Organic Chemistry 2 Lab Report: Synthesis via Aldol Condensation
VerifiedAdded on 2022/08/02
|7
|1593
|26
Report
AI Summary
This lab report details an Organic Chemistry 2 experiment focused on the Aldol Condensation reaction. The experiment aimed to synthesize an unsaturated hydrocarbon through the formation of carbon-carbon bonds, utilizing acetone and p-anisaldehyde as reactants. The experimental procedure involved setting up a reaction with acetone, p-anisaldehyde, and potassium hydroxide, followed by filtration and recrystallization to obtain the product. The report includes detailed experimental steps, observations of color changes and texture, and the use of NMR spectroscopy to confirm the formation of the carbon-carbon double bond. The discussion section analyzes the reaction mechanism, the role of each component, and the successful formation of the desired product, supported by TLC and NMR analysis, which confirmed the purity and structure of the synthesized compound. The report highlights the importance of the aldol condensation reaction in forming carbon-carbon bonds and provides a comprehensive overview of the experiment, results, and analysis.

Organic Chemistry 2 Lab
Lab Report 2: The Aldol Condensation
Group Members:
Keyon Gaines
Miasha Richardson
Francesca Worthy
Decatur Farner
Chanel Thomas
Due: April 20, 2020
Lab Report 2: The Aldol Condensation
Group Members:
Keyon Gaines
Miasha Richardson
Francesca Worthy
Decatur Farner
Chanel Thomas
Due: April 20, 2020
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Abstract(FW)
In this experiment, aspects of carbonyl chemistry will be explored. A carbonyl group is a
functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. The
objective of this experiment is to understand the formation of carbon-carbon bonds as well as
implementing the aldol condensation reaction. The use of the NMR spectrometer will determine
if the formation of the carbon-carbon double bond was created or not. This lab was designed to
form crystals from
Introduction(CT)
The purpose of this lab was to form a double bond, also known as an unsaturated hydrocarbon.
This was achieved through aldol condensation, which is a reaction occurs with two carbonyl
compound forming
Experimental(MR)
Step 1:
Step up the hot plate and the magnetic stirrer. Attach a 100 mL round bottom flask. Add 15mL
of acetone and 1.2 mL of p-anisaldehyde. Add 1.06 g of potassium hydroxide to 20mL of water
into a seperate round bottom flask. This was added in slowly over a course of 2 minutes. The
solution turned from clear to yellow. It began to thicken in texture as time went along. The
solution was allowed to stir for 20 minutes. Once it was finished, 40mL of water was added. This
ensured that all of the product precipitated. The best way to describe the change in texture was
that it went from “lemonade to soda (Mellow Yellow)”.
Step 2:
The solution was then filtered through vacuum filtration. It was having a hard time filtering, so a
stirring rod was used to help the liquid flow out. The first filter paper had a whole in it, so a new
one had to be used. Recrystallize the solid using ethanol. Ethanol boils at 77-78.37 degrees
Celsius: 173.1 degrees Fahrenheit. Without realizing the strong impact of the ethanol, 40 mL
was added to the solution. Minimum heat had to be used because the solution wasn’t supposed
to boil. The solution heated up for 6 minutes and 14 seconds. After heating, the solution was
placed in an ice bath for 30 minutes. The crystals in the solution didn’t reform due to the excess
amount of ethanol used. Extra steps had to be added to regain crystals. Instead of using 40mL
of ethanol, 10mLs of water was used. Recrystallize with 4 mLof ethanol. Boil the mixture to
evaporate ethanol. (Drying step for 5 minutes). The solution was taken off the heat, and then
back on to evaporate. Heat ethanol. Add 4mL to crystals to make them dissolve. The solution
was added into a separatory funnel along with ethyl acetate. The solution was shaken up. The
aqueous layer was drained off of the organic layer. It was then dried off with sodium sulfate.
