Alkali Metals Properties and Trends
VerifiedAdded on 2019/09/18
|6
|700
|329
Report
AI Summary
This report provides a detailed analysis of the properties and trends of alkali metals, specifically Lithium, Sodium, and Potassium. It covers various aspects such as their ionic nature, low melting and boiling points, reactivity with water, and silvery appearance. The report also examines trends in atomic radius, electronegativity, ionization energy, melting and boiling points, density, electropositive character, and reactivity. It explains how these properties change down the group due to factors like the addition of new energy shells and increased shielding. The report includes tables of physical properties for each element and concludes with a list of references.

Table of Contents
Properties.........................................................................................................................................2
Trends..............................................................................................................................................3
Nature of the Compounds............................................................................................................3
Atomic Radius.............................................................................................................................3
Electronegativity..........................................................................................................................3
Ionization energy.........................................................................................................................4
Melting and boiling points...........................................................................................................4
Density.........................................................................................................................................5
Electropositive or Metallic Character..........................................................................................5
Reactivity.....................................................................................................................................5
Properties.........................................................................................................................................2
Trends..............................................................................................................................................3
Nature of the Compounds............................................................................................................3
Atomic Radius.............................................................................................................................3
Electronegativity..........................................................................................................................3
Ionization energy.........................................................................................................................4
Melting and boiling points...........................................................................................................4
Density.........................................................................................................................................5
Electropositive or Metallic Character..........................................................................................5
Reactivity.....................................................................................................................................5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Properties
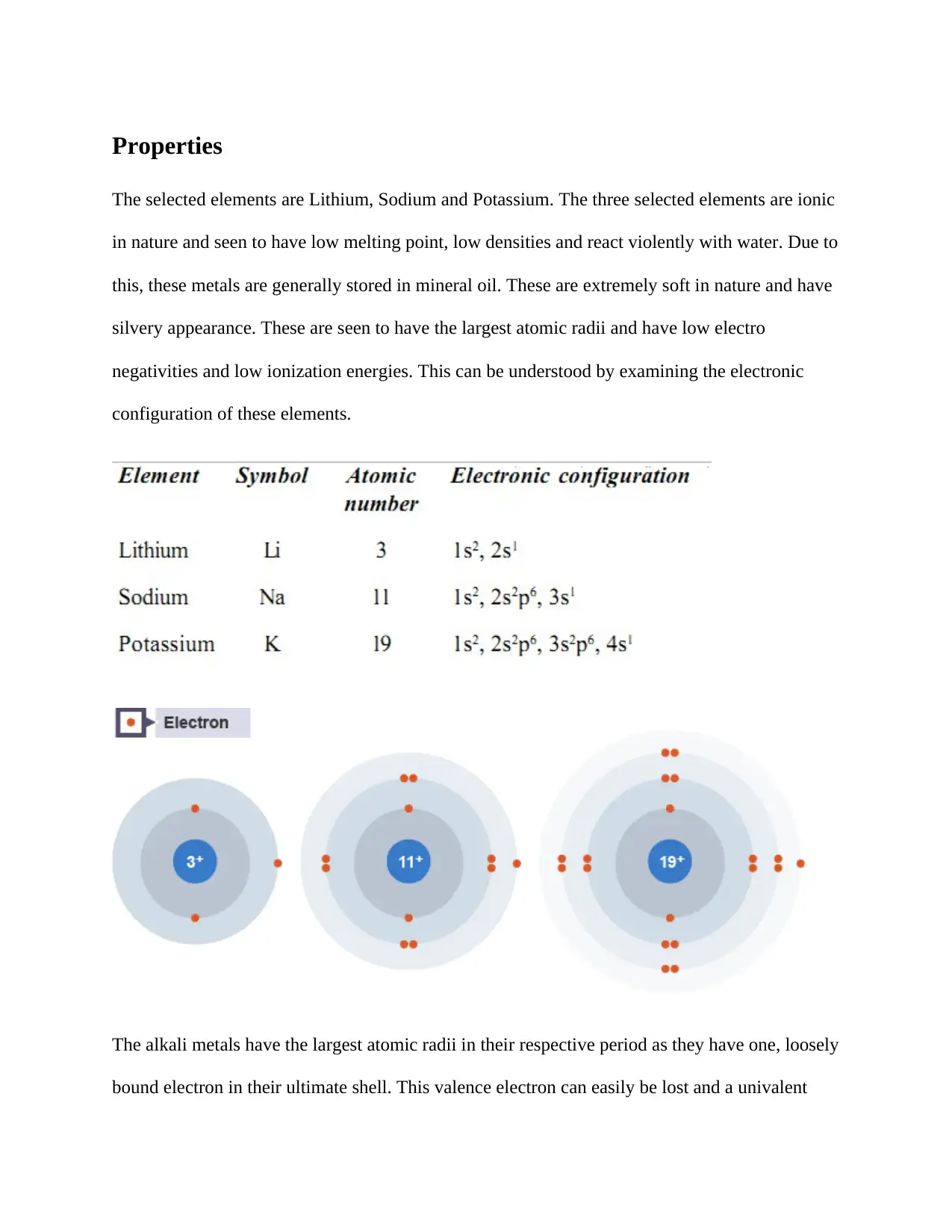
The selected elements are Lithium, Sodium and Potassium. The three selected elements are ionic
in nature and seen to have low melting point, low densities and react violently with water. Due to
this, these metals are generally stored in mineral oil. These are extremely soft in nature and have
silvery appearance. These are seen to have the largest atomic radii and have low electro
negativities and low ionization energies. This can be understood by examining the electronic
configuration of these elements.
