COIT20249 Report: 3D Printing of Anatomical Teaching Resources
VerifiedAdded on 2023/06/15
|8
|7329
|189
Report
AI Summary
This report details the use of three-dimensional (3D) printing technology in producing anatomical teaching resources, addressing the challenges associated with traditional cadaver-based anatomy education. It discusses how 3D printing enables the creation of high-resolution, accurate color reproductions of prosected human cadavers and other anatomical specimens, using data from surface scanning or CT imaging. The report illustrates the application of 3D printing to produce models of negative spaces and contrast CT radiographic data using segmentation software, comparing the accuracy of printed specimens with original specimens. It highlights the advantages of this approach over plastination, including rapid production, scalability, and suitability for diverse teaching facilities, while also mitigating cultural and ethical issues related to cadaver specimens. The study addresses data input requirements, data processing logistics, qualitative and quantitative accuracy, and relative costs, ultimately demonstrating the potential of 3D printing to enhance anatomical sciences education.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

The Production of AnatomicalTeaching Resources Using
Three-Dimensional(3D) Printing Technology
Paul G. McMenamin,* Michelle R. Quayle, Colin R. McHenry, Justin W. Adams
Centre for Human Anatomy Education, Department of Anatomy and DevelopmentalBiology,
School of BiomedicalSciences, Faculty of Medicine, Nursing and Health Sciences, Monash University,
Clayton, Victoria, Australia
The teaching of anatomy has consistently been the subject of societalcontroversy,espe-
cially in the context of employing cadaveric materials in professionalmedicaland allied
health professionaltraining.The reduction in dissection-based teaching in medicaland
allied health professionaltraining programs has been in part due to the financial consid-
erations involved in maintaining bequest programs,accessing human cadavers and con-
cerns with health and safety considerations for students and staffexposed to formalin-
containing embalming fluids.This reportdetails how additive manufacturing or three-
dimensional(3D) printing allows the creation of reproductionsof prosected human
cadaver and other anatomicalspecimens that obviates many of the above issues.These
3D prints are high resolution,accurate color reproductions of prosections based on data
acquired by surface scanning or CT imaging.The application of 3D printing to produce
models of negative spaces,contrast CT radiographic data using segmentation software is
illustrated.The accuracy of printed specimens is compared with original specimens. This
alternativeapproach to producinganatomicallyaccuratereproductionsoffers many
advantages over plastination as it allows rapid production of multiple copies of any dis-
sected specimen,at any size scale and should be suitable for any teaching facility in any
country, thereby avoiding some of the cultural and ethical issues associated with cadaver
specimens either in an embalmed or plastinated form.Anat Sci Educ 00: 000–000.VC 2014
American Association of Anatomists.
Key words: gross anatomy education;medicaleducation;human anatomy;cadavers;
image processing;3D printing; rapid prototyping;additive manufacturing;anatomical
models
INTRODUCTION
Historically,the teaching of human anatomy in medicaland
allied health curricula using cadavershas been a source of
significantsocial controversy,rivaling the mostcontentious
medico-legaland ethicaldebates across other scientific disci-
plines.One of the major recurring controversies in anatomy
education is whether dissection of cadavers is stilla relevant
and appropriate component of a modern medicalundergrad-
uate training (Parker,2002;Winkelmann,2007;Korf et al.,
2008; Chambersand Emlyn-Jones,2009; Heetun, 2009).
Many hold the view that cadaveric dissection is the key com-
ponentof teaching anatomy (Ramsey-Stewartet al., 2010;
Sugand et al., 2010) and the consequences for trainees/practi-
tioners not having competentanatomicalknowledgehas
recently been emphasized (Johnson et al.,2012).In contrast,
some institutionsin the United Kingdom and Europe have
abandoned dissection-based learning (McLachlan and Patten,
2006) and in the United States many rely on combinations of
prosection and dissection (Drake et al.,2009).The reduction
in dissection-based teaching in medicaland allied health pro-
fessionaltraining programs in developed countries has been
in part due to financialconsiderations involved in maintain-
ing a cadaver bequestprogram,accessing cadavers and the
cost of maintaining modern laboratories and storage facilities
AdditionalSupporting Information may be found in the online version of
this article.
*Correspondence to:Prof. Paul G. McMenamin,Department of Anat-
omy and DevelopmentalBiology, Monash University,Building 13C,
Wellington Rd, Clayton, Victoria, 3800, Australia.