Results(KG)
In this experiment, aspects of carbonyl chemistry will be explored. A carbonyl group is a
functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. The
objective of this experiment is to understand the formation of carbon-carbon bonds as well as
implementing the aldol condensation reaction. The use of the NMR spectrometer will determine
if the formation of the carbon-carbon double bond was created or not. This lab was designed to
form crystals from
Introduction(CT)
The purpose of this lab was to form a double bond, also known as an unsaturated hydrocarbon.
This was achieved through aldol condensation, which is a reaction occurs with two carbonyl
compound forming
Experimental(MR)
Step 1:
Step up the hot plate and the magnetic stirrer. Attach a 100 mL round bottom flask. Add 15mL
of acetone and 1.2 mL of p-anisaldehyde. Add 1.06 g of potassium hydroxide to 20mL of water
into a seperate round bottom flask. This was added in slowly over a course of 2 minutes. The
solution turned from clear to yellow. It began to thicken in texture as time went along. The
solution was allowed to stir for 20 minutes. Once it was finished, 40mL of water was added. This
ensured that all of the product precipitated. The best way to describe the change in texture was
that it went from “lemonade to soda (Mellow Yellow)”.
Step 2:
The solution was then filtered through vacuum filtration. It was having a hard time filtering, so a
stirring rod was used to help the liquid flow out. The first filter paper had a whole in it, so a new
