Advanced Research Methods: HIV Viral Load Intervention Study Analysis
VerifiedAdded on 2022/09/18
|21
|3398
|29
Report
AI Summary
This report analyzes a qualitative study on an HIV viral load intervention using SMS technology in Zimbabwe. The study, conducted in Gutu and Buhera districts, utilized a phenomenological method to understand participants' experiences with receiving their HIV viral load results via SMS. The intervention aimed to improve healthcare service delivery and reduce patient waiting times. The research involved participants aged 18-60 with a viral load greater than or equal to 1000 copies/mL, who were notified of their results readiness via SMS. Ethical considerations, including privacy preservation, were paramount. Findings highlighted mHealth's advantages, such as time-saving and enhanced privacy. Limitations included potential misunderstandings of messages and infrastructure challenges. The study concluded that the intervention was acceptable, despite needing clarifications on the new messaging system. The report includes the Johns Hopkins Nursing Evidence-Based Practice Appendix E Research Evidence Appraisal Tool to evaluate the research methodology and findings.

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Advanced Research Methods
Name
Institution
Author’s Note
1
Appendix E
Research Evidence Appraisal Tool
Advanced Research Methods
Name
Institution
Author’s Note
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
ADVANCED RESEARCH METHODS
Introduction
In the world today, a lot of efforts are put in place by many individuals to be able to improve on the
health sector. Human Immunodeficiency Virus (HIV), in particular, has been contained and can be controlled
by many ways that have been designed, ranging from sensitization to medications to minimize infections and to
reduce impacts on the lives of individuals infected (Venables et al., 2019). This article entails the
implementation of various telecommunication facilities within the healthcare sector in order to scale up various
healthcare services. In this sector of health, there is a lot of information that is confidential, and even a clue of
the health condition of an individual should not be availed to the public.
Body
Qualitative method of study
The qualitative method that is attached to this study is phenomenological method because it fully relies
on the perspectives of various participants in order to provide insights into their motives.
Participant sampling
The intervention that was used from June 2014 after lab results were sent to twenty-one health centers in
Gutu and Buhera, Zimbabwe via short messages, when the sampling process for the participants in the study
began. The sample group was picked from the result since the HIV positive individuals were needed from the
age of 18 to 60 years (Venables et al., 2019). The invitation was done through the messages as they were told to
visit the health center personally for their results. The design ensured both male and female inclusion with a
viral load more than or equal to 1000copies per milliliters. Considerations were made on the location and
distances from the health facilities to ensure wide coverage of the study within the two districts.
2
Appendix E
Research Evidence Appraisal Tool
ADVANCED RESEARCH METHODS
Introduction
In the world today, a lot of efforts are put in place by many individuals to be able to improve on the
health sector. Human Immunodeficiency Virus (HIV), in particular, has been contained and can be controlled
by many ways that have been designed, ranging from sensitization to medications to minimize infections and to
reduce impacts on the lives of individuals infected (Venables et al., 2019). This article entails the
implementation of various telecommunication facilities within the healthcare sector in order to scale up various
healthcare services. In this sector of health, there is a lot of information that is confidential, and even a clue of
the health condition of an individual should not be availed to the public.
Body
Qualitative method of study
The qualitative method that is attached to this study is phenomenological method because it fully relies
on the perspectives of various participants in order to provide insights into their motives.
Participant sampling
The intervention that was used from June 2014 after lab results were sent to twenty-one health centers in
Gutu and Buhera, Zimbabwe via short messages, when the sampling process for the participants in the study
began. The sample group was picked from the result since the HIV positive individuals were needed from the
age of 18 to 60 years (Venables et al., 2019). The invitation was done through the messages as they were told to
visit the health center personally for their results. The design ensured both male and female inclusion with a
viral load more than or equal to 1000copies per milliliters. Considerations were made on the location and
distances from the health facilities to ensure wide coverage of the study within the two districts.
2

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
The two districts’ results were part of the normal laboratory operations, and they presented 70% of the
patients on the antiretroviral therapy in the two districts. The message technology was mainly implemented to
reduce the amount of time taken by patients to gain access to their results. It also aimed towards minimizing
delays when attending to patients who required much support.
Questionnaire
Patients whose blood samples were tested received messages through their mobile phones about the
results readiness with instructions on how to get them. The content of the message was limited to notification of
the result readiness, but there was no trace of the result. This was to avoid any further complications that could
emerge as a result of phone sharing.
Ethics
Ethically, the use of the services of the short message without result details was to preserve the privacy
of the people who were involved in the study (Venables et al., 2019). Before the study, approval was provided
by the Médecins Sans Frontières Ethics Review Board Geneva, from Switzerland and the medical research
council of Zimbabwe. Everyone’s willingness to participate was confirmed as there was a verbal agreement
made by the sample group. With the literacy rate of 88.69% in Zimbabwe, the short messages were sent in
English despite the many languages spoken in the country.
Analysis of findings
From the results, mHealth was one of the best technologies to be brought in the health sector following
many of the advantages ranging from time-saving to privacy enhancement. Those with high viral loads and
maybe require to visit health centers will also be able to respond quickly.
3
Appendix E
Research Evidence Appraisal Tool
The two districts’ results were part of the normal laboratory operations, and they presented 70% of the
patients on the antiretroviral therapy in the two districts. The message technology was mainly implemented to
reduce the amount of time taken by patients to gain access to their results. It also aimed towards minimizing
delays when attending to patients who required much support.
