Aircraft Mechanics Assignment: Thermodynamics, Cycles, and Laws
VerifiedAdded on 2021/04/17
|11
|2426
|377
Homework Assignment
AI Summary
This assignment solution delves into the core principles of aircraft mechanics, providing detailed explanations and solutions to various problems. It covers fundamental concepts such as Newton's laws of motion, including inertia, and the relationship between force, mass, and acceleration. The assignment further explores thermodynamics, examining Boyle's, Charles', and the Pressure Law, leading to the Combined Gas Law. Key engine cycles, the Otto and Brayton cycles, are analyzed, along with calculations involving kinetic and potential energy. The concepts of work, power, force, and acceleration are explained, alongside their respective formulas and units. The solution provides calculations for thrust in different scenarios, including piston engine and gas turbine cycles, complete with P-V and T-S diagrams. The assignment references the Ideal Gas Law and provides a comprehensive understanding of aircraft mechanics principles.

Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Aircraft
2018
2018

Contents
Answer 1................................................................................................................................................2
Newton first law of motion................................................................................................................2
Newton second law of motion...........................................................................................................2
Newton third law of motion..............................................................................................................2
Answer 2................................................................................................................................................2
Boyle’s Law........................................................................................................................................2
Charles’ Law.......................................................................................................................................3
Pressure Law......................................................................................................................................3
Combined Gas Law............................................................................................................................3
Answer 3................................................................................................................................................4
Otto Cycle..........................................................................................................................................4
Brayton Cycle.....................................................................................................................................4
Answer 4................................................................................................................................................4
Solution 1...........................................................................................................................................5
Solution 2...........................................................................................................................................5
Answer 5................................................................................................................................................5
Energy & Work..................................................................................................................................5
Power................................................................................................................................................6
Force..................................................................................................................................................6
Acceleration.......................................................................................................................................6
Answer 6................................................................................................................................................6
Solution 1...........................................................................................................................................6
Solution 2...........................................................................................................................................7
Solution 3...........................................................................................................................................7
Answer LO 1.2.......................................................................................................................................7
Piston Engine Cycle............................................................................................................................7
Gas Turbine Cycle..............................................................................................................................8
References.............................................................................................................................................9
Answer 1................................................................................................................................................2
Newton first law of motion................................................................................................................2
Newton second law of motion...........................................................................................................2
Newton third law of motion..............................................................................................................2
Answer 2................................................................................................................................................2
Boyle’s Law........................................................................................................................................2
Charles’ Law.......................................................................................................................................3
Pressure Law......................................................................................................................................3
Combined Gas Law............................................................................................................................3
Answer 3................................................................................................................................................4
Otto Cycle..........................................................................................................................................4
Brayton Cycle.....................................................................................................................................4
Answer 4................................................................................................................................................4
Solution 1...........................................................................................................................................5
Solution 2...........................................................................................................................................5
Answer 5................................................................................................................................................5
Energy & Work..................................................................................................................................5
Power................................................................................................................................................6
Force..................................................................................................................................................6
Acceleration.......................................................................................................................................6
Answer 6................................................................................................................................................6
Solution 1...........................................................................................................................................6
Solution 2...........................................................................................................................................7
Solution 3...........................................................................................................................................7
Answer LO 1.2.......................................................................................................................................7
Piston Engine Cycle............................................................................................................................7
Gas Turbine Cycle..............................................................................................................................8
References.............................................................................................................................................9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Answer 1
Newton first law of motion
Newton first law of motion states that the body will remain in rest or motion until and unless an
external force is applied on it. This is also known as law of Inertia. Inertia is a property by virtue of
which a body remain in its state unless an unbalanced force is applied on it.
Newton second law of motion
Newton second law of motion states the acceleration produced by the net force on a body is directly
proportional to the force applied and inversely proportional to the mass of the body (Gianopoulos,
2012).
Mathematically expressed as, F=MA
Where,
M = Mass of the body
A = Acceleration of the body
In our everyday life, we do use second law of motion many a times. Let’s explain this better with an
example.
Kim was out for a car drive when suddenly it went out of fuel. He thought of pushing the car and was
able to move the car at 0.5m/s/s. Suppose the mass of the car was 120 kg. Now we can easily find
the force applied by Kim to move the car with the mathematical expression, F=MA
F = 120 ×0.5=60 N
Newton third law of motion
Whenever two bodies interact with each other there is an exchange of force. Newton’s third law of
motion states that there is always an active and reactive force whenever two bodies interact
(Gianopoulos, 2012). In simple words it is stated as, “to every action there is equal and opposite
reaction”.
The best example for this is the spring.
When we pull a spring it does feel that the spring is pulling itself back. The pulling force which we
applied on the body is the active force and the restoring force of the spring is the reactive force.
Answer 2
Boyle’s Law
Boyle’s Law which is also termed as Mariotte’s Law is a relation which is concerned with the
expansion and compression of a gas at constant temperature.
Boyle’s Law states that at constant temperature the product of pressure and volume of a given
quantity of gas is constant. In other word, the pressure and volume of a given quantity of gas varies
inversely (Given temperature is constant).
Newton first law of motion
Newton first law of motion states that the body will remain in rest or motion until and unless an
external force is applied on it. This is also known as law of Inertia. Inertia is a property by virtue of
which a body remain in its state unless an unbalanced force is applied on it.