E-mail: paul.mcmenamin@monash.edu
Received 20 January 2014;Revised 14 May 2014;Accepted 12 June
2014.
Published online 00 Month 2014 in Wiley Online Library
(wileyonlinelibrary.com). DOI 10.1002/ase.1475
VC 2014 American Association of Anatomists
AnatomicalSciences EducationMONTH 2014 Anat SciEduc 00:00–00 (2014)
DESCRIPTIVE ARTICLE
Three-Dimensional(3D) Printing Technology
Paul G. McMenamin,* Michelle R. Quayle, Colin R. McHenry, Justin W. Adams
Centre for Human Anatomy Education, Department of Anatomy and DevelopmentalBiology,
School of BiomedicalSciences, Faculty of Medicine, Nursing and Health Sciences, Monash University,
Clayton, Victoria, Australia
The teaching of anatomy has consistently been the subject of societalcontroversy,espe-
cially in the context of employing cadaveric materials in professionalmedicaland allied
health professionaltraining.The reduction in dissection-based teaching in medicaland
allied health professionaltraining programs has been in part due to the financial consid-
erations involved in maintaining bequest programs,accessing human cadavers and con-
cerns with health and safety considerations for students and staffexposed to formalin-
containing embalming fluids.This reportdetails how additive manufacturing or three-
dimensional(3D) printing allows the creation of reproductionsof prosected human
cadaver and other anatomicalspecimens that obviates many of the above issues.These
3D prints are high resolution,accurate color reproductions of prosections based on data
acquired by surface scanning or CT imaging.The application of 3D printing to produce
models of negative spaces,contrast CT radiographic data using segmentation software is
illustrated.The accuracy of printed specimens is compared with original specimens. This
alternativeapproach to producinganatomicallyaccuratereproductionsoffers many
advantages over plastination as it allows rapid production of multiple copies of any dis-
sected specimen,at any size scale and should be suitable for any teaching facility in any
country, thereby avoiding some of the cultural and ethical issues associated with cadaver
specimens either in an embalmed or plastinated form.Anat Sci Educ 00: 000–000.VC 2014
American Association of Anatomists.
Key words: gross anatomy education;medicaleducation;human anatomy;cadavers;
image processing;3D printing; rapid prototyping;additive manufacturing;anatomical
models
INTRODUCTION
Historically,the teaching of human anatomy in medicaland
allied health curricula using cadavershas been a source of
significantsocial controversy,rivaling the mostcontentious
medico-legaland ethicaldebates across other scientific disci-
plines.One of the major recurring controversies in anatomy
education is whether dissection of cadavers is stilla relevant
and appropriate component of a modern medicalundergrad-
uate training (Parker,2002;Winkelmann,2007;Korf et al.,
2008; Chambersand Emlyn-Jones,2009; Heetun, 2009).
Many hold the view that cadaveric dissection is the key com-
ponentof teaching anatomy (Ramsey-Stewartet al., 2010;
Sugand et al., 2010) and the consequences for trainees/practi-
tioners not having competentanatomicalknowledgehas
recently been emphasized (Johnson et al.,2012).In contrast,
some institutionsin the United Kingdom and Europe have
abandoned dissection-based learning (McLachlan and Patten,
2006) and in the United States many rely on combinations of
prosection and dissection (Drake et al.,2009).The reduction
in dissection-based teaching in medicaland allied health pro-
fessionaltraining programs in developed countries has been
in part due to financialconsiderations involved in maintain-
ing a cadaver bequestprogram,accessing cadavers and the
cost of maintaining modern laboratories and storage facilities
AdditionalSupporting Information may be found in the online version of
this article.
*Correspondence to:Prof. Paul G. McMenamin,Department of Anat-
omy and DevelopmentalBiology, Monash University,Building 13C,
Wellington Rd, Clayton, Victoria, 3800, Australia.
E-mail: paul.mcmenamin@monash.edu
Received 20 January 2014;Revised 14 May 2014;Accepted 12 June
2014.