Questionnaire
Patients whose blood samples were tested received messages through their mobile phones about the
results readiness with instructions on how to get them. The content of the message was limited to notification of
the result readiness, but there was no trace of the result. This was to avoid any further complications that could
emerge as a result of phone sharing.
Ethics
Ethically, the use of the services of the short message without result details was to preserve the privacy
of the people who were involved in the study (Venables et al., 2019). Before the study, approval was provided
by the Médecins Sans Frontières Ethics Review Board Geneva, from Switzerland and the medical research
council of Zimbabwe. Everyone’s willingness to participate was confirmed as there was a verbal agreement
made by the sample group. With the literacy rate of 88.69% in Zimbabwe, the short messages were sent in
English despite the many languages spoken in the country.
Analysis of findings
From the results, mHealth was one of the best technologies to be brought in the health sector following
many of the advantages ranging from time-saving to privacy enhancement. Those with high viral loads and
maybe require to visit health centers will also be able to respond quickly.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Limitations
The challenges here are a misunderstanding of the message and improper infrastructure to facilitate
movement to the health centers.
Discussion
It was realized that there should be an alert in the event there is any intervention so as to make people
understand the possible changes in the system. This study was conducted in a proper way that ensures the
security of the information and the participants. The sample was inclusive with a lot of considerations on the
vast area and the relevance of the sample to the new intervention of the message services in the health sector in
Zimbabwe to notify on the viral load of the HIV patients.
Summary
This intervention was acceptable despite the few issues that were to be ironed out, such as notification
and explanations on the new intervention and the reasons for the messages that patients were to expect.
4
Appendix E
Research Evidence Appraisal Tool
Limitations
The challenges here are a misunderstanding of the message and improper infrastructure to facilitate
movement to the health centers.
Discussion
It was realized that there should be an alert in the event there is any intervention so as to make people
understand the possible changes in the system. This study was conducted in a proper way that ensures the
security of the information and the participants. The sample was inclusive with a lot of considerations on the
vast area and the relevance of the sample to the new intervention of the message services in the health sector in
Zimbabwe to notify on the viral load of the HIV patients.
Summary
This intervention was acceptable despite the few issues that were to be ironed out, such as notification
and explanations on the new intervention and the reasons for the messages that patients were to expect.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
References
Venables, E. et al. (2019). Patient and health-care worker experiences of an HIV viral load
intervention using SMS: A qualitative study.
5
Appendix E
Research Evidence Appraisal Tool
References
Venables, E. et al. (2019). Patient and health-care worker experiences of an HIV viral load
intervention using SMS: A qualitative study.
5
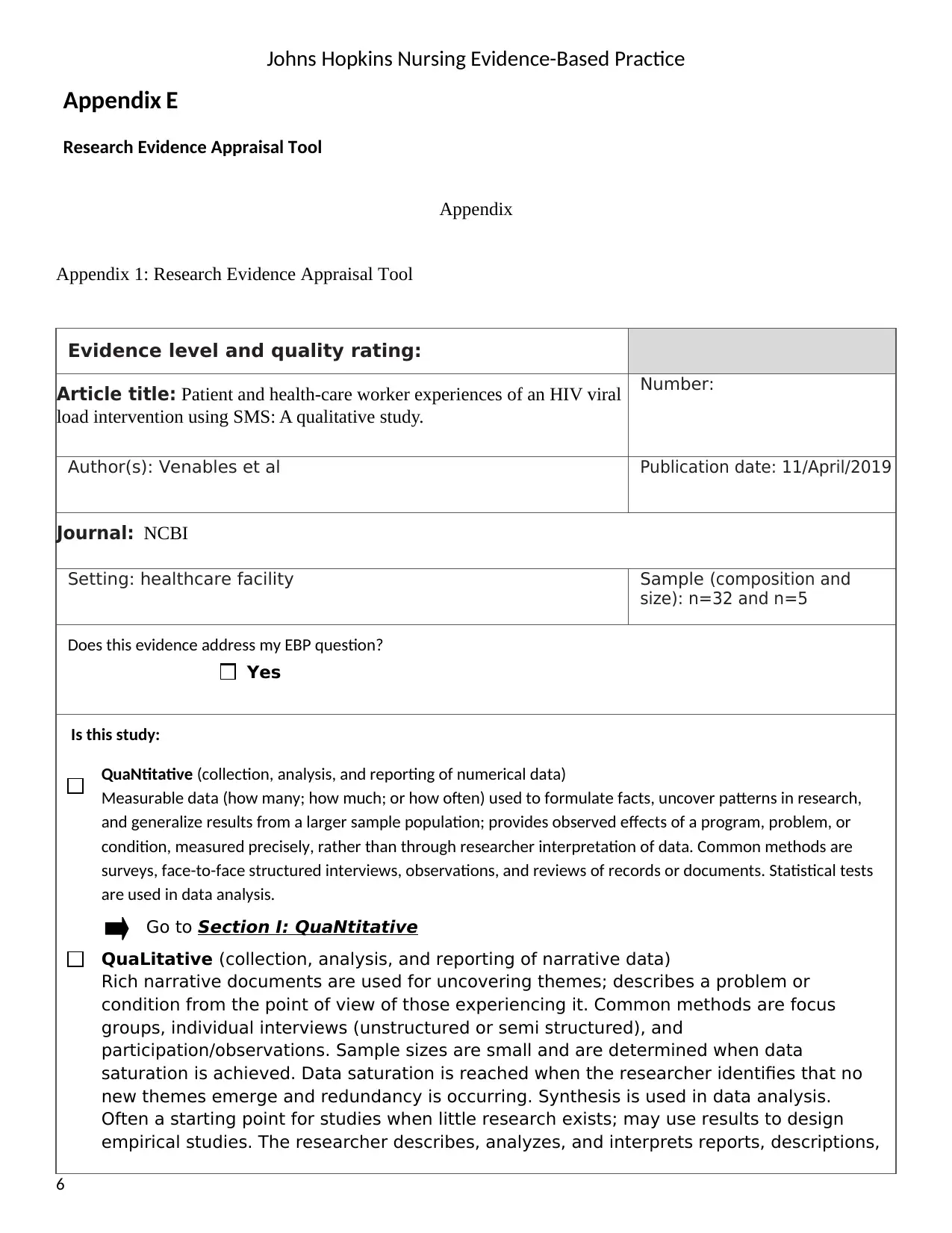
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Appendix
Appendix 1: Research Evidence Appraisal Tool
Evidence level and quality rating:
Article title: Patient and health-care worker experiences of an HIV viral
load intervention using SMS: A qualitative study.