one had to be used. Recrystallize the solid using ethanol. Ethanol boils at 77-78.37 degrees
Celsius: 173.1 degrees Fahrenheit. Without realizing the strong impact of the ethanol, 40 mL
was added to the solution. Minimum heat had to be used because the solution wasn’t supposed
to boil. The solution heated up for 6 minutes and 14 seconds. After heating, the solution was
placed in an ice bath for 30 minutes. The crystals in the solution didn’t reform due to the excess
amount of ethanol used. Extra steps had to be added to regain crystals. Instead of using 40mL
of ethanol, 10mLs of water was used. Recrystallize with 4 mLof ethanol. Boil the mixture to
evaporate ethanol. (Drying step for 5 minutes). The solution was taken off the heat, and then
back on to evaporate. Heat ethanol. Add 4mL to crystals to make them dissolve. The solution
was added into a separatory funnel along with ethyl acetate. The solution was shaken up. The
aqueous layer was drained off of the organic layer. It was then dried off with sodium sulfate.
Results(KG)
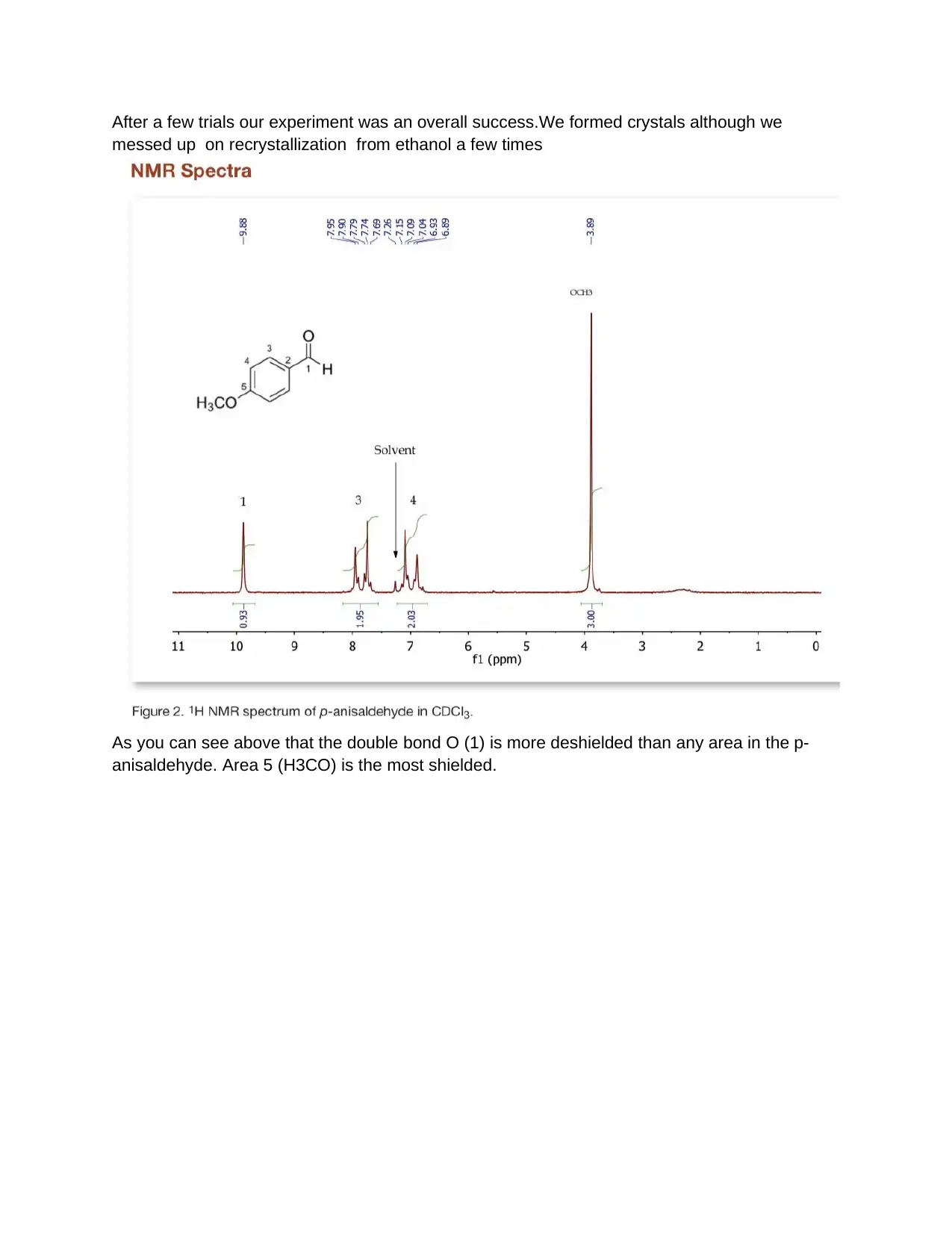
After a few trials our experiment was an overall success.We formed crystals although we
messed up on recrystallization from ethanol a few times
As you can see above that the double bond O (1) is more deshielded than any area in the p-
anisaldehyde. Area 5 (H3CO) is the most shielded.
messed up on recrystallization from ethanol a few times