Published online 00 Month 2014 in Wiley Online Library
(wileyonlinelibrary.com). DOI 10.1002/ase.1475
VC 2014 American Association of Anatomists
AnatomicalSciences EducationMONTH 2014 Anat SciEduc 00:00–00 (2014)
DESCRIPTIVE ARTICLE
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

that comply with current health and safety considerations for
studentsand staff is also a financial burden (AAA, 2012;
Raja and Sultana, 2012). Furthermore, in some countries cul-
tural and ethicalconsiderations,and the rural location of
some institutions,mean thatmany medicalschoolsor col-
legesinvolved in educating doctorsand other allied disci-
plines have difficulties accessing human cadaver specimens.
Many medicalschoolsand anatomydepartmentshave
soughtalternativesor adjunctsto cadaver-based instruction
through the use ofalternative techniques including plastina-
tion (von Hagenset al., 1979), two-dimensional(2D) and
three-dimensional(3D) imaging (Estevez etal., 2010), and
body painting (McMenamin, 2008).
Rapid prototyping via 3D printing is a rapidly expanding
technology that is now a critical part of the iterative design pro-
cess in engineering,producing physicalmodels quickly,easily
and inexpensively from computer-aided design (CAD)and
other digital data (Pham and Dimov, 2001). Additive manufac-
turing or 3D printing is often promoted as one of the most sig-
nificant technologicaladvancesin our modern era.In the
medical and health care arena, 3D printing was identified as a
technology with great promise as early as 1997 (McGurk et al.,
1997) and has already had an impact in the domain of oromax-
illary and facial surgery (Isolan et al., 2007; Cohen et al., 2009)
and orthopedic surgery (Esses et al., 2011) by allowing the pro-
duction of bespoke prefabricated bone models for presurgical
planning or the creation of patient-specificprosthesesfor
implantation (Tam et al., 2013), surgical simulation (Monfared
et al.,2012,Waran et al.,2013) or as a patient educational
tools (see review, Rengier et al., 2010). The use of 3D printing
in forensic medicine to create models of bone fractures, vessels,
cardiac infarctions, ruptured organs and bite-mark wounds has
also been reported (Ebert et al., 2011). As 3D prints can be gen-
erated from medical CT/MRI data, it is logically possible to use
3D print outs from common imaging studies to augment the
teaching of topographic and applied clinical anatomy.
Some issuesremain unresolved regarding the application
of this emerging technology foranatomicalscienceseduca-
tion. In this study we wanted to addressthe following
questions:
(1) What data inputsare required orcan be potentially
utilized? (2) What are the logistics of data processing and 3D
print production? (3) What is the qualitative and quantitative
accuracy of the 3D prints compared with the originalspeci-
mens?(4) What are the relativecostswhen compared to
alternatives?
METHODS
Image Data Acquisition
The precise threshold ofresolution required for 3D printed
models to be usefulfor haptic teaching aids is not presently
known, but the majority of3D printers are capable of100
mm isometric resolution,and latestgeneration 3D scanning
equipment(such as fixed or hand-held surface scanners)are
capableof comparable(or higher)resolution during data
acquisition.A modern 64 slice CT scanner typically involves
lower resolutions;for example a CT scan of a limb segment
would produce pixel sizes (i.e., X and Y resolutions) of 0.15–
0.5 mm and interslice distances (Z resolution) of 0.4–1.0 mm
(Kalender,2006).Thus as long as printer resolution is higher
than the scan resolution,3D printing will not resultin any
loss of accuracy.For initial testing of3D printing as a tool
for anatomy teaching and learning,we aimed to produce a
3D model that displayed the surface features visible in a pro-
sected specimen. To obtain high quality 3D printed models of
cadaver specimens it is vitalthat the originalcadaver prosec-
tion be of high quality.For the initial“proof of concept” we
scanned a prosected upperlimb (Fig. 1A), using a Philips
Brilliance64 CT scanner (Olympic Park Radiology, Mel-
bourne,Australia).The scannerfield of view was set to
150 mm,giving a per-slice pixelsize of 0.195 mm,and slice
distance wasset to 0.4 mm (near maximalresolutionsfor
this scanner).Using these parameters on a fixed prosection
we were essentially using the CT scanner for the purpose of
digitizing 3D surface geometry only,and either soft- or hard-
tissue optimized algorithms are suitable for subsequent gener-
ation of the 3D data.
