostic Samples and Imaging
Blood
Faeces
Urine
Sputum
Swab
Fluids
Tissue
Cytology
Other, namely:
Tests / Profiles Requested
Biochemistry Haematology Microbiology Histology/Cytology
Blood pH to be
determined.
CO2 partial
pressure and
HCO3- tests.
arterial gas tests
Chest X ray
Metabolic panel
Indicates whether blood is in
acidosis normal range or in
alkalosis state.
Indicate whether the
imbalance is respiratory or
metabolic.
Urgency: Normal Sample taken from patient:
URGENT Date: 12/4/2018 (dd/mm/yyyy)
Time: 11.00 am (hh/mm)
Fasting Non-fasting
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
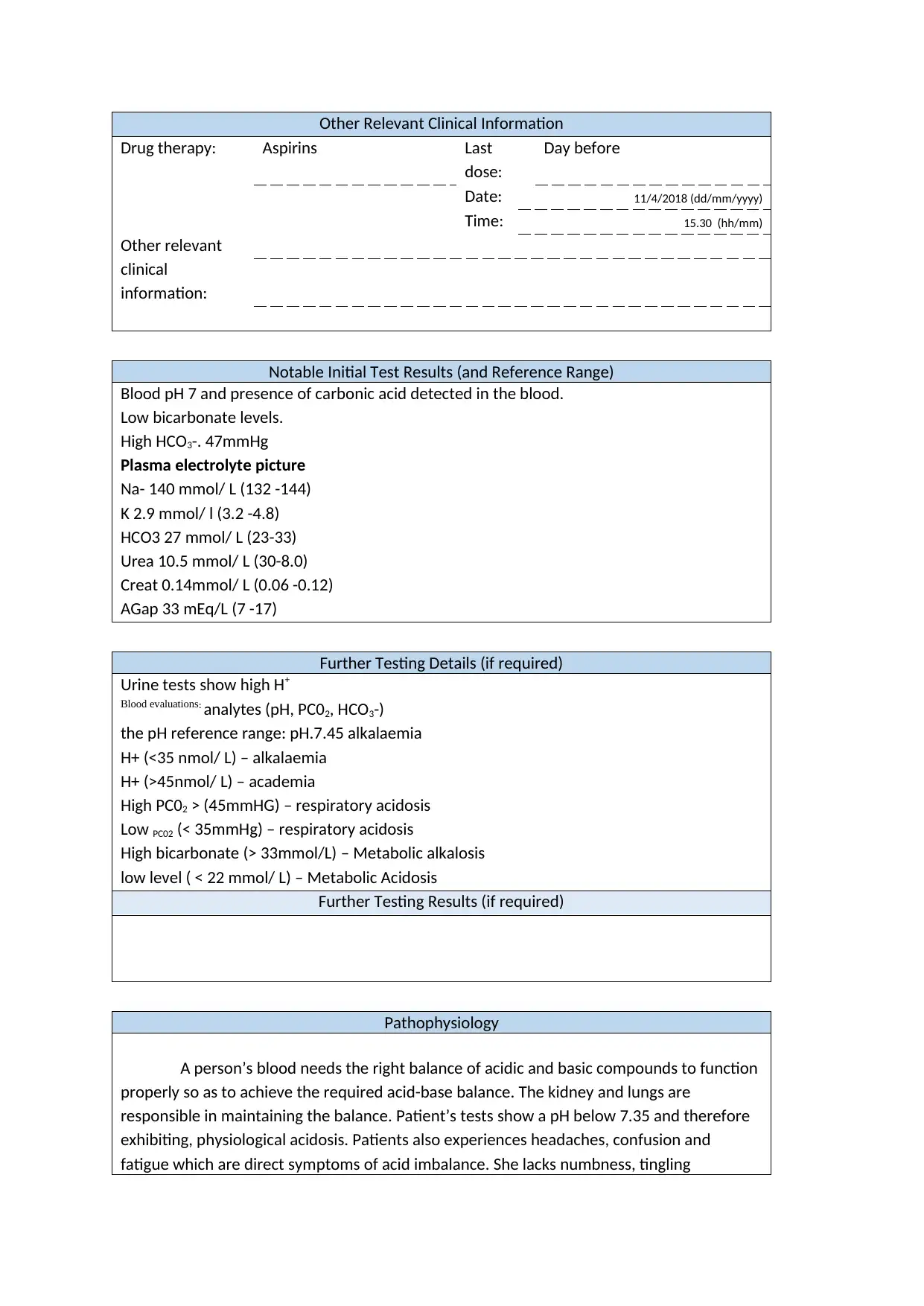
Other Relevant Clinical Information
Drug therapy: Aspirins Last
dose:
Day before
Date: 11/4/2018 (dd/mm/yyyy)
Time: 15.30 (hh/mm)
Other relevant
clinical
information:
Notable Initial Test Results (and Reference Range)
Blood pH 7 and presence of carbonic acid detected in the blood.