Number:
Author(s): Venables et al Publication date: 11/April/2019
Journal: NCBI
Setting: healthcare facility Sample (composition and
size): n=32 and n=5
Does this evidence address my EBP question?
Yes
Is this study:
QuaNtitative (collection, analysis, and reporting of numerical data)
Measurable data (how many; how much; or how often) used to formulate facts, uncover patterns in research,
and generalize results from a larger sample population; provides observed effects of a program, problem, or
condition, measured precisely, rather than through researcher interpretation of data. Common methods are
surveys, face-to-face structured interviews, observations, and reviews of records or documents. Statistical tests
are used in data analysis.
Go to Section I: QuaNtitative
QuaLitative (collection, analysis, and reporting of narrative data)
Rich narrative documents are used for uncovering themes; describes a problem or
condition from the point of view of those experiencing it. Common methods are focus
groups, individual interviews (unstructured or semi structured), and
participation/observations. Sample sizes are small and are determined when data
saturation is achieved. Data saturation is reached when the researcher identifies that no
new themes emerge and redundancy is occurring. Synthesis is used in data analysis.
Often a starting point for studies when little research exists; may use results to design
empirical studies. The researcher describes, analyzes, and interprets reports, descriptions,
6
Y
Appendix E
Research Evidence Appraisal Tool
Appendix
Appendix 1: Research Evidence Appraisal Tool
Evidence level and quality rating:
Article title: Patient and health-care worker experiences of an HIV viral
load intervention using SMS: A qualitative study.
Number:
Author(s): Venables et al Publication date: 11/April/2019
Journal: NCBI
Setting: healthcare facility Sample (composition and
size): n=32 and n=5
Does this evidence address my EBP question?
Yes
Is this study:
QuaNtitative (collection, analysis, and reporting of numerical data)
Measurable data (how many; how much; or how often) used to formulate facts, uncover patterns in research,
and generalize results from a larger sample population; provides observed effects of a program, problem, or
condition, measured precisely, rather than through researcher interpretation of data. Common methods are
surveys, face-to-face structured interviews, observations, and reviews of records or documents. Statistical tests
are used in data analysis.
Go to Section I: QuaNtitative
QuaLitative (collection, analysis, and reporting of narrative data)
Rich narrative documents are used for uncovering themes; describes a problem or
condition from the point of view of those experiencing it. Common methods are focus
groups, individual interviews (unstructured or semi structured), and
participation/observations. Sample sizes are small and are determined when data
saturation is achieved. Data saturation is reached when the researcher identifies that no
new themes emerge and redundancy is occurring. Synthesis is used in data analysis.
Often a starting point for studies when little research exists; may use results to design
empirical studies. The researcher describes, analyzes, and interprets reports, descriptions,
6
Y
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
and observations from participants.
Go to Section II: QuaLitative
Mixed methods (results reported both numerically and narratively)
Both quaNtitative and quaLitative methods are used in the study design. Using both
approaches, in combination, provides a better understanding of research problems than
using either approach alone. Sample sizes vary based on methods used. Data collection
involves collecting and analyzing both quaNtitative and quaLitative data in a single study
or series of studies. Interpretation is continual and can influence stages in the research
process.
Go to Section III: Mixed Methods
7
Appendix E
Research Evidence Appraisal Tool
and observations from participants.
Go to Section II: QuaLitative
Mixed methods (results reported both numerically and narratively)
Both quaNtitative and quaLitative methods are used in the study design. Using both
approaches, in combination, provides a better understanding of research problems than
using either approach alone. Sample sizes vary based on methods used. Data collection
involves collecting and analyzing both quaNtitative and quaLitative data in a single study
or series of studies. Interpretation is continual and can influence stages in the research
process.