Newton second law of motion
Newton second law of motion states the acceleration produced by the net force on a body is directly
proportional to the force applied and inversely proportional to the mass of the body (Gianopoulos,
2012).
Mathematically expressed as, F=MA
Where,
M = Mass of the body
A = Acceleration of the body
In our everyday life, we do use second law of motion many a times. Let’s explain this better with an
example.
Kim was out for a car drive when suddenly it went out of fuel. He thought of pushing the car and was
able to move the car at 0.5m/s/s. Suppose the mass of the car was 120 kg. Now we can easily find
the force applied by Kim to move the car with the mathematical expression, F=MA
F = 120 ×0.5=60 N
Newton third law of motion
Whenever two bodies interact with each other there is an exchange of force. Newton’s third law of
motion states that there is always an active and reactive force whenever two bodies interact
(Gianopoulos, 2012). In simple words it is stated as, “to every action there is equal and opposite
reaction”.
The best example for this is the spring.
When we pull a spring it does feel that the spring is pulling itself back. The pulling force which we
applied on the body is the active force and the restoring force of the spring is the reactive force.
Answer 2
Boyle’s Law
Boyle’s Law which is also termed as Mariotte’s Law is a relation which is concerned with the
expansion and compression of a gas at constant temperature.
Boyle’s Law states that at constant temperature the product of pressure and volume of a given
quantity of gas is constant. In other word, the pressure and volume of a given quantity of gas varies
inversely (Given temperature is constant).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
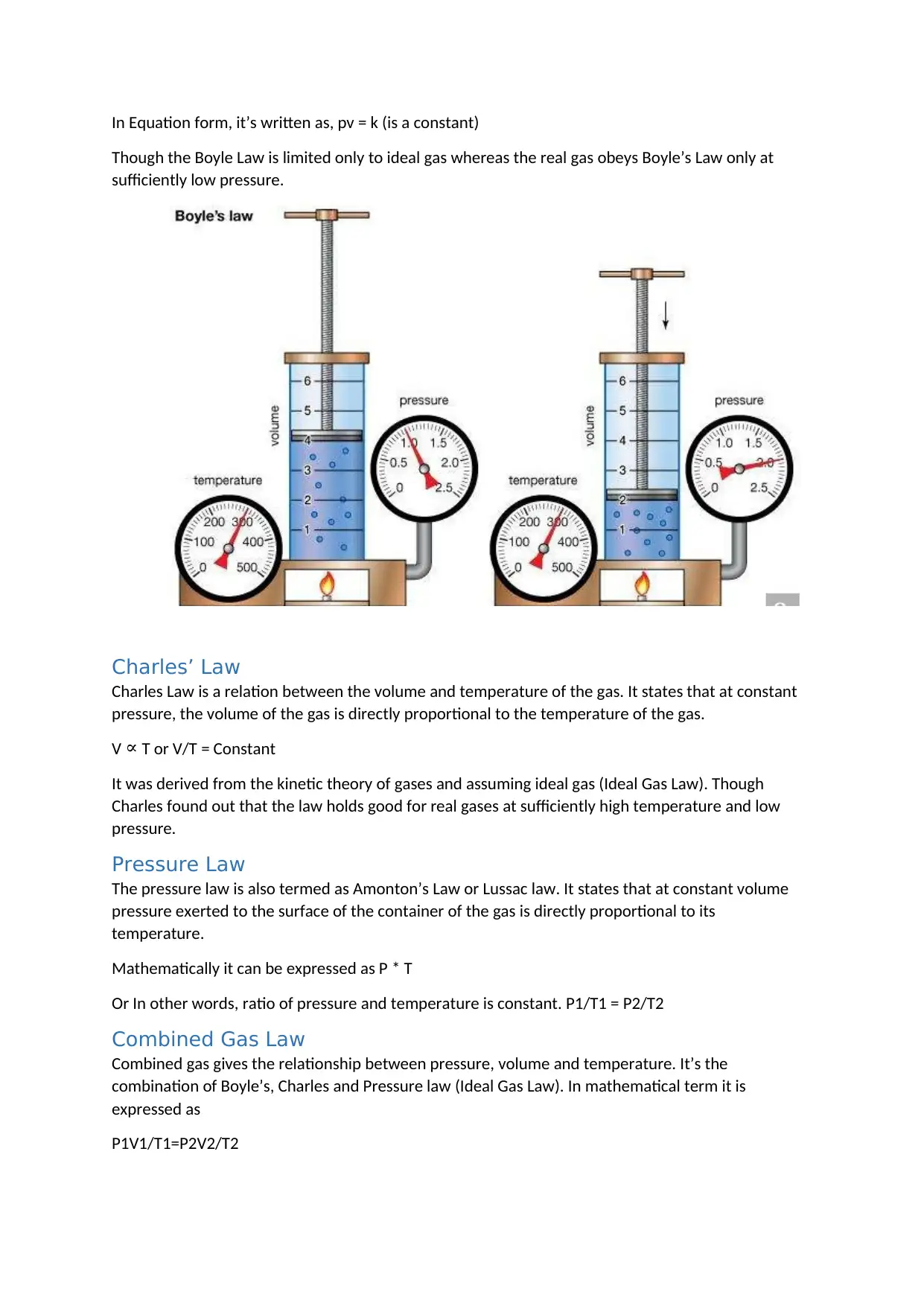
In Equation form, it’s written as, pv = k (is a constant)
Though the Boyle Law is limited only to ideal gas whereas the real gas obeys Boyle’s Law only at
sufficiently low pressure.