As you can see above that the double bond O (1) is more deshielded than any area in the p-
anisaldehyde. Area 5 (H3CO) is the most shielded.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
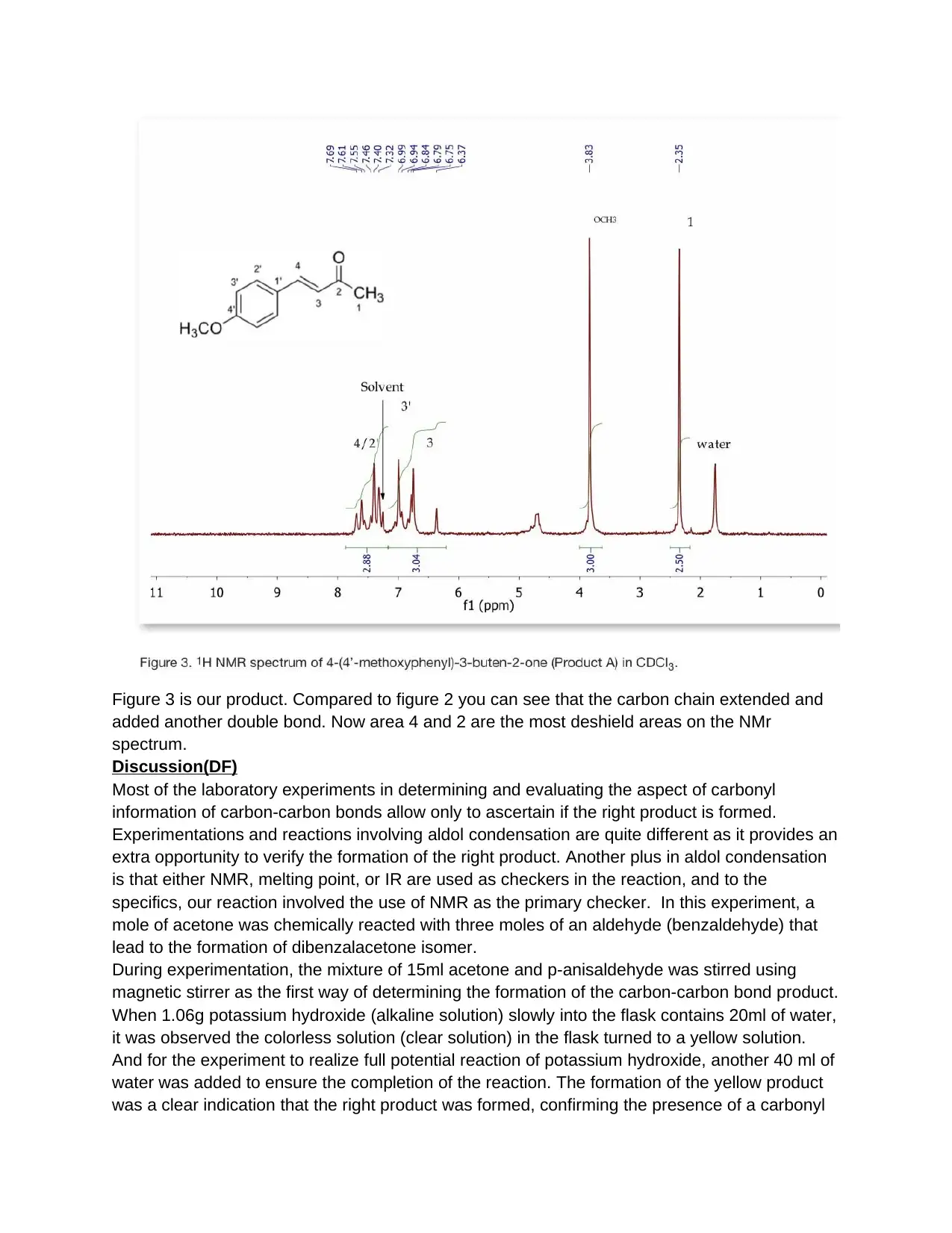
Figure 3 is our product. Compared to figure 2 you can see that the carbon chain extended and
added another double bond. Now area 4 and 2 are the most deshield areas on the NMr
spectrum.
Discussion(DF)
Most of the laboratory experiments in determining and evaluating the aspect of carbonyl
information of carbon-carbon bonds allow only to ascertain if the right product is formed.
Experimentations and reactions involving aldol condensation are quite different as it provides an
extra opportunity to verify the formation of the right product. Another plus in aldol condensation
is that either NMR, melting point, or IR are used as checkers in the reaction, and to the
specifics, our reaction involved the use of NMR as the primary checker. In this experiment, a
mole of acetone was chemically reacted with three moles of an aldehyde (benzaldehyde) that
lead to the formation of dibenzalacetone isomer.
During experimentation, the mixture of 15ml acetone and p-anisaldehyde was stirred using
magnetic stirrer as the first way of determining the formation of the carbon-carbon bond product.
When 1.06g potassium hydroxide (alkaline solution) slowly into the flask contains 20ml of water,
it was observed the colorless solution (clear solution) in the flask turned to a yellow solution.