Go to Section III: Mixed Methods
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
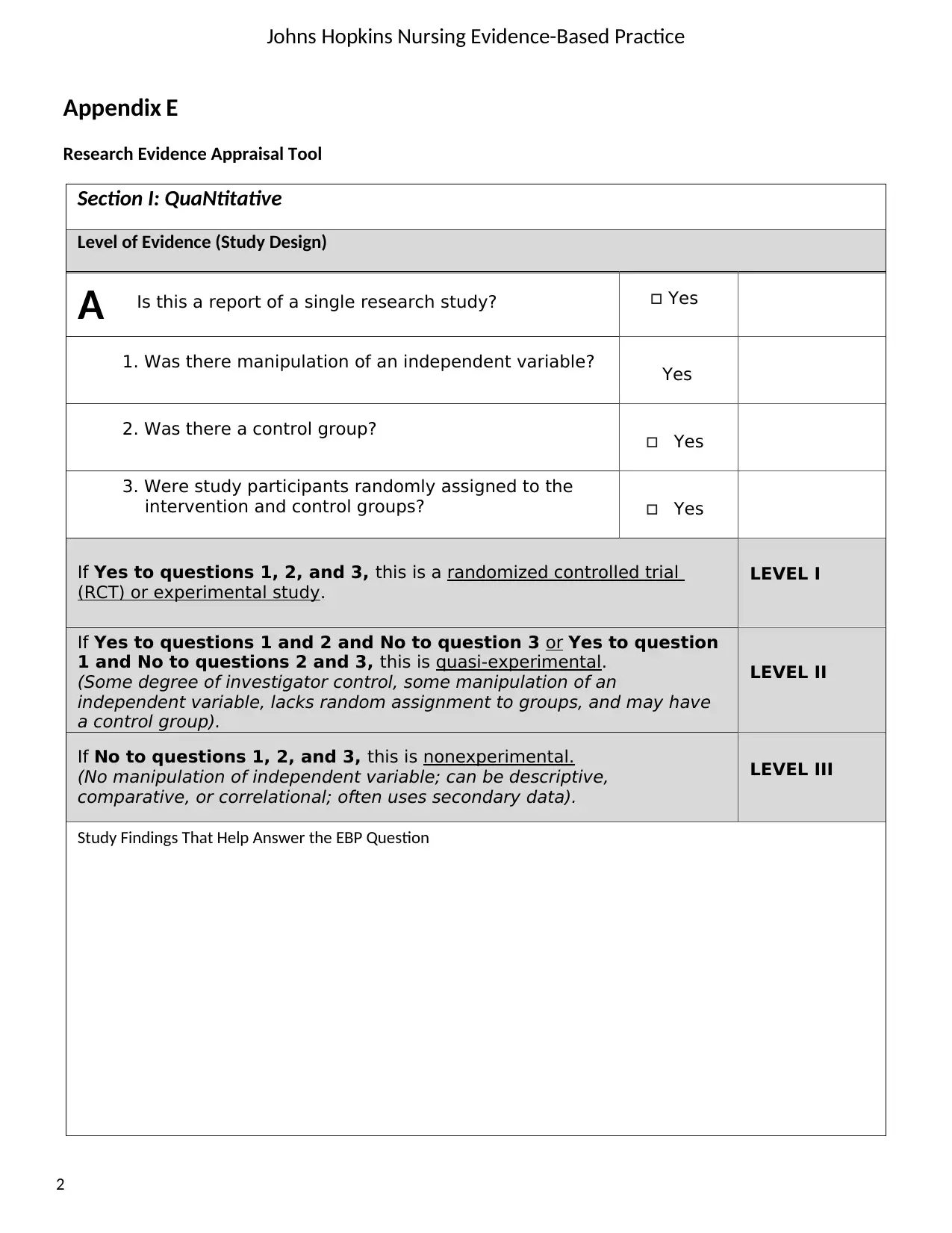
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Section I: QuaNtitative
Level of Evidence (Study Design)
Is this a report of a single research study? Yes
1. Was there manipulation of an independent variable? Yes
2. Was there a control group? Yes
3. Were study participants randomly assigned to the
intervention and control groups? Yes
If Yes to questions 1, 2, and 3, this is a randomized controlled trial
(RCT) or experimental study.
LEVEL I
If Yes to questions 1 and 2 and No to question 3 or Yes to question
1 and No to questions 2 and 3, this is quasi-experimental.
(Some degree of investigator control, some manipulation of an
independent variable, lacks random assignment to groups, and may have
a control group).
LEVEL II
If No to questions 1, 2, and 3, this is nonexperimental.
(No manipulation of independent variable; can be descriptive,
comparative, or correlational; often uses secondary data).
LEVEL III
Study Findings That Help Answer the EBP Question
2
A
Appendix E
Research Evidence Appraisal Tool
Section I: QuaNtitative
Level of Evidence (Study Design)
Is this a report of a single research study? Yes
1. Was there manipulation of an independent variable? Yes
2. Was there a control group? Yes
3. Were study participants randomly assigned to the
intervention and control groups? Yes
If Yes to questions 1, 2, and 3, this is a randomized controlled trial
(RCT) or experimental study.
LEVEL I
If Yes to questions 1 and 2 and No to question 3 or Yes to question
1 and No to questions 2 and 3, this is quasi-experimental.
(Some degree of investigator control, some manipulation of an
independent variable, lacks random assignment to groups, and may have
a control group).
LEVEL II
If No to questions 1, 2, and 3, this is nonexperimental.
(No manipulation of independent variable; can be descriptive,
comparative, or correlational; often uses secondary data).