Charles’ Law
Charles Law is a relation between the volume and temperature of the gas. It states that at constant
pressure, the volume of the gas is directly proportional to the temperature of the gas.
V ∝ T or V/T = Constant
It was derived from the kinetic theory of gases and assuming ideal gas (Ideal Gas Law). Though
Charles found out that the law holds good for real gases at sufficiently high temperature and low
pressure.
Pressure Law
The pressure law is also termed as Amonton’s Law or Lussac law. It states that at constant volume
pressure exerted to the surface of the container of the gas is directly proportional to its
temperature.
Mathematically it can be expressed as P * T
Or In other words, ratio of pressure and temperature is constant. P1/T1 = P2/T2
Combined Gas Law
Combined gas gives the relationship between pressure, volume and temperature. It’s the
combination of Boyle’s, Charles and Pressure law (Ideal Gas Law). In mathematical term it is
expressed as
P1V1/T1=P2V2/T2
Though the Boyle Law is limited only to ideal gas whereas the real gas obeys Boyle’s Law only at
sufficiently low pressure.
Charles’ Law
Charles Law is a relation between the volume and temperature of the gas. It states that at constant
pressure, the volume of the gas is directly proportional to the temperature of the gas.
V ∝ T or V/T = Constant
It was derived from the kinetic theory of gases and assuming ideal gas (Ideal Gas Law). Though
Charles found out that the law holds good for real gases at sufficiently high temperature and low
pressure.
Pressure Law
The pressure law is also termed as Amonton’s Law or Lussac law. It states that at constant volume
pressure exerted to the surface of the container of the gas is directly proportional to its
temperature.
Mathematically it can be expressed as P * T
Or In other words, ratio of pressure and temperature is constant. P1/T1 = P2/T2
Combined Gas Law
Combined gas gives the relationship between pressure, volume and temperature. It’s the
combination of Boyle’s, Charles and Pressure law (Ideal Gas Law). In mathematical term it is
expressed as
P1V1/T1=P2V2/T2

Answer 3
Otto Cycle
Its a thermodynamic cycle which showcases the functioning of a 4-stroke piston engine. It consists of
4 processes.
Two Isochoric process (Volume Constant), &
Two Isentropic process (Adiabatic Process)
During both the isochoric process, volume remains constant but the temperature and pressure have
a directly proportional relationship which means as the temperature increases the pressure also
increases. And similarly with decrease in temperature, the pressure as well decreases.
P ∝ T
Also, during the isentropic process the pressure and volume shows inverse relationship to each
other. This means that as the pressure increases, the volume decreases and vice versa.
V ∝ 1/P
Brayton Cycle
The Brayton Cycle is considered the Ideal Cycle for the Gas Turbine Engine which constitutes a
compressor, combustion chamber and turbine.
It consists of 4 Process
Two Isentropic Compression (Entropy constant)
Two Adiabatic process
During both the isentropic process, the pressure and temperature shows direct relationship with
each other. This means that as the pressure increases the temperature also increases.
P ∝ T
And during both adiabatic process the volume is kept constant and heat is supplied/released.
Both the Otto Cycle and Brayton Cycle follows the gas laws. The ideal gas law is stated as
PV = nRT
Now, if we check both the cycle, it can easily identified that pressure is directly proportional to
Temperature and inversely proportional to Volume.
For Ex – 1st Process of both the cycle, the volume is decreased by compressing the air with the help
of piston and compressor which results in increasing the pressure of the air (Achuthan, 2009).
Answer 4
Kinetic Energy is the energy possessed by a body because of its movement whereas potential energy
is possessed by the body because of its position (Penner, 2011).
Mathematically, the Kinetic energy and Potential energy is explained as follows:
Kinetic Energy - 1
2 mv2
Otto Cycle
Its a thermodynamic cycle which showcases the functioning of a 4-stroke piston engine. It consists of
4 processes.
Two Isochoric process (Volume Constant), &
Two Isentropic process (Adiabatic Process)
During both the isochoric process, volume remains constant but the temperature and pressure have
a directly proportional relationship which means as the temperature increases the pressure also
increases. And similarly with decrease in temperature, the pressure as well decreases.
P ∝ T
Also, during the isentropic process the pressure and volume shows inverse relationship to each
other. This means that as the pressure increases, the volume decreases and vice versa.
V ∝ 1/P
Brayton Cycle
The Brayton Cycle is considered the Ideal Cycle for the Gas Turbine Engine which constitutes a
compressor, combustion chamber and turbine.
It consists of 4 Process
Two Isentropic Compression (Entropy constant)
Two Adiabatic process
During both the isentropic process, the pressure and temperature shows direct relationship with
each other. This means that as the pressure increases the temperature also increases.
P ∝ T
And during both adiabatic process the volume is kept constant and heat is supplied/released.
Both the Otto Cycle and Brayton Cycle follows the gas laws. The ideal gas law is stated as
PV = nRT
Now, if we check both the cycle, it can easily identified that pressure is directly proportional to
Temperature and inversely proportional to Volume.
