CHEM 801 (Module 5) Experiment 5.3: Aldol Reaction Analysis Report
VerifiedAdded on 2020/04/29
|7
|982
|349
Report
AI Summary
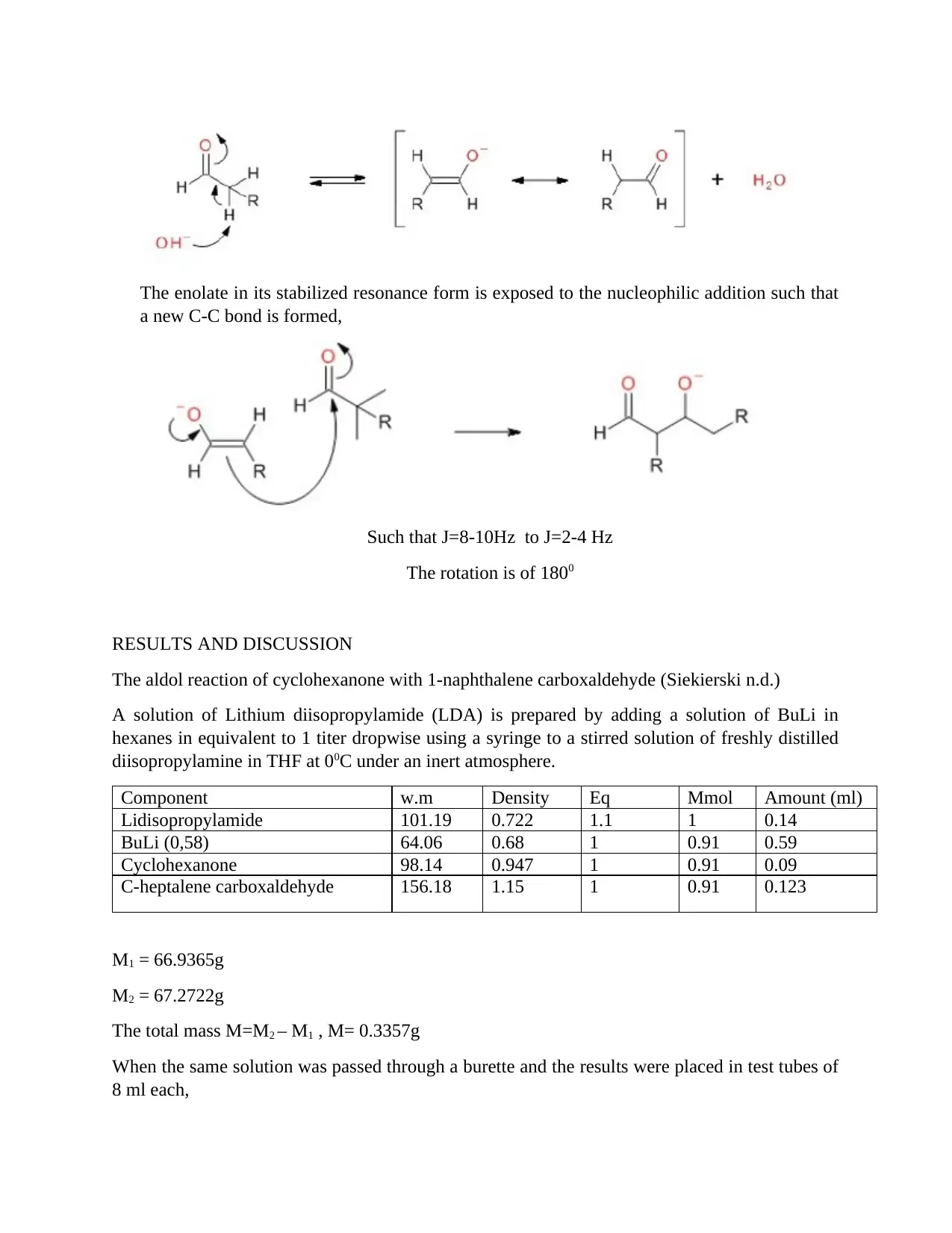
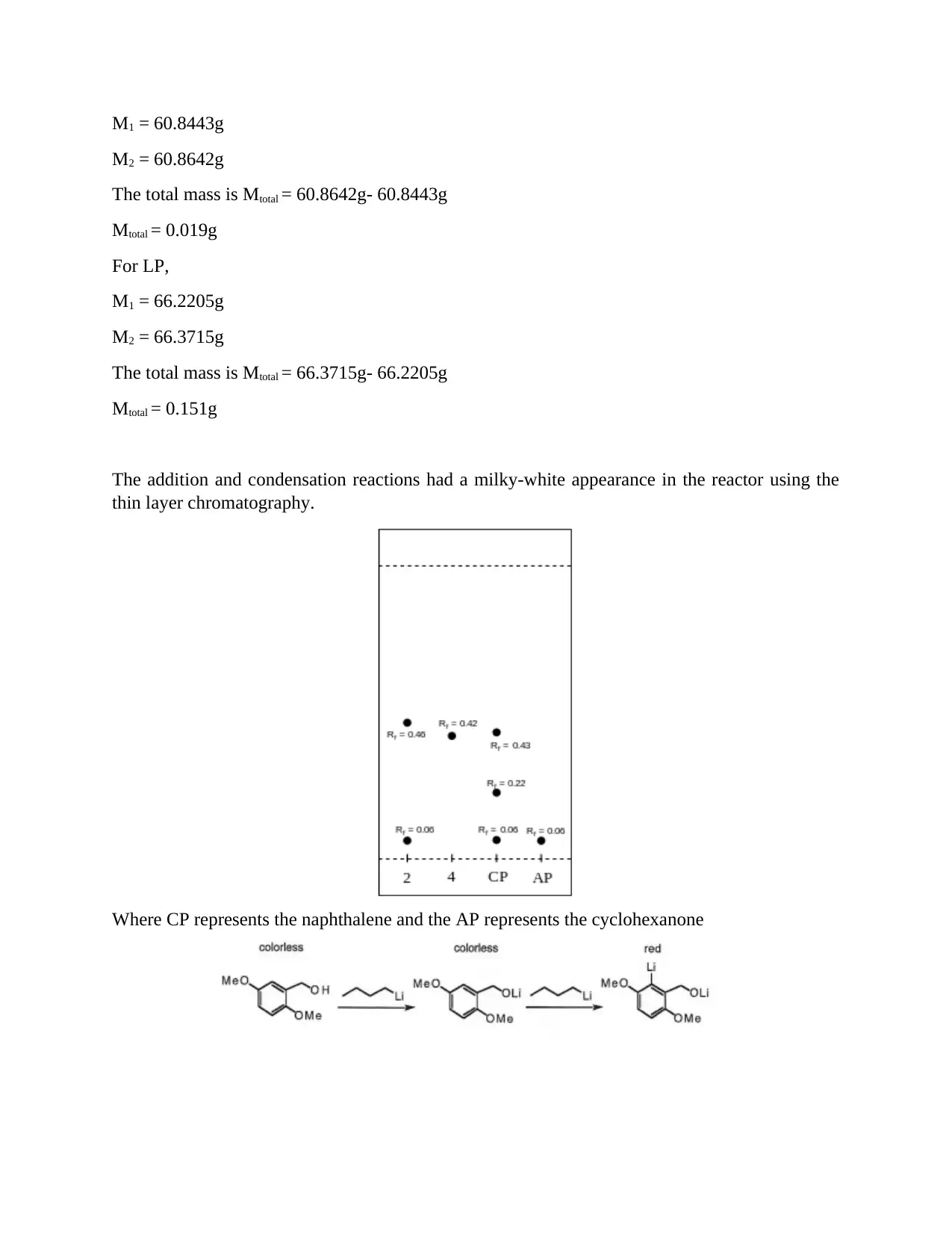
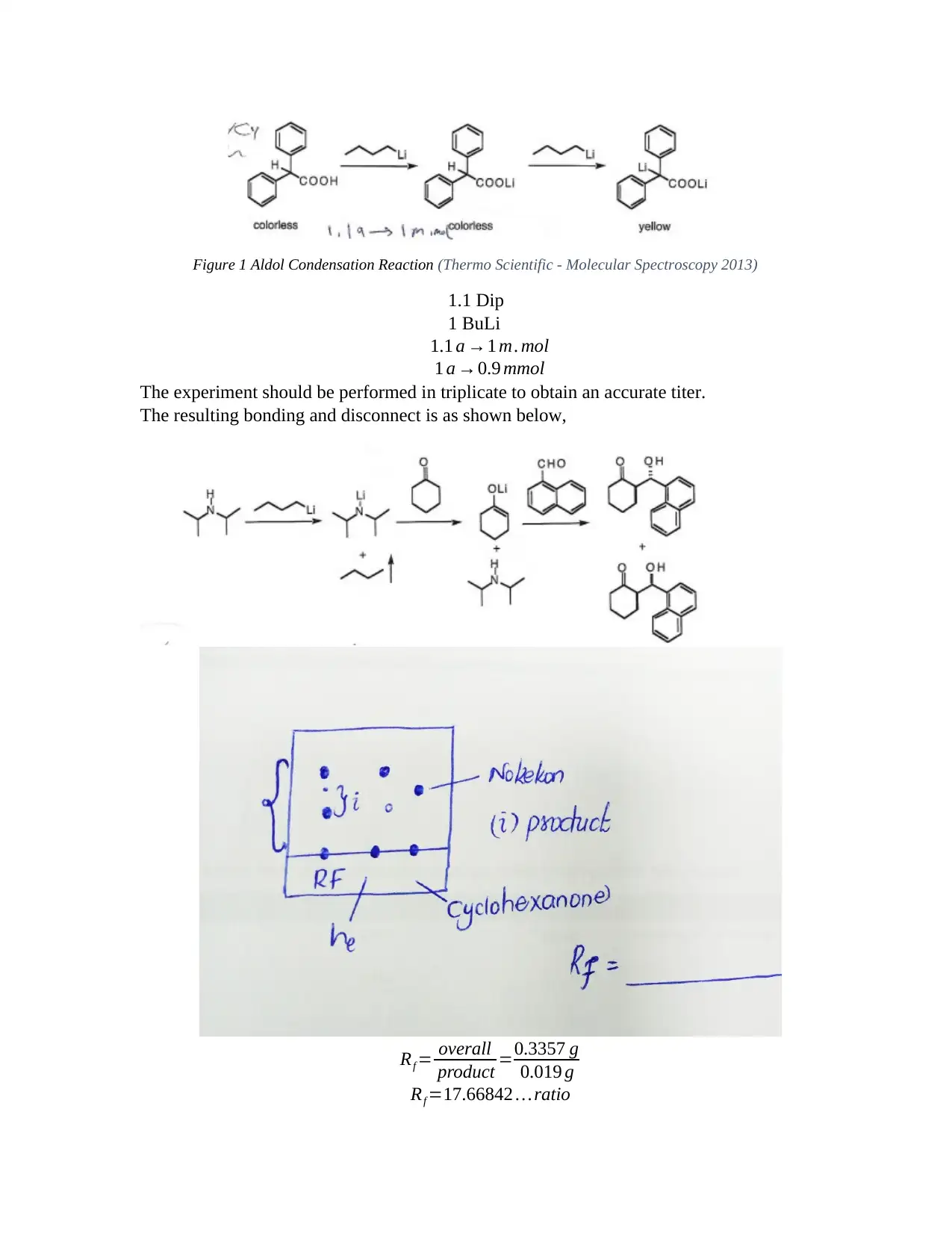
This report details an aldol reaction experiment (CHEM 801, Module 5) conducted to demonstrate the influence of reaction conditions on the aldol reaction of cyclohexanone with 1-naphthalene carboxaldehyde. The experiment utilized solvent extraction, TLC plate analysis, and IR spectroscopy to analyze the reaction. The report includes the reaction scheme, detailed procedures, and results, including mass calculations and NMR analysis. The experiment aimed to determine the yield, identify the product, and assess the reaction's selectivity. The results and discussion section covers the preparation of LDA, the addition and condensation reactions, and the use of thin-layer chromatography. The report concludes with an overview of the aldol reaction, highlighting the purity of the products and the use of NMR for further separation. The experiment also discusses the advantages of solvent extraction techniques, including separation funnels and batch-mode extraction, along with the importance of drying the organic layer to remove water contamination.
1 out of 7





![[object Object]](/_next/static/media/star-bottom.7253800d.svg)