LEVEL III
Study Findings That Help Answer the EBP Question
2
A
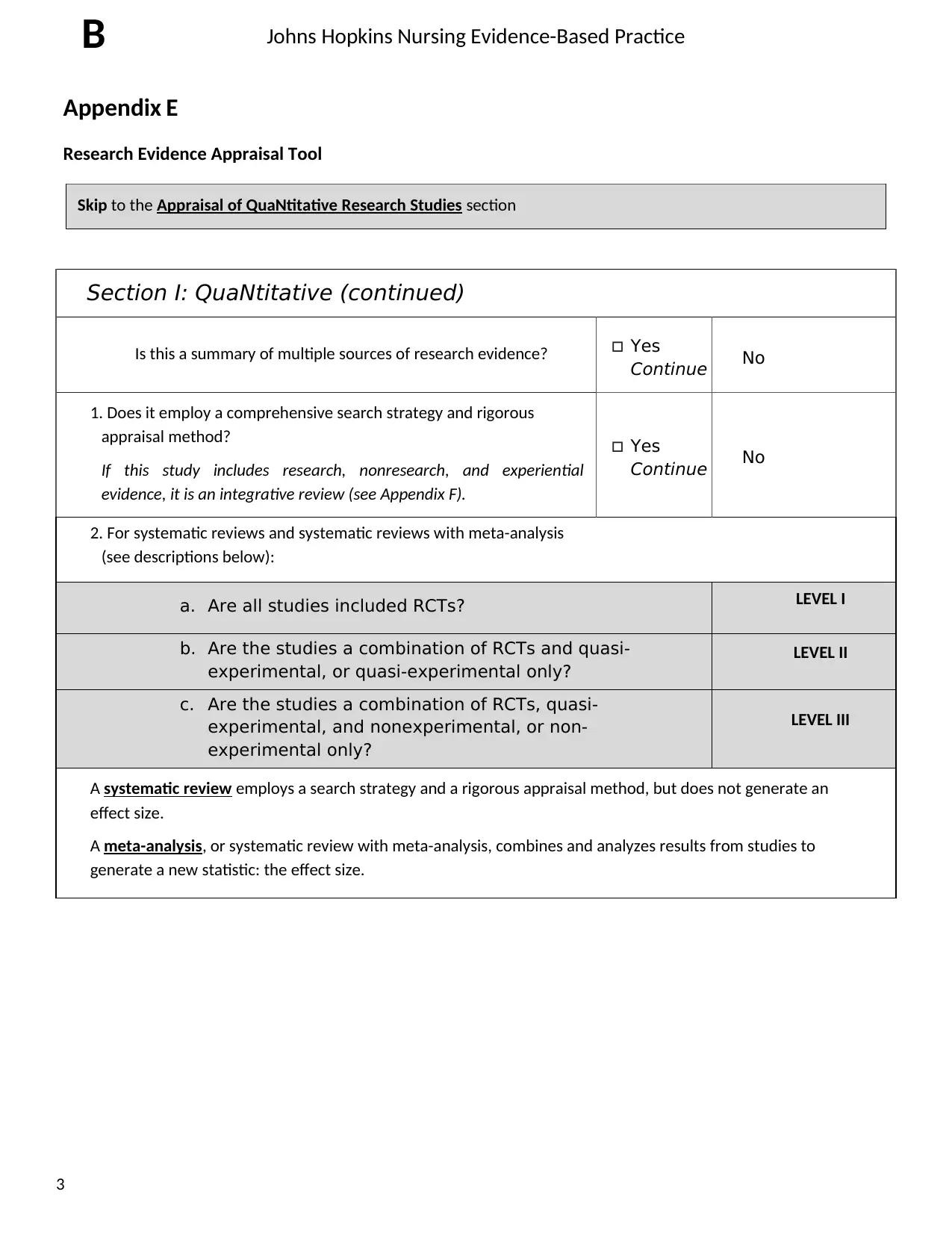
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Skip to the Appraisal of QuaNtitative Research Studies section
Section I: QuaNtitative (continued)
Is this a summary of multiple sources of research evidence? Yes
Continue No
1. Does it employ a comprehensive search strategy and rigorous
appraisal method?
If this study includes research, nonresearch, and experiential
evidence, it is an integrative review (see Appendix F).
Yes
Continue No
2. For systematic reviews and systematic reviews with meta-analysis
(see descriptions below):
a. Are all studies included RCTs? LEVEL I
b. Are the studies a combination of RCTs and quasi-
experimental, or quasi-experimental only?
LEVEL II
c. Are the studies a combination of RCTs, quasi-
experimental, and nonexperimental, or non-
experimental only?
LEVEL III
A systematic review employs a search strategy and a rigorous appraisal method, but does not generate an
effect size.
A meta-analysis, or systematic review with meta-analysis, combines and analyzes results from studies to
generate a new statistic: the effect size.
3
B
Appendix E
Research Evidence Appraisal Tool
Skip to the Appraisal of QuaNtitative Research Studies section
Section I: QuaNtitative (continued)
Is this a summary of multiple sources of research evidence? Yes
Continue No
1. Does it employ a comprehensive search strategy and rigorous
appraisal method?
If this study includes research, nonresearch, and experiential
evidence, it is an integrative review (see Appendix F).
Yes
Continue No
2. For systematic reviews and systematic reviews with meta-analysis
(see descriptions below):
a. Are all studies included RCTs? LEVEL I
b. Are the studies a combination of RCTs and quasi-
experimental, or quasi-experimental only?
LEVEL II
c. Are the studies a combination of RCTs, quasi-
experimental, and nonexperimental, or non-
experimental only?
LEVEL III
A systematic review employs a search strategy and a rigorous appraisal method, but does not generate an
effect size.
A meta-analysis, or systematic review with meta-analysis, combines and analyzes results from studies to
generate a new statistic: the effect size.