For Ex – 1st Process of both the cycle, the volume is decreased by compressing the air with the help
of piston and compressor which results in increasing the pressure of the air (Achuthan, 2009).
Answer 4
Kinetic Energy is the energy possessed by a body because of its movement whereas potential energy
is possessed by the body because of its position (Penner, 2011).
Mathematically, the Kinetic energy and Potential energy is explained as follows:
Kinetic Energy - 1
2 mv2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
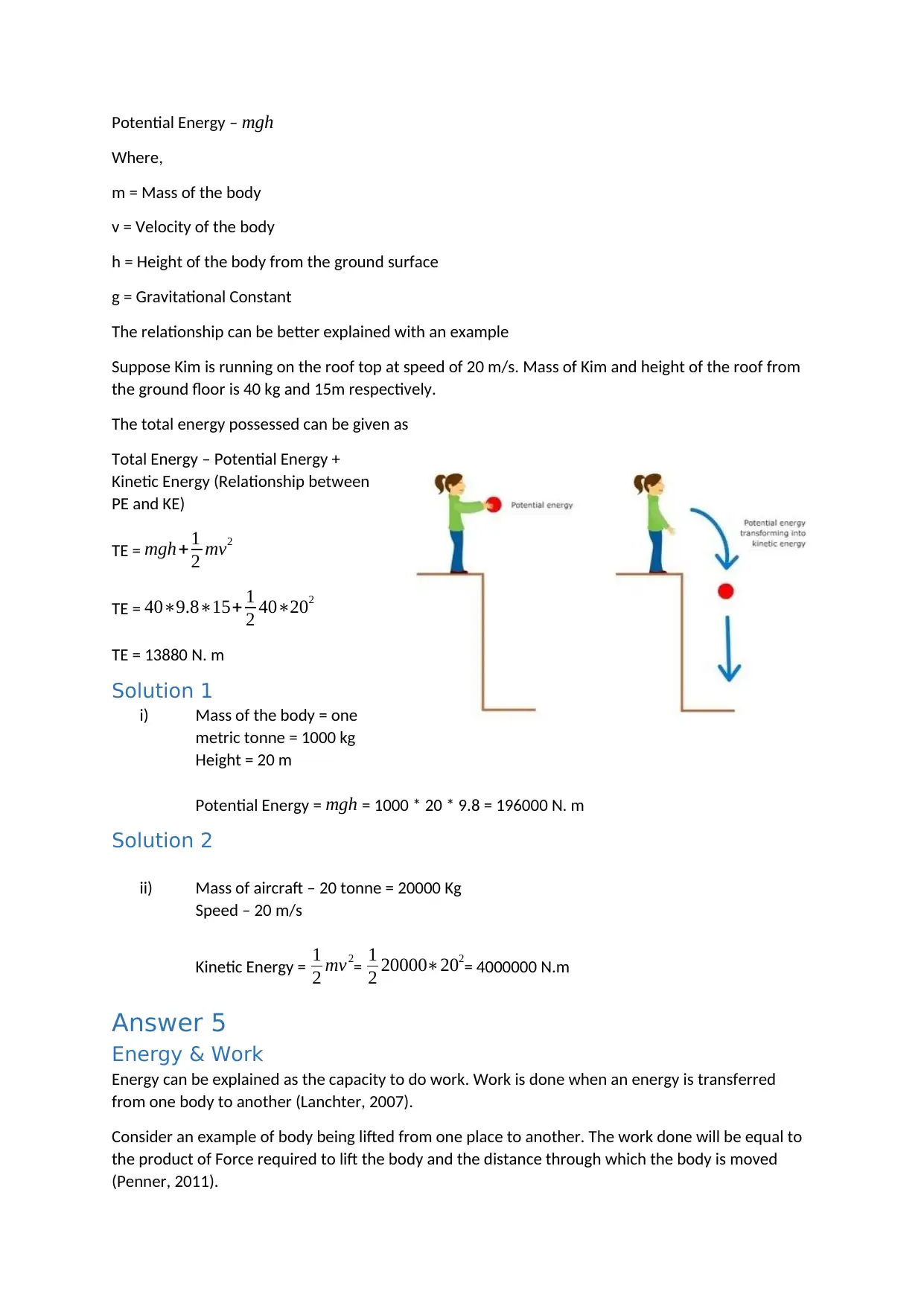
Potential Energy – mgh
Where,
m = Mass of the body
v = Velocity of the body
h = Height of the body from the ground surface
g = Gravitational Constant
The relationship can be better explained with an example
Suppose Kim is running on the roof top at speed of 20 m/s. Mass of Kim and height of the roof from
the ground floor is 40 kg and 15m respectively.
The total energy possessed can be given as
Total Energy – Potential Energy +
Kinetic Energy (Relationship between
PE and KE)
TE = mgh+ 1
2 mv2
TE = 40∗9.8∗15+ 1
2 40∗202
TE = 13880 N. m
Solution 1
i) Mass of the body = one
metric tonne = 1000 kg
Height = 20 m
Potential Energy = mgh = 1000 * 20 * 9.8 = 196000 N. m
Solution 2
ii) Mass of aircraft – 20 tonne = 20000 Kg
Speed – 20 m/s
Kinetic Energy = 1
2 mv2= 1
2 20000∗202= 4000000 N.m
Answer 5
Energy & Work
Energy can be explained as the capacity to do work. Work is done when an energy is transferred
from one body to another (Lanchter, 2007).