And for the experiment to realize full potential reaction of potassium hydroxide, another 40 ml of
water was added to ensure the completion of the reaction. The formation of the yellow product
was a clear indication that the right product was formed, confirming the presence of a carbonyl
added another double bond. Now area 4 and 2 are the most deshield areas on the NMr
spectrum.
Discussion(DF)
Most of the laboratory experiments in determining and evaluating the aspect of carbonyl
information of carbon-carbon bonds allow only to ascertain if the right product is formed.
Experimentations and reactions involving aldol condensation are quite different as it provides an
extra opportunity to verify the formation of the right product. Another plus in aldol condensation
is that either NMR, melting point, or IR are used as checkers in the reaction, and to the
specifics, our reaction involved the use of NMR as the primary checker. In this experiment, a
mole of acetone was chemically reacted with three moles of an aldehyde (benzaldehyde) that
lead to the formation of dibenzalacetone isomer.
During experimentation, the mixture of 15ml acetone and p-anisaldehyde was stirred using
magnetic stirrer as the first way of determining the formation of the carbon-carbon bond product.
When 1.06g potassium hydroxide (alkaline solution) slowly into the flask contains 20ml of water,
it was observed the colorless solution (clear solution) in the flask turned to a yellow solution.
And for the experiment to realize full potential reaction of potassium hydroxide, another 40 ml of
water was added to ensure the completion of the reaction. The formation of the yellow product
was a clear indication that the right product was formed, confirming the presence of a carbonyl
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

compound. If, after the addition of water into the compound and white precipitate formed, it
could be an indication that the wrong product was produced, but in this experiment up to the first
stage of experimentation, yellow solution reaffirmed the validity of aldol condensation reaction
by confirming the presence of carbonyl compound.
The aldol condensation reaction between benzaldehyde was successful, as confirmed by the H-
NMR spectrum obtained. The essential feature in the starting procedures that led to the
specificity of the reaction product was that acetanisole always has alpha hydrogen freely
available for reaction deprotonation. The availability of alpha hydrogen in the reaction resulted in
the formation of new pi electrons that attack the carbon carbonyl in p-anisaldehyde. In the
experiment, the alpha carbon deprotonation was facilitated by the potassium hydroxide solution.
The reaction was facilitated to unsaturation by adding 40ml water, which furthers the reaction to
form alpha and beta-unsaturated products. Then a copious amount of water used in washing
the product leads to more protonation, allowing the product to react with water molecules to
form the alpha-beta double bonds, confirming the formation of the carbon-carbon bond
formation.
The second phase of the experiment involved a solution obtained in the first phase through the
use of vacuum filtration. The solution formed in the first phase was thick due to the formation of
a strong carbon-carbon (C=C) double bond; therefore, a little stirring was necessary to ease the
filtration process. In eternity the second phase reactions and experimentations were aimed at
the formation of the crystalline organic compound. The yellow solution obtained in the first part
of the experiment was filtered to separate water molecules from the yellow precipitate. The
yellow precipice was then subjected to a series of testing samples, as illustrated in step 2 of the
experimentation section. The essence of step 2 was to verify the presence of a ketone or
aldehyde. First, the use of ethanol was to verify the formation of an aldehyde. This required
minimal temperature to boost the reaction process, thus facilitating the protonation of hydrogen
ions for the reaction; however, this process was conducted with precaution in temperature, not
heat the solution to boiling. Another 40ml of water was added to facilitate reaction at safety
conditions since with the addition of water, the solution could be heated to vaporization then
crystallization carrie4d thereof.
After crystallization, the experiment confirmed the formation of the unknown crystalline product;
this was facilitated by the use of ethanol acid in the reaction. It then concluded the unknown
crystalline substance produced was an aldehyde compound. Since acids often give a false test
on ketones, and iodination test was conducted on the substrate that confirmed the positive for
ketone compound. In addition, a bromine test was conducted on the alpha-beta unsaturated
solution sample to verify the presence of an alkene, and the test turns positive, reaffirming the
conclusion that the alpha-beta was unsaturated, confirming the formation of the carbon-carbon
bond. The purity assessment of the final product, H-NMR data, was recorded, and TLC was
used. TLC plate analysis showed that the final product had an Rf value of 0.37 different from the
values of initial materials. The TLC test verified the purity of the product formed since there were
no observable spots in the plate line. The NMR spectrum further affirms the findings. However,
the spectrum did not confirm the presence of acetanisole in the final compound.