3
B
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
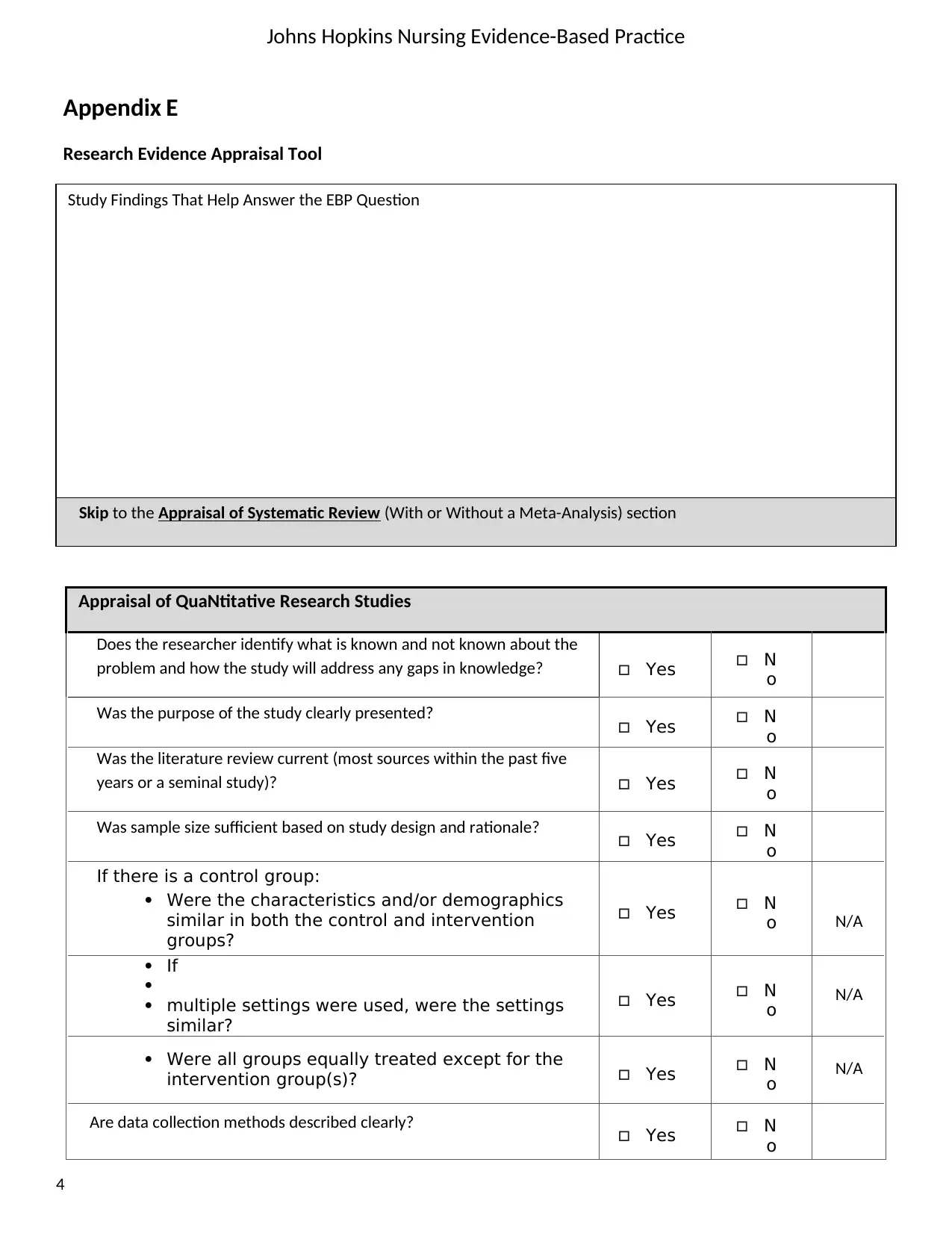
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
Study Findings That Help Answer the EBP Question
Skip to the Appraisal of Systematic Review (With or Without a Meta-Analysis) section
Appraisal of QuaNtitative Research Studies
Does the researcher identify what is known and not known about the
problem and how the study will address any gaps in knowledge? Yes N
o
Was the purpose of the study clearly presented? Yes N
o
Was the literature review current (most sources within the past five
years or a seminal study)? Yes N
o
Was sample size sufficient based on study design and rationale? Yes N
o
If there is a control group:
Were the characteristics and/or demographics
similar in both the control and intervention
groups?
Yes N
o N/A
If
multiple settings were used, were the settings
similar?
Yes N
o N/A
Were all groups equally treated except for the
intervention group(s)? Yes N
o N/A
Are data collection methods described clearly? Yes N
o
4
Appendix E
Research Evidence Appraisal Tool
Study Findings That Help Answer the EBP Question
Skip to the Appraisal of Systematic Review (With or Without a Meta-Analysis) section
Appraisal of QuaNtitative Research Studies
Does the researcher identify what is known and not known about the
problem and how the study will address any gaps in knowledge? Yes N
o
Was the purpose of the study clearly presented? Yes N
o
Was the literature review current (most sources within the past five
years or a seminal study)? Yes N
o
Was sample size sufficient based on study design and rationale? Yes N
o
If there is a control group:
Were the characteristics and/or demographics
similar in both the control and intervention
groups?
Yes N
o N/A
If
multiple settings were used, were the settings
similar?