Consider an example of body being lifted from one place to another. The work done will be equal to
the product of Force required to lift the body and the distance through which the body is moved
(Penner, 2011).
Where,
m = Mass of the body
v = Velocity of the body
h = Height of the body from the ground surface
g = Gravitational Constant
The relationship can be better explained with an example
Suppose Kim is running on the roof top at speed of 20 m/s. Mass of Kim and height of the roof from
the ground floor is 40 kg and 15m respectively.
The total energy possessed can be given as
Total Energy – Potential Energy +
Kinetic Energy (Relationship between
PE and KE)
TE = mgh+ 1
2 mv2
TE = 40∗9.8∗15+ 1
2 40∗202
TE = 13880 N. m
Solution 1
i) Mass of the body = one
metric tonne = 1000 kg
Height = 20 m
Potential Energy = mgh = 1000 * 20 * 9.8 = 196000 N. m
Solution 2
ii) Mass of aircraft – 20 tonne = 20000 Kg
Speed – 20 m/s
Kinetic Energy = 1
2 mv2= 1
2 20000∗202= 4000000 N.m
Answer 5
Energy & Work
Energy can be explained as the capacity to do work. Work is done when an energy is transferred
from one body to another (Lanchter, 2007).
Consider an example of body being lifted from one place to another. The work done will be equal to
the product of Force required to lift the body and the distance through which the body is moved
(Penner, 2011).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Mathematically,
W = F * S
Where,
F = Force applied
S = Distance moved
The Unit of both Energy and Work is Joule (J), can also be written as Newton. Metre (N. m)
Power
Power is defined as the rate of work done. In other words, it is a measure of how quickly the work
was accomplished (Lanchter, 2007).
Mathematically,
Power = Work Done/Time = W/t
The unit of Power is Watt (W), can also be written as Joule/Second (J/s)
Force
By Newton’s 2nd Law of motion, we know that Force is the product of Mass and Acceleration.
Mathematically,
F = m * a
Where,
m = Mass of the body
a = Acceleration
The unit of Force is Newton (N), also can be written as kg . m/s2
Acceleration
Acceleration is defined as the rate of change of velocity.
Mathematically,
Acceleration = Velocity/Time
Its unit is m/s2
Answer 6
Solution 1
1) Mass flow rate of air, m = 260kg/s
Outlet Velocity, v = 500 m/s
Thrust, F = m (v – u)
= 260 (500 – 0)
= 130000 N
W = F * S
Where,
F = Force applied
S = Distance moved
The Unit of both Energy and Work is Joule (J), can also be written as Newton. Metre (N. m)
Power
Power is defined as the rate of work done. In other words, it is a measure of how quickly the work
was accomplished (Lanchter, 2007).
Mathematically,
Power = Work Done/Time = W/t
The unit of Power is Watt (W), can also be written as Joule/Second (J/s)
Force
By Newton’s 2nd Law of motion, we know that Force is the product of Mass and Acceleration.
Mathematically,
F = m * a
Where,
m = Mass of the body
a = Acceleration
The unit of Force is Newton (N), also can be written as kg . m/s2
Acceleration
Acceleration is defined as the rate of change of velocity.