As many interesting anatomicalfeaturesare fluid or air
filled spaces(e.g.,ventricles,paranasalair sinuses,vessels,
heart chambers) we also provide three examples of “negative
spaces” to demonstrate the capability of this method for visu-
alizing such anatomicalfeaturesof interestand producing
haptic models.First, to obtain a print of mammalian cranial
sinuses we scanned an adult common warthog (Phacochoerus
africanus;TM 738) specimen from theDitsong National
Museum NaturalHistory Departmentof Vertebrates (Preto-
ria, South Africa) collection usinga Phillips Brilliance 6
180P3 CT Scanner (Philips Healthcare,Best, The Nether-
lands)with a per-slice pixelsize of 0.5mm and a slice dis-
tance of 1.0 mm. Second, to prove 3D vascular data could be
printed contrast CT coronary angiogram data set was chosen
for segmentation.Third, to obtain a printof a mammalian
cochlea and vestibularapparatusof the dried skull of an
adult king colobus monkey (Colobus polykomos;ZA 1038)
was scanned using the Nikon XT H 225 ST micro-focus X-
ray tomography systems (Nikon Metrology, Leuven, Belgium)
housed atthe South African NationalCentre for Radiogra-
phy and Tomography thatobtained an isometric voxel(3D
pixel) size of 66 mm.
Image Processing
The CT data output for the upperlimb prosection wasin
DICOM (Digital Imaging and COmmunications in Medicine)
image stack of 1,343 slices. To generate a file that can be 3D
printed requiresspecialized 3D imageprocessing software
that can import a DICOM stack. Various ‘segmentation’
tools are then used to produce a 3D isosurface that is essen-
tially a 3D visualization of segmented structures. In this study
Avizo software,version 7.0,for 3D analysis of scientific and
industrial data (Visualization Science Group/FEI Comp,
M erignac Cedex,France)was chosen.As only the surface
features ofthe specimen were initially ofinterestsegmenta-
tion required only thatvoxels in the datasetwith an X-ray
density close to orhigherthan thatof water be separated
from voxels with a density corresponding to air.Automatic
thresholding tools(which segmentvoxels based upon CT
attenuation values,i.e., Hounsfield numbers)are a fastand
effective means of achieving this outcome. We found it valua-
ble to use low-density foam to hold the specimen clear of the
scanning table allowing the prosected specimen to be seg-
mented and digitally separated from the scanning table.The
scan was cropped in the long axis so that only the hand and
wrist were included in the final isosurface (Fig. 1B). The reso-
lutions and reconstruction algorithm used in the scan allow
2 McMenamin et al.
studentsand staff is also a financial burden (AAA, 2012;
Raja and Sultana, 2012). Furthermore, in some countries cul-
tural and ethicalconsiderations,and the rural location of
some institutions,mean thatmany medicalschoolsor col-
legesinvolved in educating doctorsand other allied disci-
plines have difficulties accessing human cadaver specimens.
Many medicalschoolsand anatomydepartmentshave
soughtalternativesor adjunctsto cadaver-based instruction
through the use ofalternative techniques including plastina-
tion (von Hagenset al., 1979), two-dimensional(2D) and
three-dimensional(3D) imaging (Estevez etal., 2010), and
body painting (McMenamin, 2008).
Rapid prototyping via 3D printing is a rapidly expanding
technology that is now a critical part of the iterative design pro-
cess in engineering,producing physicalmodels quickly,easily
and inexpensively from computer-aided design (CAD)and
other digital data (Pham and Dimov, 2001). Additive manufac-
turing or 3D printing is often promoted as one of the most sig-
nificant technologicaladvancesin our modern era.In the
medical and health care arena, 3D printing was identified as a
technology with great promise as early as 1997 (McGurk et al.,
1997) and has already had an impact in the domain of oromax-
illary and facial surgery (Isolan et al., 2007; Cohen et al., 2009)
and orthopedic surgery (Esses et al., 2011) by allowing the pro-
duction of bespoke prefabricated bone models for presurgical
planning or the creation of patient-specificprosthesesfor
implantation (Tam et al., 2013), surgical simulation (Monfared
et al.,2012,Waran et al.,2013) or as a patient educational
tools (see review, Rengier et al., 2010). The use of 3D printing
in forensic medicine to create models of bone fractures, vessels,
cardiac infarctions, ruptured organs and bite-mark wounds has
also been reported (Ebert et al., 2011). As 3D prints can be gen-
erated from medical CT/MRI data, it is logically possible to use
3D print outs from common imaging studies to augment the
teaching of topographic and applied clinical anatomy.