Yes N
o N/A
Were all groups equally treated except for the
intervention group(s)? Yes N
o N/A
Are data collection methods described clearly? Yes N
o
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
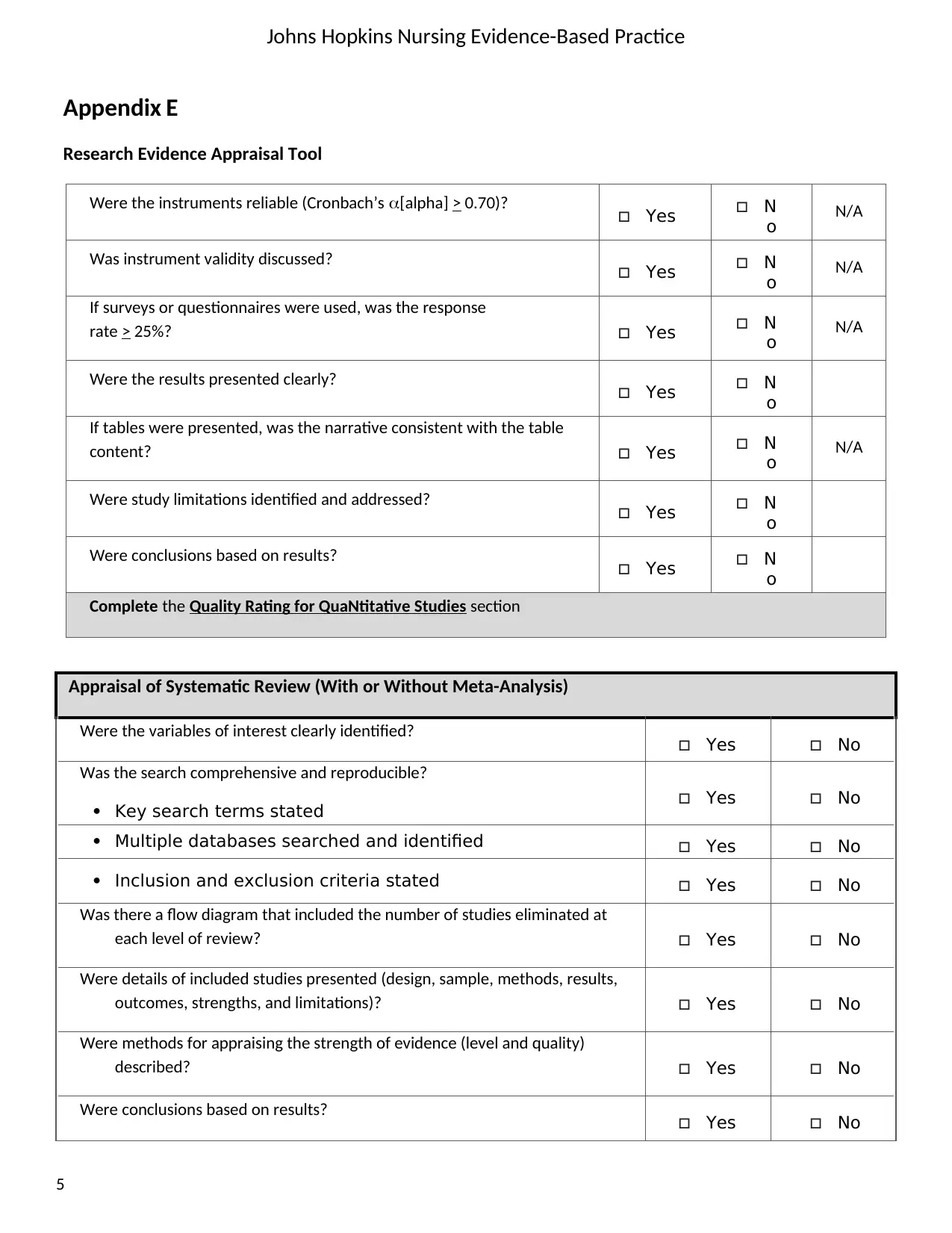
Were the instruments reliable (Cronbach’s [alpha] > 0.70)? Yes N
o N/A
Was instrument validity discussed? Yes N
o N/A
If surveys or questionnaires were used, was the response
rate > 25%? Yes N
o N/A
Were the results presented clearly? Yes N
o
If tables were presented, was the narrative consistent with the table
content? Yes N
o N/A
Were study limitations identified and addressed? Yes N
o
Were conclusions based on results? Yes N
o
Complete the Quality Rating for QuaNtitative Studies section
Appraisal of Systematic Review (With or Without Meta-Analysis)
Were the variables of interest clearly identified? Yes No
Was the search comprehensive and reproducible?
Key search terms stated Yes No
Multiple databases searched and identified Yes No
Inclusion and exclusion criteria stated Yes No
Was there a flow diagram that included the number of studies eliminated at
each level of review? Yes No
Were details of included studies presented (design, sample, methods, results,
outcomes, strengths, and limitations)? Yes No
Were methods for appraising the strength of evidence (level and quality)
described? Yes No
Were conclusions based on results? Yes No
5
Appendix E
Research Evidence Appraisal Tool
Were the instruments reliable (Cronbach’s [alpha] > 0.70)? Yes N
o N/A
Was instrument validity discussed? Yes N
o N/A
If surveys or questionnaires were used, was the response
rate > 25%? Yes N
o N/A
Were the results presented clearly? Yes N
o
If tables were presented, was the narrative consistent with the table
content? Yes N
o N/A
Were study limitations identified and addressed? Yes N
o
Were conclusions based on results? Yes N
o
Complete the Quality Rating for QuaNtitative Studies section
Appraisal of Systematic Review (With or Without Meta-Analysis)
Were the variables of interest clearly identified? Yes No
Was the search comprehensive and reproducible?