Generally, the experiment and reactions were successful, with the results obtained confirming to
the objectives and purpose of the experimentation. NMR and TCL tests verified the unique
could be an indication that the wrong product was produced, but in this experiment up to the first
stage of experimentation, yellow solution reaffirmed the validity of aldol condensation reaction
by confirming the presence of carbonyl compound.
The aldol condensation reaction between benzaldehyde was successful, as confirmed by the H-
NMR spectrum obtained. The essential feature in the starting procedures that led to the
specificity of the reaction product was that acetanisole always has alpha hydrogen freely
available for reaction deprotonation. The availability of alpha hydrogen in the reaction resulted in
the formation of new pi electrons that attack the carbon carbonyl in p-anisaldehyde. In the
experiment, the alpha carbon deprotonation was facilitated by the potassium hydroxide solution.
The reaction was facilitated to unsaturation by adding 40ml water, which furthers the reaction to
form alpha and beta-unsaturated products. Then a copious amount of water used in washing
the product leads to more protonation, allowing the product to react with water molecules to
form the alpha-beta double bonds, confirming the formation of the carbon-carbon bond
formation.
The second phase of the experiment involved a solution obtained in the first phase through the
use of vacuum filtration. The solution formed in the first phase was thick due to the formation of
a strong carbon-carbon (C=C) double bond; therefore, a little stirring was necessary to ease the
filtration process. In eternity the second phase reactions and experimentations were aimed at
the formation of the crystalline organic compound. The yellow solution obtained in the first part
of the experiment was filtered to separate water molecules from the yellow precipitate. The
yellow precipice was then subjected to a series of testing samples, as illustrated in step 2 of the
experimentation section. The essence of step 2 was to verify the presence of a ketone or
aldehyde. First, the use of ethanol was to verify the formation of an aldehyde. This required
minimal temperature to boost the reaction process, thus facilitating the protonation of hydrogen
ions for the reaction; however, this process was conducted with precaution in temperature, not
heat the solution to boiling. Another 40ml of water was added to facilitate reaction at safety
conditions since with the addition of water, the solution could be heated to vaporization then
crystallization carrie4d thereof.
After crystallization, the experiment confirmed the formation of the unknown crystalline product;
this was facilitated by the use of ethanol acid in the reaction. It then concluded the unknown
crystalline substance produced was an aldehyde compound. Since acids often give a false test
on ketones, and iodination test was conducted on the substrate that confirmed the positive for
ketone compound. In addition, a bromine test was conducted on the alpha-beta unsaturated
solution sample to verify the presence of an alkene, and the test turns positive, reaffirming the
conclusion that the alpha-beta was unsaturated, confirming the formation of the carbon-carbon
bond. The purity assessment of the final product, H-NMR data, was recorded, and TLC was
used. TLC plate analysis showed that the final product had an Rf value of 0.37 different from the
values of initial materials. The TLC test verified the purity of the product formed since there were
no observable spots in the plate line. The NMR spectrum further affirms the findings. However,
the spectrum did not confirm the presence of acetanisole in the final compound.
Generally, the experiment and reactions were successful, with the results obtained confirming to
the objectives and purpose of the experimentation. NMR and TCL tests verified the unique
properties of the final product being different from the specs of the initial compounds, affirming
the purity of the final product.