The alkali metals have the largest atomic radii in their respective period as they have one, loosely
bound electron in their ultimate shell. This valence electron can easily be lost and a univalent
The selected elements are Lithium, Sodium and Potassium. The three selected elements are ionic
in nature and seen to have low melting point, low densities and react violently with water. Due to
this, these metals are generally stored in mineral oil. These are extremely soft in nature and have
silvery appearance. These are seen to have the largest atomic radii and have low electro
negativities and low ionization energies. This can be understood by examining the electronic
configuration of these elements.
The alkali metals have the largest atomic radii in their respective period as they have one, loosely
bound electron in their ultimate shell. This valence electron can easily be lost and a univalent
cation can be formed, making these elements highly reactive in nature. Due to this, they have
low effective charge owning to low ionization energies.
Trends
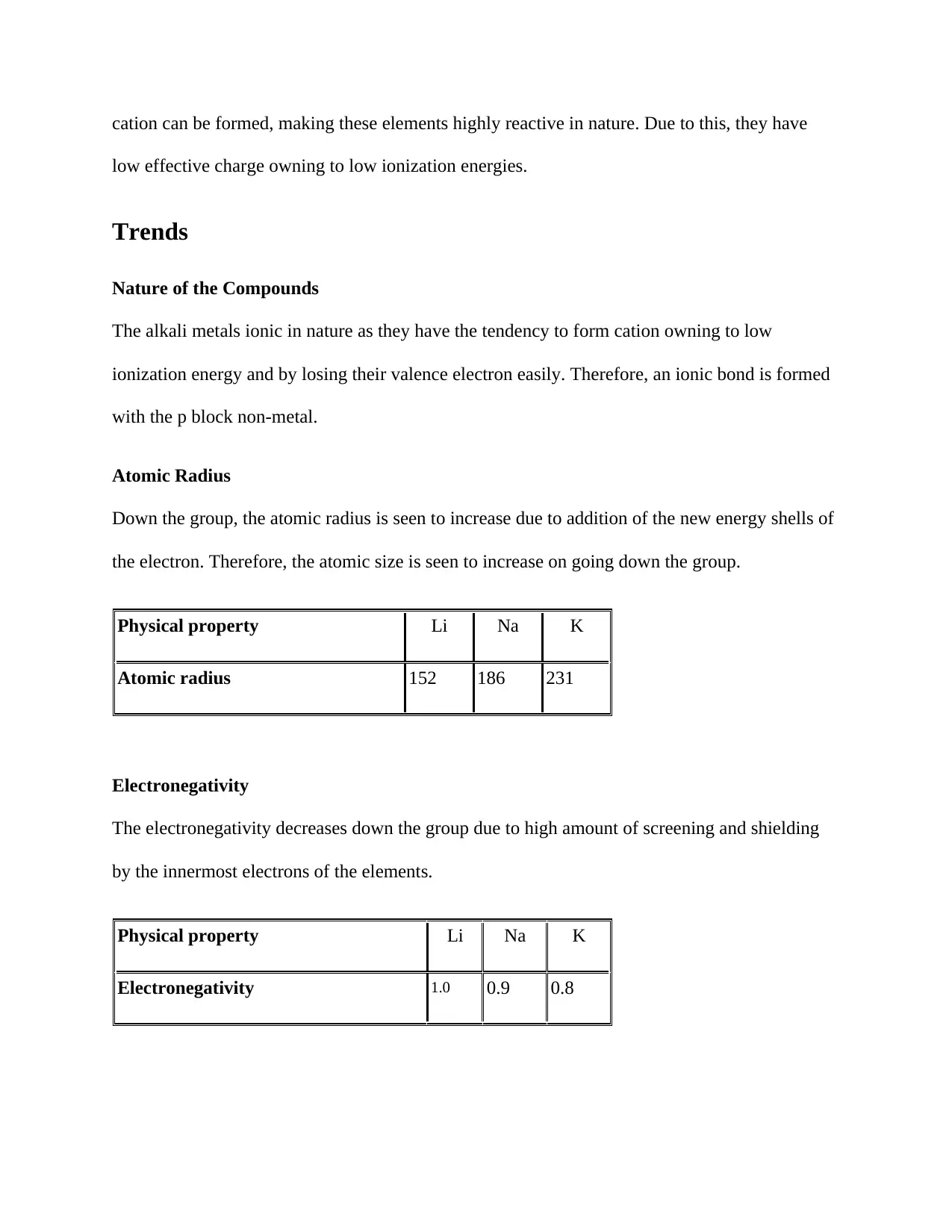
Nature of the Compounds
The alkali metals ionic in nature as they have the tendency to form cation owning to low
ionization energy and by losing their valence electron easily. Therefore, an ionic bond is formed
with the p block non-metal.