Mathematically,
Acceleration = Velocity/Time
Its unit is m/s2
Answer 6
Solution 1
1) Mass flow rate of air, m = 260kg/s
Outlet Velocity, v = 500 m/s
Thrust, F = m (v – u)
= 260 (500 – 0)
= 130000 N
Solution 2
2) Mass flow rate of air, m = 650kg/s
Outlet Velocity, V1 = 200 m/s
Initial Velocity, V0 = 0 m/s
Thrust of Propeller, F = m (V1 – V0)
= 650 (200 -0)
= 130000 N
Solution 3
3) Mass of cold Stream, mc = 500 kg/s
Mass of hot stream, mh = 100 kg/s
Velocity of cold stream, V c = 200 m/s
Velocity of hot stream, V h = 300 m/s
Thrust of Bypass Engine is given by,
F = Thrust of Fan + Thrust of Core (Gunston, 2010)
F = mc V c−mc V 0 +mh V h−mi V 0
The scenario doesn’t have the value ofV 0, therefore removing the terms we have
F = mc V c+mh V h
F = 500 * 200 + 100 * 300
F = 130000 N
Answer LO 1.2
Piston Engine Cycle
Piston Engine cycle can be best understood with the help of P-V and T-S diagram. The complete
process includes
Two Isochoric process (Volume Constant), &
Two Isentropic process (Adiabatic Process)
1st Process (Isentropic Process)
In this process, compression takes
place as the piston moves from
Bottom Dead Centre (BDC) to Top
Dead Centre (TDC) (Otto Cycle,
2013). As the piston compresses,
the volume of the air decreases
which result is increasing of the
pressure. The pressure increases
2) Mass flow rate of air, m = 650kg/s
Outlet Velocity, V1 = 200 m/s
Initial Velocity, V0 = 0 m/s
Thrust of Propeller, F = m (V1 – V0)
= 650 (200 -0)
= 130000 N
Solution 3
3) Mass of cold Stream, mc = 500 kg/s
Mass of hot stream, mh = 100 kg/s
Velocity of cold stream, V c = 200 m/s
Velocity of hot stream, V h = 300 m/s
Thrust of Bypass Engine is given by,
F = Thrust of Fan + Thrust of Core (Gunston, 2010)
F = mc V c−mc V 0 +mh V h−mi V 0
The scenario doesn’t have the value ofV 0, therefore removing the terms we have
F = mc V c+mh V h
F = 500 * 200 + 100 * 300
F = 130000 N
Answer LO 1.2
Piston Engine Cycle
Piston Engine cycle can be best understood with the help of P-V and T-S diagram. The complete
process includes
Two Isochoric process (Volume Constant), &
Two Isentropic process (Adiabatic Process)
1st Process (Isentropic Process)
In this process, compression takes
place as the piston moves from
Bottom Dead Centre (BDC) to Top
Dead Centre (TDC) (Otto Cycle,
2013). As the piston compresses,
the volume of the air decreases
which result is increasing of the
pressure. The pressure increases
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
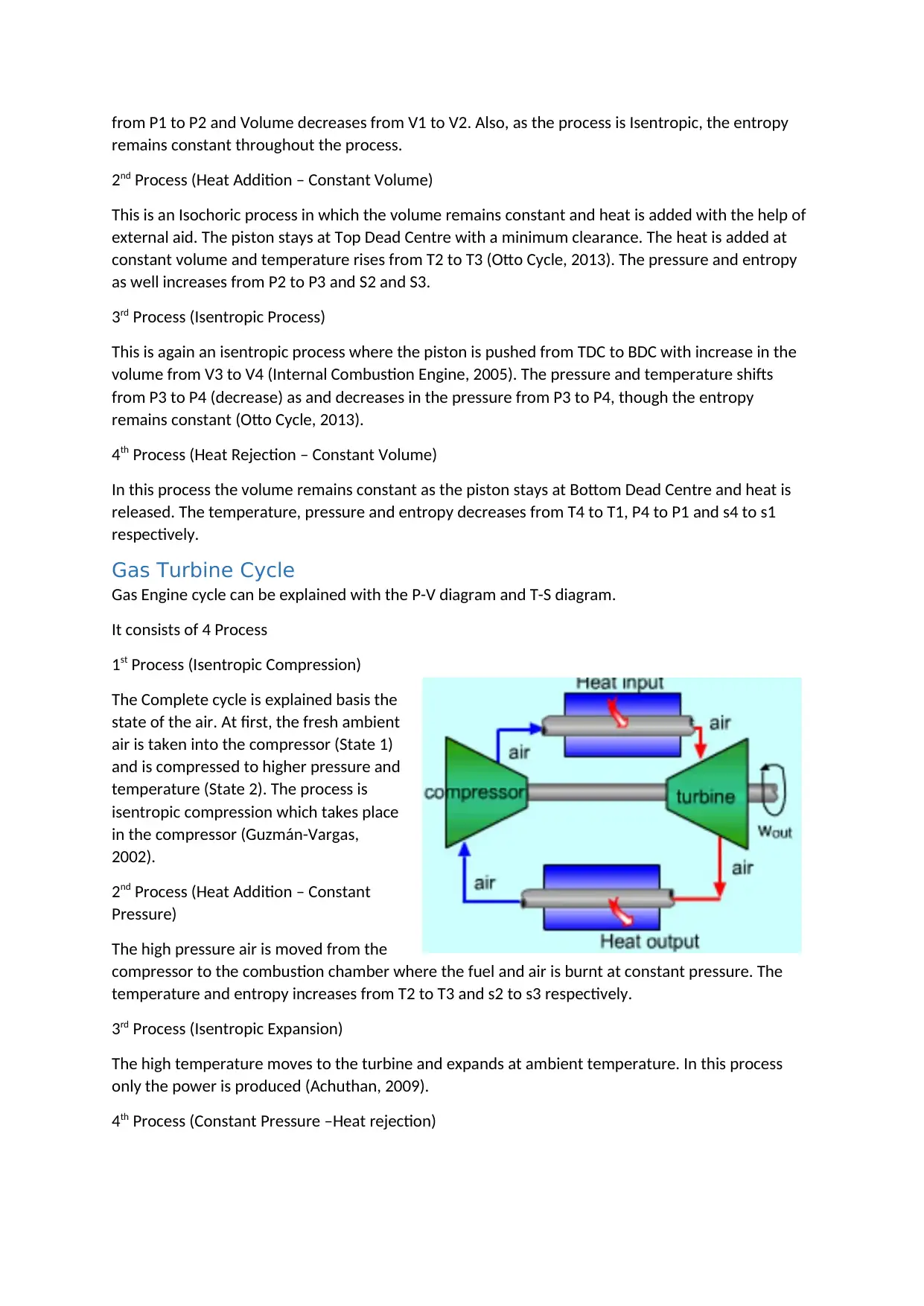
from P1 to P2 and Volume decreases from V1 to V2. Also, as the process is Isentropic, the entropy
remains constant throughout the process.
2nd Process (Heat Addition – Constant Volume)
This is an Isochoric process in which the volume remains constant and heat is added with the help of
external aid. The piston stays at Top Dead Centre with a minimum clearance. The heat is added at
constant volume and temperature rises from T2 to T3 (Otto Cycle, 2013). The pressure and entropy
as well increases from P2 to P3 and S2 and S3.