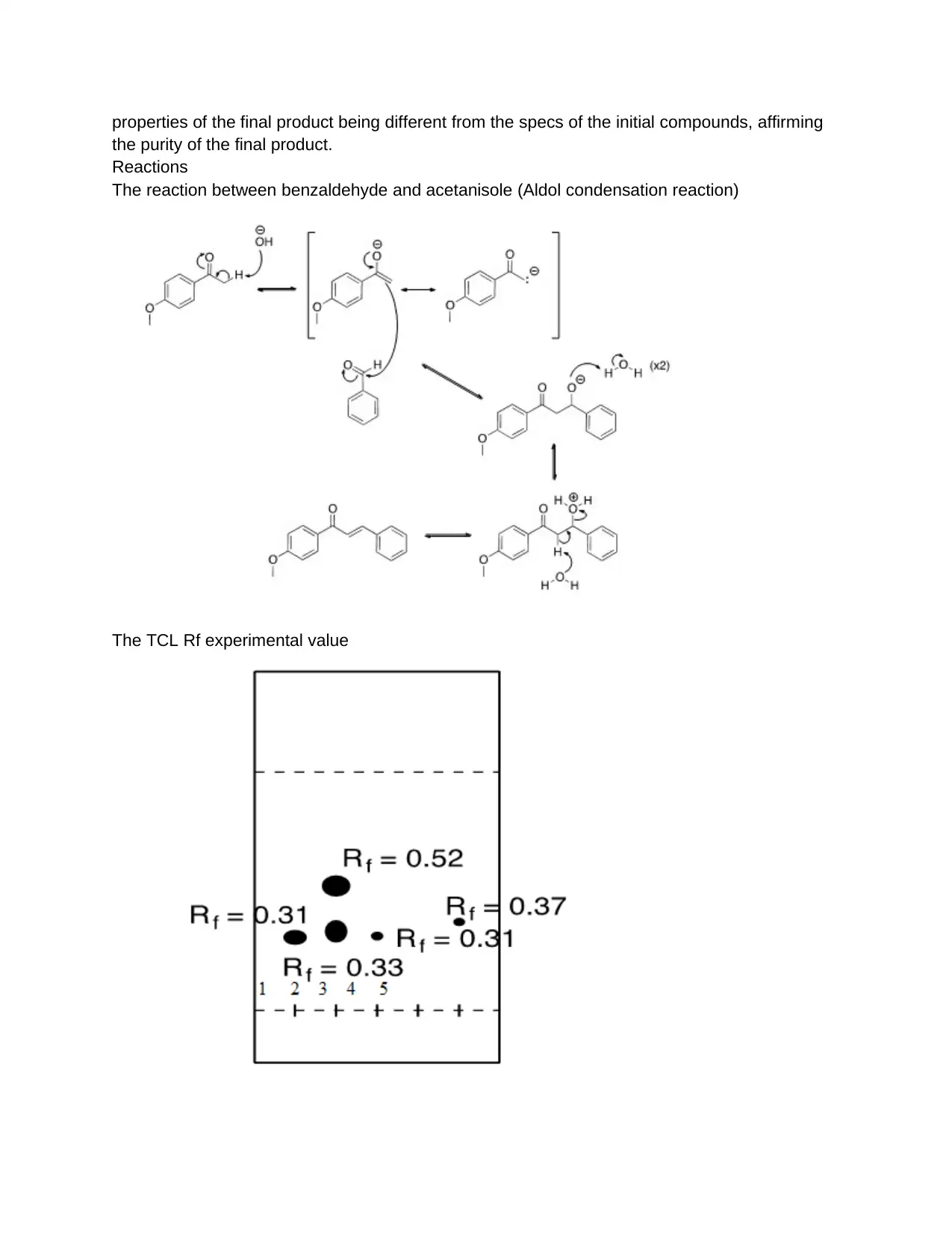
Reactions
The reaction between benzaldehyde and acetanisole (Aldol condensation reaction)
The TCL Rf experimental value
the purity of the final product.