Atomic Radius
Down the group, the atomic radius is seen to increase due to addition of the new energy shells of
the electron. Therefore, the atomic size is seen to increase on going down the group.
Physical property Li Na K
Atomic radius 152 186 231
Electronegativity
The electronegativity decreases down the group due to high amount of screening and shielding
by the innermost electrons of the elements.
Physical property Li Na K
Electronegativity 1.0 0.9 0.8
low effective charge owning to low ionization energies.
Trends
Nature of the Compounds
The alkali metals ionic in nature as they have the tendency to form cation owning to low
ionization energy and by losing their valence electron easily. Therefore, an ionic bond is formed
with the p block non-metal.
Atomic Radius
Down the group, the atomic radius is seen to increase due to addition of the new energy shells of
the electron. Therefore, the atomic size is seen to increase on going down the group.
Physical property Li Na K
Atomic radius 152 186 231
Electronegativity
The electronegativity decreases down the group due to high amount of screening and shielding
by the innermost electrons of the elements.
Physical property Li Na K
Electronegativity 1.0 0.9 0.8
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
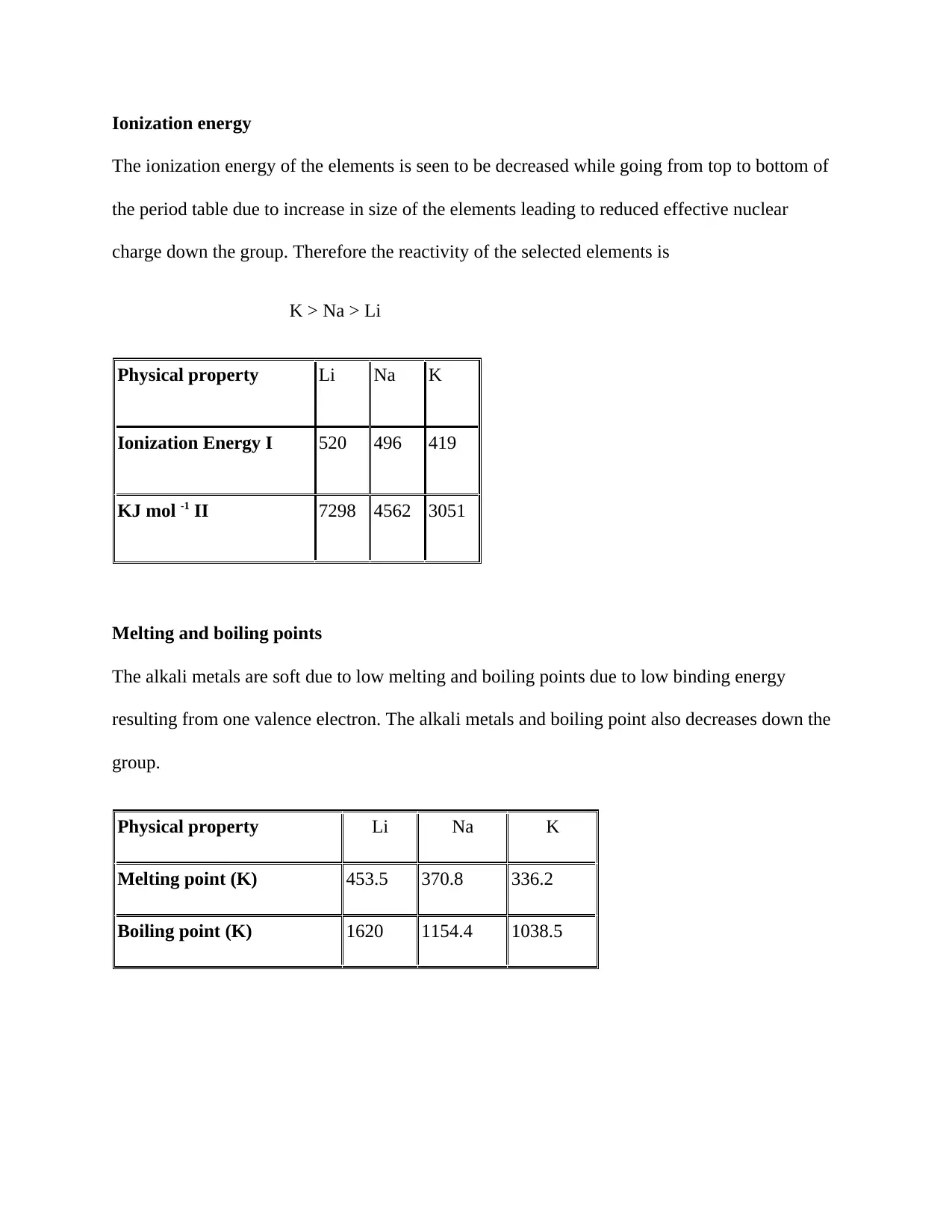
Ionization energy
The ionization energy of the elements is seen to be decreased while going from top to bottom of
the period table due to increase in size of the elements leading to reduced effective nuclear
charge down the group. Therefore the reactivity of the selected elements is
K > Na > Li
Physical property Li Na K
Ionization Energy I 520 496 419
KJ mol -1 II 7298 4562 3051
Melting and boiling points
The alkali metals are soft due to low melting and boiling points due to low binding energy
resulting from one valence electron. The alkali metals and boiling point also decreases down the
group.
Physical property Li Na K
Melting point (K) 453.5 370.8 336.2
Boiling point (K) 1620 1154.4 1038.5
The ionization energy of the elements is seen to be decreased while going from top to bottom of
the period table due to increase in size of the elements leading to reduced effective nuclear
charge down the group. Therefore the reactivity of the selected elements is
K > Na > Li
Physical property Li Na K
Ionization Energy I 520 496 419