Some issuesremain unresolved regarding the application
of this emerging technology foranatomicalscienceseduca-
tion. In this study we wanted to addressthe following
questions:
(1) What data inputsare required orcan be potentially
utilized? (2) What are the logistics of data processing and 3D
print production? (3) What is the qualitative and quantitative
accuracy of the 3D prints compared with the originalspeci-
mens?(4) What are the relativecostswhen compared to
alternatives?
METHODS
Image Data Acquisition
The precise threshold ofresolution required for 3D printed
models to be usefulfor haptic teaching aids is not presently
known, but the majority of3D printers are capable of100
mm isometric resolution,and latestgeneration 3D scanning
equipment(such as fixed or hand-held surface scanners)are
capableof comparable(or higher)resolution during data
acquisition.A modern 64 slice CT scanner typically involves
lower resolutions;for example a CT scan of a limb segment
would produce pixel sizes (i.e., X and Y resolutions) of 0.15–
0.5 mm and interslice distances (Z resolution) of 0.4–1.0 mm
(Kalender,2006).Thus as long as printer resolution is higher
than the scan resolution,3D printing will not resultin any
loss of accuracy.For initial testing of3D printing as a tool
for anatomy teaching and learning,we aimed to produce a
3D model that displayed the surface features visible in a pro-
sected specimen. To obtain high quality 3D printed models of
cadaver specimens it is vitalthat the originalcadaver prosec-
tion be of high quality.For the initial“proof of concept” we
scanned a prosected upperlimb (Fig. 1A), using a Philips
Brilliance64 CT scanner (Olympic Park Radiology, Mel-
bourne,Australia).The scannerfield of view was set to
150 mm,giving a per-slice pixelsize of 0.195 mm,and slice
distance wasset to 0.4 mm (near maximalresolutionsfor
this scanner).Using these parameters on a fixed prosection
we were essentially using the CT scanner for the purpose of
digitizing 3D surface geometry only,and either soft- or hard-
tissue optimized algorithms are suitable for subsequent gener-
ation of the 3D data.
As many interesting anatomicalfeaturesare fluid or air
filled spaces(e.g.,ventricles,paranasalair sinuses,vessels,
heart chambers) we also provide three examples of “negative
spaces” to demonstrate the capability of this method for visu-
alizing such anatomicalfeaturesof interestand producing
haptic models.First, to obtain a print of mammalian cranial
sinuses we scanned an adult common warthog (Phacochoerus
africanus;TM 738) specimen from theDitsong National
Museum NaturalHistory Departmentof Vertebrates (Preto-
ria, South Africa) collection usinga Phillips Brilliance 6
180P3 CT Scanner (Philips Healthcare,Best, The Nether-
lands)with a per-slice pixelsize of 0.5mm and a slice dis-
tance of 1.0 mm. Second, to prove 3D vascular data could be
printed contrast CT coronary angiogram data set was chosen
for segmentation.Third, to obtain a printof a mammalian
cochlea and vestibularapparatusof the dried skull of an
adult king colobus monkey (Colobus polykomos;ZA 1038)
was scanned using the Nikon XT H 225 ST micro-focus X-
ray tomography systems (Nikon Metrology, Leuven, Belgium)
housed atthe South African NationalCentre for Radiogra-
phy and Tomography thatobtained an isometric voxel(3D
pixel) size of 66 mm.
Image Processing
The CT data output for the upperlimb prosection wasin
DICOM (Digital Imaging and COmmunications in Medicine)
image stack of 1,343 slices. To generate a file that can be 3D
printed requiresspecialized 3D imageprocessing software
that can import a DICOM stack. Various ‘segmentation’