Low bicarbonate levels.
High HCO3-. 47mmHg
Plasma electrolyte picture
Na- 140 mmol/ L (132 -144)
K 2.9 mmol/ l (3.2 -4.8)
HCO3 27 mmol/ L (23-33)
Urea 10.5 mmol/ L (30-8.0)
Creat 0.14mmol/ L (0.06 -0.12)
AGap 33 mEq/L (7 -17)
Further Testing Details (if required)
Urine tests show high H+
Blood evaluations: analytes (pH, PC02, HCO3-)
the pH reference range: pH.7.45 alkalaemia
H+ (<35 nmol/ L) – alkalaemia
H+ (>45nmol/ L) – academia
High PC02 > (45mmHG) – respiratory acidosis
Low PC02 (< 35mmHg) – respiratory acidosis
High bicarbonate (> 33mmol/L) – Metabolic alkalosis
low level ( < 22 mmol/ L) – Metabolic Acidosis
Further Testing Results (if required)
Pathophysiology
A person’s blood needs the right balance of acidic and basic compounds to function
properly so as to achieve the required acid-base balance. The kidney and lungs are
responsible in maintaining the balance. Patient’s tests show a pH below 7.35 and therefore
exhibiting, physiological acidosis. Patients also experiences headaches, confusion and
fatigue which are direct symptoms of acid imbalance. She lacks numbness, tingling
Drug therapy: Aspirins Last
dose:
Day before
Date: 11/4/2018 (dd/mm/yyyy)
Time: 15.30 (hh/mm)
Other relevant
clinical
information:
Notable Initial Test Results (and Reference Range)
Blood pH 7 and presence of carbonic acid detected in the blood.
Low bicarbonate levels.
High HCO3-. 47mmHg
Plasma electrolyte picture
Na- 140 mmol/ L (132 -144)
K 2.9 mmol/ l (3.2 -4.8)
HCO3 27 mmol/ L (23-33)
Urea 10.5 mmol/ L (30-8.0)
Creat 0.14mmol/ L (0.06 -0.12)
AGap 33 mEq/L (7 -17)
Further Testing Details (if required)
Urine tests show high H+
Blood evaluations: analytes (pH, PC02, HCO3-)
the pH reference range: pH.7.45 alkalaemia
H+ (<35 nmol/ L) – alkalaemia
H+ (>45nmol/ L) – academia
High PC02 > (45mmHG) – respiratory acidosis
Low PC02 (< 35mmHg) – respiratory acidosis
High bicarbonate (> 33mmol/L) – Metabolic alkalosis
low level ( < 22 mmol/ L) – Metabolic Acidosis
Further Testing Results (if required)
Pathophysiology
A person’s blood needs the right balance of acidic and basic compounds to function
properly so as to achieve the required acid-base balance. The kidney and lungs are
responsible in maintaining the balance. Patient’s tests show a pH below 7.35 and therefore
exhibiting, physiological acidosis. Patients also experiences headaches, confusion and
fatigue which are direct symptoms of acid imbalance. She lacks numbness, tingling

sensation, nausea, vomiting, muscle twitching and spasm which are signs of alkalosis (Yun
Kyu, 2010).
The high amount of carbonic acid in the patient’s blood system show signs of
oxygen poisoning. The patient seems to be exposed to an environment where she breathes
in a lot of carbon dioxide gas. Her low bicarbonate levels bring about the diarrhoea she is
experiencing. Being that she works in a coal production company, she is exposed to high
levels of carbon dioxide which have increased her carbonic acid in the blood. This has
increases her respiratory acidosis. Her strenuous activities have led to metabolic acidosis
(Jain, 2018).
In order to relief the discomfort and pain she experiences due to her constant
headaches, she explained that she takes in a lot of aspirins to help her relieve the headache.
In high doses, her drug routine leads to metabolic acidosis. Similarly, being that she
diarrhoeas, this could lead to low bicarbonate ions which increase her metabolic acidosis.
Her high levels of carbonic acid caused by the high amounts of CO2 in her blood, causes her
to suffer from respiratory acidosis (Jayara, 2016).