3rd Process (Isentropic Process)
This is again an isentropic process where the piston is pushed from TDC to BDC with increase in the
volume from V3 to V4 (Internal Combustion Engine, 2005). The pressure and temperature shifts
from P3 to P4 (decrease) as and decreases in the pressure from P3 to P4, though the entropy
remains constant (Otto Cycle, 2013).
4th Process (Heat Rejection – Constant Volume)
In this process the volume remains constant as the piston stays at Bottom Dead Centre and heat is
released. The temperature, pressure and entropy decreases from T4 to T1, P4 to P1 and s4 to s1
respectively.
Gas Turbine Cycle
Gas Engine cycle can be explained with the P-V diagram and T-S diagram.
It consists of 4 Process
1st Process (Isentropic Compression)
The Complete cycle is explained basis the
state of the air. At first, the fresh ambient
air is taken into the compressor (State 1)
and is compressed to higher pressure and
temperature (State 2). The process is
isentropic compression which takes place
in the compressor (Guzmán-Vargas,
2002).
2nd Process (Heat Addition – Constant
Pressure)
The high pressure air is moved from the
compressor to the combustion chamber where the fuel and air is burnt at constant pressure. The
temperature and entropy increases from T2 to T3 and s2 to s3 respectively.
3rd Process (Isentropic Expansion)
The high temperature moves to the turbine and expands at ambient temperature. In this process
only the power is produced (Achuthan, 2009).
4th Process (Constant Pressure –Heat rejection)
remains constant throughout the process.
2nd Process (Heat Addition – Constant Volume)
This is an Isochoric process in which the volume remains constant and heat is added with the help of
external aid. The piston stays at Top Dead Centre with a minimum clearance. The heat is added at
constant volume and temperature rises from T2 to T3 (Otto Cycle, 2013). The pressure and entropy
as well increases from P2 to P3 and S2 and S3.
3rd Process (Isentropic Process)
This is again an isentropic process where the piston is pushed from TDC to BDC with increase in the
volume from V3 to V4 (Internal Combustion Engine, 2005). The pressure and temperature shifts
from P3 to P4 (decrease) as and decreases in the pressure from P3 to P4, though the entropy
remains constant (Otto Cycle, 2013).
4th Process (Heat Rejection – Constant Volume)
In this process the volume remains constant as the piston stays at Bottom Dead Centre and heat is
released. The temperature, pressure and entropy decreases from T4 to T1, P4 to P1 and s4 to s1
respectively.
Gas Turbine Cycle
Gas Engine cycle can be explained with the P-V diagram and T-S diagram.
It consists of 4 Process
1st Process (Isentropic Compression)
The Complete cycle is explained basis the
state of the air. At first, the fresh ambient
air is taken into the compressor (State 1)
and is compressed to higher pressure and
temperature (State 2). The process is
isentropic compression which takes place
in the compressor (Guzmán-Vargas,
2002).
2nd Process (Heat Addition – Constant
Pressure)
The high pressure air is moved from the