tools are then used to produce a 3D isosurface that is essen-
tially a 3D visualization of segmented structures. In this study
Avizo software,version 7.0,for 3D analysis of scientific and
industrial data (Visualization Science Group/FEI Comp,
M erignac Cedex,France)was chosen.As only the surface
features ofthe specimen were initially ofinterestsegmenta-
tion required only thatvoxels in the datasetwith an X-ray
density close to orhigherthan thatof water be separated
from voxels with a density corresponding to air.Automatic
thresholding tools(which segmentvoxels based upon CT
attenuation values,i.e., Hounsfield numbers)are a fastand
effective means of achieving this outcome. We found it valua-
ble to use low-density foam to hold the specimen clear of the
scanning table allowing the prosected specimen to be seg-
mented and digitally separated from the scanning table.The
scan was cropped in the long axis so that only the hand and
wrist were included in the final isosurface (Fig. 1B). The reso-
lutions and reconstruction algorithm used in the scan allow
2 McMenamin et al.
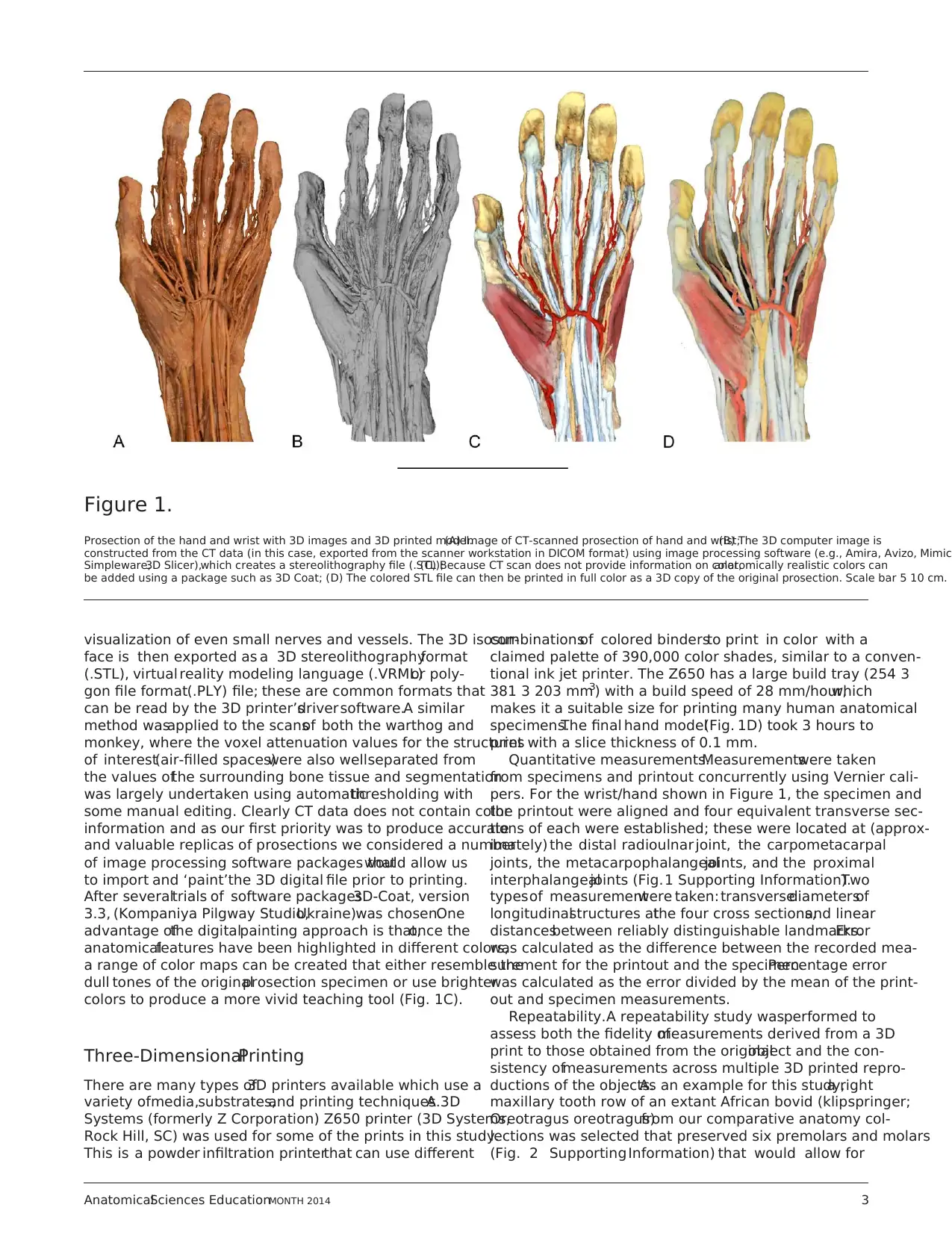
visualization of even small nerves and vessels. The 3D isosur-
face is then exported as a 3D stereolithographyformat
(.STL), virtual reality modeling language (.VRML)or poly-
gon file format(.PLY) file; these are common formats that
can be read by the 3D printer’sdriver software.A similar
method wasapplied to the scansof both the warthog and
monkey, where the voxel attenuation values for the structures
of interest(air-filled spaces)were also wellseparated from
the values ofthe surrounding bone tissue and segmentation
was largely undertaken using automaticthresholding with
some manual editing. Clearly CT data does not contain color
information and as our first priority was to produce accurate
and valuable replicas of prosections we considered a number
of image processing software packages thatwould allow us
to import and ‘paint’the 3D digital file prior to printing.