The high gap in anions is indicate of acidosis within the upper limit reference range
of 17 mEg/ L, at 16 mEg/ L difference. Then an assumption that there has been an addition
of hydrogen ions to the ECF resulting to a decrease in plasma bicarbonate of about 16
mmo/L. this leads to the development of acidosis. Her decreased plasma could lead to
acidaemia disorder that may contribute a lot to renal issues (R. N. Walmsley, 1999).
She is in need of respiratory compensation to improve her metabolic acidosis which
increases her respiratory rate in order to drive out the excess CO2 in her blood and readjust
the bicarbonate to carbonic acid ratio to 20:1 level. Her low plasma HCO3- (<14 mEq/L
[mmol/L) causes the decreased cardiac activity or contractility and arrhythmia. It also
causes depressed central nervous system depressed functions. It’s important to note that
her metabolic acidosis is generated by her renal compensation to chronic respiratory
alkalosis. Her serum Ag shows increased unmeasured anions (UA) which assists in
determining the cause of her metabolic acidosis (Kellum, 2014).
Being that the bicarbonate levels are low, there is high indication of hyperchloremic
metabolic acidosis which reflect on the blood pH and blood gases tests. The low pH levels
show the metabolic acidosis. The HCO3- indicate that she has bad respiratory acidosis and
metabolic acidosis. The high level of acidity shown in her blood indicate that the kidney is
unable to react fast in buffering the concertation using the hydrogen buffers. This may cause
renal failure. The respiratory system regulates the PaCO2 at the recommend 40mmHg levels
and thus the chemoreceptors at the central medulla rise at high rates and the depth of
breathing rises as well causing normal breathes. The hypoventilation causes secretion of CO2
to be reduced and retained PaCO2; hypercapnia to fall in pH leading to respiratory acidosis.
The kidney regulates pH and HCO3 levels to normal range. The high HCO3 exhibited in the
patient will result to HCO3 renal excretion in urine. The uniform change of PC02 and
bicarbonate shows respiratory and metabolic acidosis. The change is one is always the
opposite in the other due to compensatory responses (McNamara, 2001).
Only buffers like trothemanine and can alter the strong difference in ions as they do
not affect the plasma. Metabolic acidosis can lead to uraemia which is retention of urea and
uric acid or ingestion of diabetic ketoacidosis where the ketones become present in the
blood system. This are only managed by compensatory mechanisms and when one fails, it
Kyu, 2010).
The high amount of carbonic acid in the patient’s blood system show signs of
oxygen poisoning. The patient seems to be exposed to an environment where she breathes
in a lot of carbon dioxide gas. Her low bicarbonate levels bring about the diarrhoea she is
experiencing. Being that she works in a coal production company, she is exposed to high
levels of carbon dioxide which have increased her carbonic acid in the blood. This has
increases her respiratory acidosis. Her strenuous activities have led to metabolic acidosis
(Jain, 2018).
In order to relief the discomfort and pain she experiences due to her constant
headaches, she explained that she takes in a lot of aspirins to help her relieve the headache.
In high doses, her drug routine leads to metabolic acidosis. Similarly, being that she
diarrhoeas, this could lead to low bicarbonate ions which increase her metabolic acidosis.
Her high levels of carbonic acid caused by the high amounts of CO2 in her blood, causes her
to suffer from respiratory acidosis (Jayara, 2016).
The high gap in anions is indicate of acidosis within the upper limit reference range
of 17 mEg/ L, at 16 mEg/ L difference. Then an assumption that there has been an addition
of hydrogen ions to the ECF resulting to a decrease in plasma bicarbonate of about 16
mmo/L. this leads to the development of acidosis. Her decreased plasma could lead to
acidaemia disorder that may contribute a lot to renal issues (R. N. Walmsley, 1999).
She is in need of respiratory compensation to improve her metabolic acidosis which
increases her respiratory rate in order to drive out the excess CO2 in her blood and readjust
the bicarbonate to carbonic acid ratio to 20:1 level. Her low plasma HCO3- (<14 mEq/L
[mmol/L) causes the decreased cardiac activity or contractility and arrhythmia. It also
causes depressed central nervous system depressed functions. It’s important to note that
her metabolic acidosis is generated by her renal compensation to chronic respiratory
alkalosis. Her serum Ag shows increased unmeasured anions (UA) which assists in
determining the cause of her metabolic acidosis (Kellum, 2014).