Reactions
The reaction between benzaldehyde and acetanisole (Aldol condensation reaction)
The TCL Rf experimental value
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
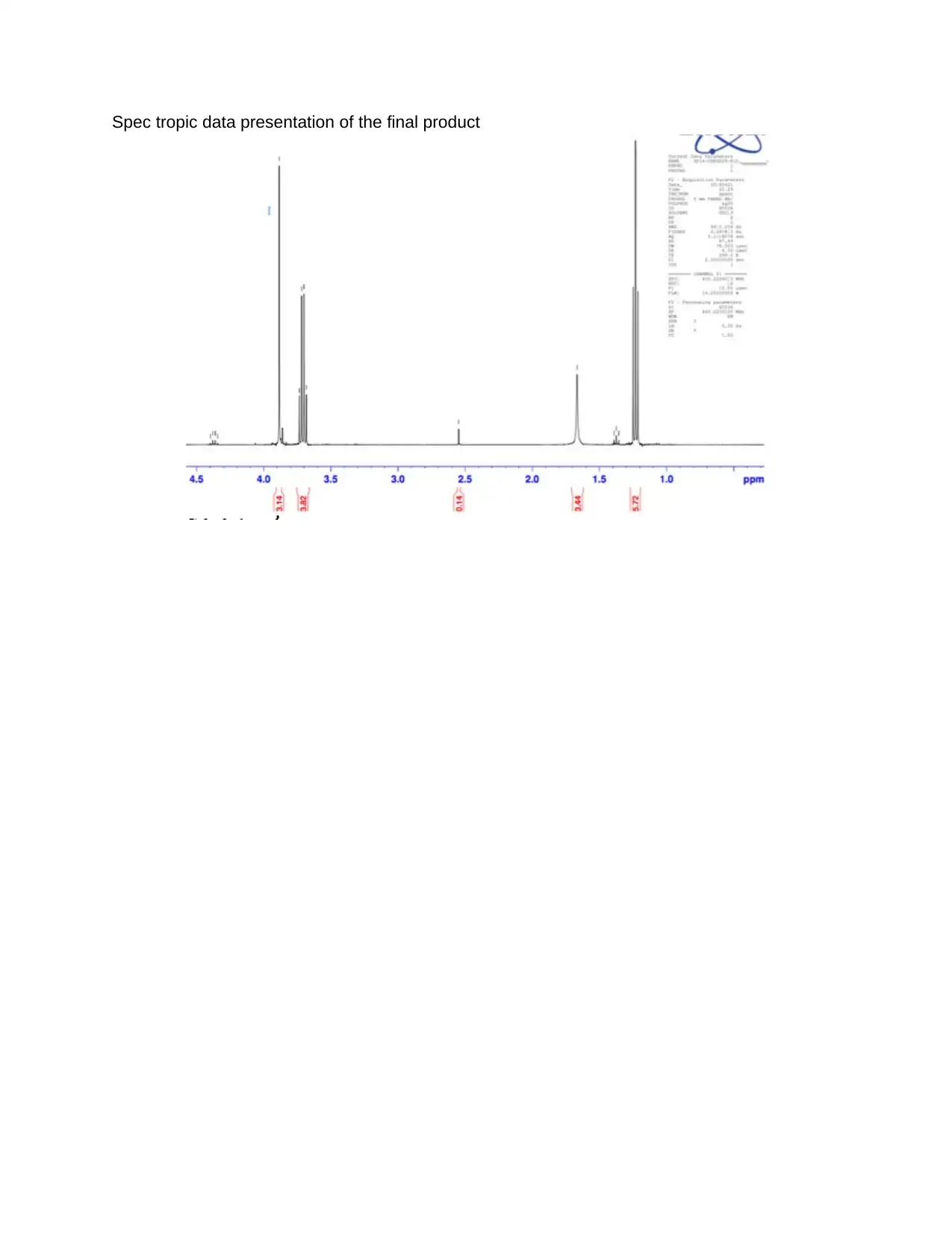
Spec tropic data presentation of the final product
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