KJ mol -1 II 7298 4562 3051
Melting and boiling points
The alkali metals are soft due to low melting and boiling points due to low binding energy
resulting from one valence electron. The alkali metals and boiling point also decreases down the
group.
Physical property Li Na K
Melting point (K) 453.5 370.8 336.2
Boiling point (K) 1620 1154.4 1038.5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Density
The alkali atoms have largest size in the respective period leading to low density. While going
down the group, the gain in atomic weight is counterbalanced by the increase in the atomic size
leading to increased densities down the group.
Physical property Li Na K
Density (g cm -1) 0.53 0.97 0.86
Electropositive or Metallic Character
All the alkali metals are metallic or electropositive in character. With the high tendency of losing
the valence electrons, the electropositive characteristics of the elements are seen to increase
down the group.
Physical property Li Na K
Eo value (V) - 3.03 - 2.71 - 2.93
Reactivity
The energy required for forming positive ion is seen to decrease leading to high reactivity. Due
to decreasing ionization energy on going down the group and consequently weaker metallic
bonds are formed. This results in lower activation energies and high reactivity.
Physical property Li Na K
Enthalpy change (kJ/mol) - 222 - 184 - 196
The alkali atoms have largest size in the respective period leading to low density. While going
down the group, the gain in atomic weight is counterbalanced by the increase in the atomic size
leading to increased densities down the group.
Physical property Li Na K
Density (g cm -1) 0.53 0.97 0.86
Electropositive or Metallic Character
All the alkali metals are metallic or electropositive in character. With the high tendency of losing
the valence electrons, the electropositive characteristics of the elements are seen to increase
down the group.
Physical property Li Na K
Eo value (V) - 3.03 - 2.71 - 2.93
Reactivity
The energy required for forming positive ion is seen to decrease leading to high reactivity. Due
to decreasing ionization energy on going down the group and consequently weaker metallic
bonds are formed. This results in lower activation energies and high reactivity.
Physical property Li Na K
Enthalpy change (kJ/mol) - 222 - 184 - 196
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6