compressor to the combustion chamber where the fuel and air is burnt at constant pressure. The
temperature and entropy increases from T2 to T3 and s2 to s3 respectively.
3rd Process (Isentropic Expansion)
The high temperature moves to the turbine and expands at ambient temperature. In this process
only the power is produced (Achuthan, 2009).
4th Process (Constant Pressure –Heat rejection)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
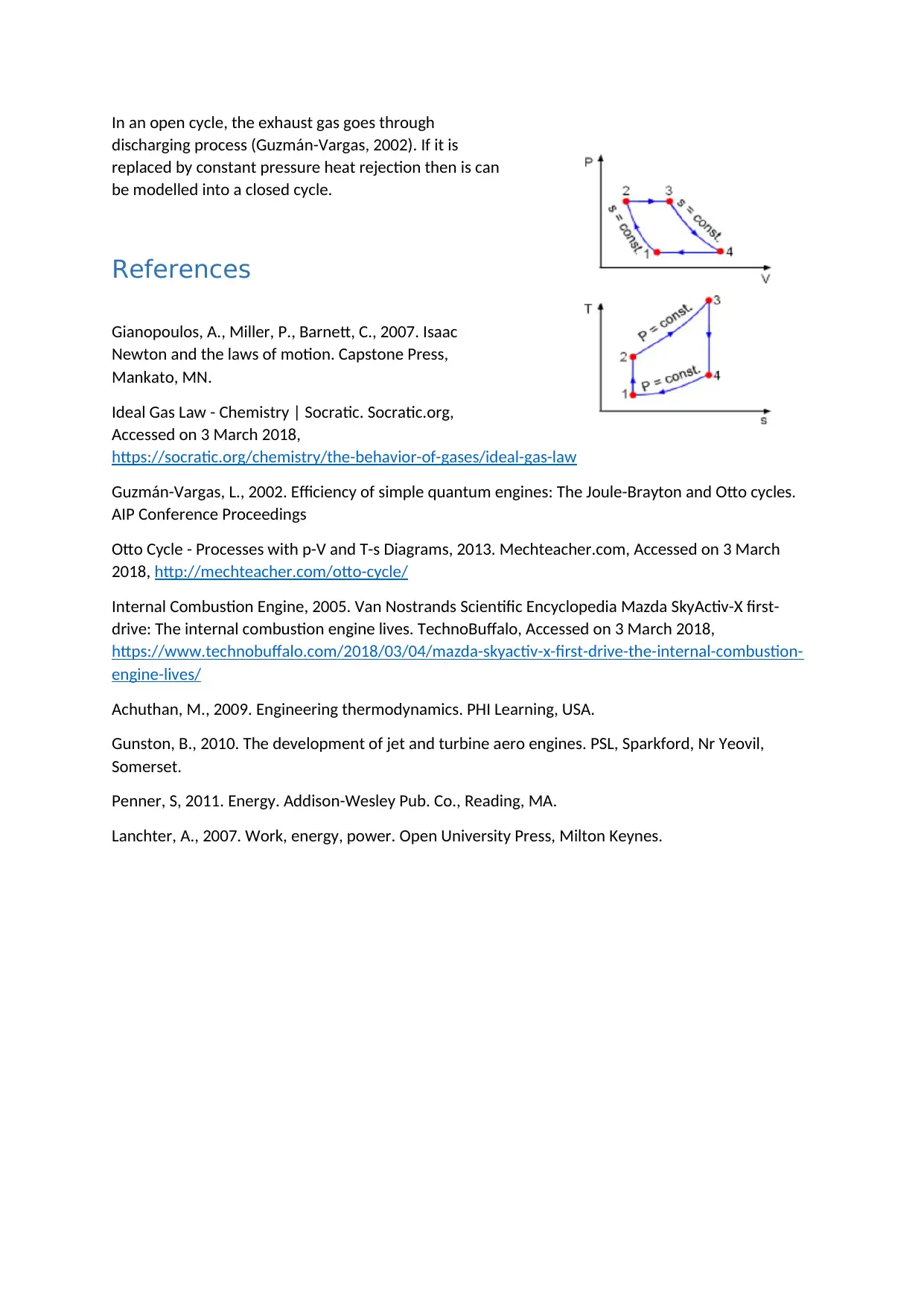
In an open cycle, the exhaust gas goes through
discharging process (Guzmán-Vargas, 2002). If it is
replaced by constant pressure heat rejection then is can
be modelled into a closed cycle.
References
Gianopoulos, A., Miller, P., Barnett, C., 2007. Isaac
Newton and the laws of motion. Capstone Press,
Mankato, MN.
Ideal Gas Law - Chemistry | Socratic. Socratic.org,
Accessed on 3 March 2018,
https://socratic.org/chemistry/the-behavior-of-gases/ideal-gas-law
Guzmán-Vargas, L., 2002. Efficiency of simple quantum engines: The Joule-Brayton and Otto cycles.
AIP Conference Proceedings
Otto Cycle - Processes with p-V and T-s Diagrams, 2013. Mechteacher.com, Accessed on 3 March
2018, http://mechteacher.com/otto-cycle/
Internal Combustion Engine, 2005. Van Nostrands Scientific Encyclopedia Mazda SkyActiv-X first-
drive: The internal combustion engine lives. TechnoBuffalo, Accessed on 3 March 2018,
https://www.technobuffalo.com/2018/03/04/mazda-skyactiv-x-first-drive-the-internal-combustion-
engine-lives/
Achuthan, M., 2009. Engineering thermodynamics. PHI Learning, USA.
Gunston, B., 2010. The development of jet and turbine aero engines. PSL, Sparkford, Nr Yeovil,
Somerset.
Penner, S, 2011. Energy. Addison-Wesley Pub. Co., Reading, MA.
Lanchter, A., 2007. Work, energy, power. Open University Press, Milton Keynes.
discharging process (Guzmán-Vargas, 2002). If it is
replaced by constant pressure heat rejection then is can
be modelled into a closed cycle.
References
Gianopoulos, A., Miller, P., Barnett, C., 2007. Isaac
Newton and the laws of motion. Capstone Press,
Mankato, MN.
Ideal Gas Law - Chemistry | Socratic. Socratic.org,
Accessed on 3 March 2018,
https://socratic.org/chemistry/the-behavior-of-gases/ideal-gas-law
Guzmán-Vargas, L., 2002. Efficiency of simple quantum engines: The Joule-Brayton and Otto cycles.
AIP Conference Proceedings
Otto Cycle - Processes with p-V and T-s Diagrams, 2013. Mechteacher.com, Accessed on 3 March
2018, http://mechteacher.com/otto-cycle/
Internal Combustion Engine, 2005. Van Nostrands Scientific Encyclopedia Mazda SkyActiv-X first-
drive: The internal combustion engine lives. TechnoBuffalo, Accessed on 3 March 2018,
https://www.technobuffalo.com/2018/03/04/mazda-skyactiv-x-first-drive-the-internal-combustion-
engine-lives/
Achuthan, M., 2009. Engineering thermodynamics. PHI Learning, USA.
Gunston, B., 2010. The development of jet and turbine aero engines. PSL, Sparkford, Nr Yeovil,
Somerset.
Penner, S, 2011. Energy. Addison-Wesley Pub. Co., Reading, MA.
Lanchter, A., 2007. Work, energy, power. Open University Press, Milton Keynes.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