Key search terms stated Yes No
Multiple databases searched and identified Yes No
Inclusion and exclusion criteria stated Yes No
Was there a flow diagram that included the number of studies eliminated at
each level of review? Yes No
Were details of included studies presented (design, sample, methods, results,
outcomes, strengths, and limitations)? Yes No
Were methods for appraising the strength of evidence (level and quality)
described? Yes No
Were conclusions based on results? Yes No
5
Johns Hopkins Nursing Evidence-Based Practice
Appendix E
Research Evidence Appraisal Tool
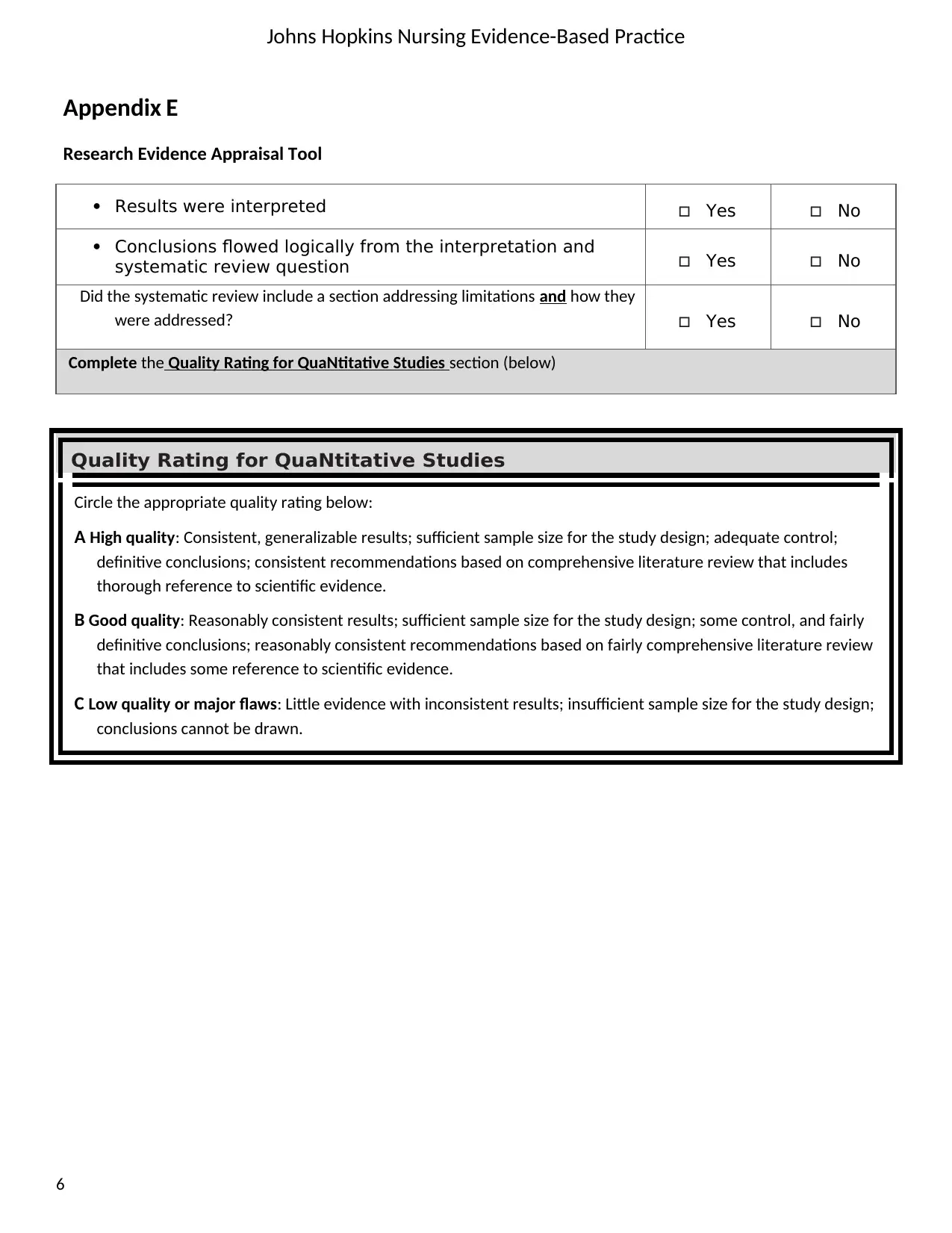
Results were interpreted Yes No
Conclusions flowed logically from the interpretation and
systematic review question Yes No
Did the systematic review include a section addressing limitations and how they
were addressed? Yes No
Complete the Quality Rating for QuaNtitative Studies section (below)
Quality Rating for QuaNtitative Studies
Circle the appropriate quality rating below:
A High quality: Consistent, generalizable results; sufficient sample size for the study design; adequate control;
definitive conclusions; consistent recommendations based on comprehensive literature review that includes
thorough reference to scientific evidence.
B Good quality: Reasonably consistent results; sufficient sample size for the study design; some control, and fairly
definitive conclusions; reasonably consistent recommendations based on fairly comprehensive literature review
that includes some reference to scientific evidence.
C Low quality or major flaws: Little evidence with inconsistent results; insufficient sample size for the study design;
conclusions cannot be drawn.
6
Appendix E
Research Evidence Appraisal Tool
Results were interpreted Yes No
Conclusions flowed logically from the interpretation and
systematic review question Yes No
Did the systematic review include a section addressing limitations and how they
were addressed? Yes No
Complete the Quality Rating for QuaNtitative Studies section (below)
Quality Rating for QuaNtitative Studies
Circle the appropriate quality rating below:
A High quality: Consistent, generalizable results; sufficient sample size for the study design; adequate control;
definitive conclusions; consistent recommendations based on comprehensive literature review that includes
thorough reference to scientific evidence.
B Good quality: Reasonably consistent results; sufficient sample size for the study design; some control, and fairly
definitive conclusions; reasonably consistent recommendations based on fairly comprehensive literature review
that includes some reference to scientific evidence.
C Low quality or major flaws: Little evidence with inconsistent results; insufficient sample size for the study design;
conclusions cannot be drawn.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 21
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