After severaltrials of software packages3D-Coat, version
3.3, (Kompaniya Pilgway Studio,Ukraine)was chosen.One
advantage ofthe digitalpainting approach is that,once the
anatomicalfeatures have been highlighted in different colors,
a range of color maps can be created that either resemble the
dull tones of the originalprosection specimen or use brighter
colors to produce a more vivid teaching tool (Fig. 1C).
Three-DimensionalPrinting
There are many types of3D printers available which use a
variety ofmedia,substrates,and printing techniques.A 3D
Systems (formerly Z Corporation) Z650 printer (3D Systems,
Rock Hill, SC) was used for some of the prints in this study.
This is a powder infiltration printerthat can use different
combinationsof colored bindersto print in color with a
claimed palette of 390,000 color shades, similar to a conven-
tional ink jet printer. The Z650 has a large build tray (254 3
381 3 203 mm3) with a build speed of 28 mm/hour,which
makes it a suitable size for printing many human anatomical
specimens.The final hand model(Fig. 1D) took 3 hours to
print with a slice thickness of 0.1 mm.
Quantitative measurements.Measurementswere taken
from specimens and printout concurrently using Vernier cali-
pers. For the wrist/hand shown in Figure 1, the specimen and
the printout were aligned and four equivalent transverse sec-
tions of each were established; these were located at (approx-
imately) the distal radioulnar joint, the carpometacarpal
joints, the metacarpophalangealjoints, and the proximal
interphalangealjoints (Fig.1 Supporting Information).Two
typesof measurementwere taken:transversediametersof
longitudinalstructures atthe four cross sections,and linear
distancesbetween reliably distinguishable landmarks.Error
was calculated as the difference between the recorded mea-
surement for the printout and the specimen.Percentage error
was calculated as the error divided by the mean of the print-
out and specimen measurements.
Repeatability.A repeatability study wasperformed to
assess both the fidelity ofmeasurements derived from a 3D
print to those obtained from the originalobject and the con-
sistency ofmeasurements across multiple 3D printed repro-
ductions of the objects.As an example for this study,a right
maxillary tooth row of an extant African bovid (klipspringer;
Oreotragus oreotragus)from our comparative anatomy col-
lections was selected that preserved six premolars and molars
(Fig. 2 Supporting Information) that would allow for
Figure 1.
Prosection of the hand and wrist with 3D images and 3D printed model.(A) Image of CT-scanned prosection of hand and wrist;(B) The 3D computer image is
constructed from the CT data (in this case, exported from the scanner workstation in DICOM format) using image processing software (e.g., Amira, Avizo, Mimics
Simpleware,3D Slicer),which creates a stereolithography file (.STL);(C) Because CT scan does not provide information on color,anatomically realistic colors can
be added using a package such as 3D Coat; (D) The colored STL file can then be printed in full color as a 3D copy of the original prosection. Scale bar 5 10 cm.
AnatomicalSciences EducationMONTH 2014 3
face is then exported as a 3D stereolithographyformat
(.STL), virtual reality modeling language (.VRML)or poly-
gon file format(.PLY) file; these are common formats that
can be read by the 3D printer’sdriver software.A similar
method wasapplied to the scansof both the warthog and
monkey, where the voxel attenuation values for the structures
of interest(air-filled spaces)were also wellseparated from
the values ofthe surrounding bone tissue and segmentation
was largely undertaken using automaticthresholding with
some manual editing. Clearly CT data does not contain color
information and as our first priority was to produce accurate
and valuable replicas of prosections we considered a number
of image processing software packages thatwould allow us
to import and ‘paint’the 3D digital file prior to printing.