Being that the bicarbonate levels are low, there is high indication of hyperchloremic
metabolic acidosis which reflect on the blood pH and blood gases tests. The low pH levels
show the metabolic acidosis. The HCO3- indicate that she has bad respiratory acidosis and
metabolic acidosis. The high level of acidity shown in her blood indicate that the kidney is
unable to react fast in buffering the concertation using the hydrogen buffers. This may cause
renal failure. The respiratory system regulates the PaCO2 at the recommend 40mmHg levels
and thus the chemoreceptors at the central medulla rise at high rates and the depth of
breathing rises as well causing normal breathes. The hypoventilation causes secretion of CO2
to be reduced and retained PaCO2; hypercapnia to fall in pH leading to respiratory acidosis.
The kidney regulates pH and HCO3 levels to normal range. The high HCO3 exhibited in the
patient will result to HCO3 renal excretion in urine. The uniform change of PC02 and
bicarbonate shows respiratory and metabolic acidosis. The change is one is always the
opposite in the other due to compensatory responses (McNamara, 2001).
Only buffers like trothemanine and can alter the strong difference in ions as they do
not affect the plasma. Metabolic acidosis can lead to uraemia which is retention of urea and
uric acid or ingestion of diabetic ketoacidosis where the ketones become present in the
blood system. This are only managed by compensatory mechanisms and when one fails, it
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

affects the rest as they all have their limits. If the kidney fails and the acid ratio are changed
drastically, the body may be unable to compensate. The change in pH can also denature
proteins and worsening the metabolic processes that cause serious tissue damage and even
death in worst case scenarios. The patient’s urine samples showed high levels of hydrogen
ion due to retention of bicarbonate ions by the kidney. This is the compensation mechanism
for respiratory diseases which increases carbonic acid levels in the respiratory system and
blood (Yun Kyu, 2010).
drastically, the body may be unable to compensate. The change in pH can also denature
proteins and worsening the metabolic processes that cause serious tissue damage and even
death in worst case scenarios. The patient’s urine samples showed high levels of hydrogen
ion due to retention of bicarbonate ions by the kidney. This is the compensation mechanism
for respiratory diseases which increases carbonic acid levels in the respiratory system and
blood (Yun Kyu, 2010).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Jain, N. (2018). NEPHROLOGY HYPERTENSION: Acid/Base Disorders: Metabolic Alkalosi.
Renal And Urology News, 1. Retrieved 4 12, 2018, from
https://www.renalandurologynews.com/nephrology-hypertension/acidbase-
disorders-metabolic-alkalosis/article/616248/
Jayara, A. (2016). Diagnosis and Treatment of acid base disorders. Health and Medicine, 1.
Kellum, A. J.-J. (2014, September 11). Acid–base disturbances in intensive care patients:
etiology, pathophysiology and treatmen. Oxford Academic, 30(7), 1104-1111.
doi:10.1093/ndt/gfu289
McNamara, J. W. (2001). Acid-base balance: part I. Physiology. National Institute of Health.
Retrieved 4 12, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/16573501
Wiseman, C. A. (2005). Disorders of Potassium and Acid-Base Balance. AJKD, 45(5), 941-949.
doi:10.1053/j.ajkd.2005.01.042
Yun Kyu, O. (2010). Electrolytes and Blood Pressure: Acid-Base Disorders in ICU Patients.
National Institute of Health, 1. Retrieved 4 12, 2018, from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043757/
Jain, N. (2018). NEPHROLOGY HYPERTENSION: Acid/Base Disorders: Metabolic Alkalosi.
Renal And Urology News, 1. Retrieved 4 12, 2018, from
https://www.renalandurologynews.com/nephrology-hypertension/acidbase-
disorders-metabolic-alkalosis/article/616248/
Jayara, A. (2016). Diagnosis and Treatment of acid base disorders. Health and Medicine, 1.
Kellum, A. J.-J. (2014, September 11). Acid–base disturbances in intensive care patients:
etiology, pathophysiology and treatmen. Oxford Academic, 30(7), 1104-1111.
doi:10.1093/ndt/gfu289
McNamara, J. W. (2001). Acid-base balance: part I. Physiology. National Institute of Health.
Retrieved 4 12, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/16573501
Wiseman, C. A. (2005). Disorders of Potassium and Acid-Base Balance. AJKD, 45(5), 941-949.
doi:10.1053/j.ajkd.2005.01.042
Yun Kyu, O. (2010). Electrolytes and Blood Pressure: Acid-Base Disorders in ICU Patients.
National Institute of Health, 1. Retrieved 4 12, 2018, from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043757/
1 out of 5
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