After severaltrials of software packages3D-Coat, version
3.3, (Kompaniya Pilgway Studio,Ukraine)was chosen.One
advantage ofthe digitalpainting approach is that,once the
anatomicalfeatures have been highlighted in different colors,
a range of color maps can be created that either resemble the
dull tones of the originalprosection specimen or use brighter
colors to produce a more vivid teaching tool (Fig. 1C).
Three-DimensionalPrinting
There are many types of3D printers available which use a
variety ofmedia,substrates,and printing techniques.A 3D
Systems (formerly Z Corporation) Z650 printer (3D Systems,
Rock Hill, SC) was used for some of the prints in this study.
This is a powder infiltration printerthat can use different
combinationsof colored bindersto print in color with a
claimed palette of 390,000 color shades, similar to a conven-
tional ink jet printer. The Z650 has a large build tray (254 3
381 3 203 mm3) with a build speed of 28 mm/hour,which
makes it a suitable size for printing many human anatomical
specimens.The final hand model(Fig. 1D) took 3 hours to
print with a slice thickness of 0.1 mm.
Quantitative measurements.Measurementswere taken
from specimens and printout concurrently using Vernier cali-
pers. For the wrist/hand shown in Figure 1, the specimen and
the printout were aligned and four equivalent transverse sec-
tions of each were established; these were located at (approx-
imately) the distal radioulnar joint, the carpometacarpal
joints, the metacarpophalangealjoints, and the proximal
interphalangealjoints (Fig.1 Supporting Information).Two
typesof measurementwere taken:transversediametersof
longitudinalstructures atthe four cross sections,and linear
distancesbetween reliably distinguishable landmarks.Error
was calculated as the difference between the recorded mea-
surement for the printout and the specimen.Percentage error
was calculated as the error divided by the mean of the print-
out and specimen measurements.
Repeatability.A repeatability study wasperformed to
assess both the fidelity ofmeasurements derived from a 3D
print to those obtained from the originalobject and the con-
sistency ofmeasurements across multiple 3D printed repro-
ductions of the objects.As an example for this study,a right
maxillary tooth row of an extant African bovid (klipspringer;
Oreotragus oreotragus)from our comparative anatomy col-
lections was selected that preserved six premolars and molars
(Fig. 2 Supporting Information) that would allow for
Figure 1.
Prosection of the hand and wrist with 3D images and 3D printed model.(A) Image of CT-scanned prosection of hand and wrist;(B) The 3D computer image is
constructed from the CT data (in this case, exported from the scanner workstation in DICOM format) using image processing software (e.g., Amira, Avizo, Mimics
Simpleware,3D Slicer),which creates a stereolithography file (.STL);(C) Because CT scan does not provide information on color,anatomically realistic colors can
be added using a package such as 3D Coat; (D) The colored STL file can then be printed in full color as a 3D copy of the original prosection. Scale bar 5 10 cm.
AnatomicalSciences EducationMONTH 2014 3
standard dentalmetricsto be acquired (overallmesiodistal
length and overallbuccolingualwidth; Janis, 1988).A sur-
face mesh ofthe maxillary dentition was captured using an
Artec SpiderTM hand-held 3D scanner (Artec Group,Luxen-
bourg)with a stated resolution of0.1 mm and accuracy up
to 0.03 mm. The resulting STL (STereoLithography)mesh
was imported into the 3D modelling software package Rhi-
noceros 3D,version 5.0 (Robert McNeel& Associates,Seat-
tle, WA) to merge the maxillary surfacewithin a solid
platform and then five copies of the final mesh (Maxilla 1–5)
were printed using the Z650 printer at 100% scaling (Fig.2
Supporting Information).
The originalspecimen of the maxillary tooth row and all
five 3D printed reproductions were measured using Mitutoyo
500 series calipers (Mitutoyo America,Aurora,IL) with pre-
cision of 0.01 mm and accuracy of0.02 mm.The dentition
of the originaland each printed specimen was measured by
one of the authors(J.W.A.) five timesover the span of a
week (average intervalof 24 hours between specimen meas-
urements) to establish a range of intraobserver error for each
dentalmeasurement.These data were used fora seriesof
intraclasscorrelation coefficients(ICC) calculated in SPSS
statisticalpackage,version 20.0 (IBM SPSS,Chicago,IL) to
assessthe intraobserverreliability ofthe repeated measure-
ments on the original and each of the printed maxillary denti-
tions.In addition,the calculated mean measurements for the
original and reproductions was used to calculate concordance
correlation coefficients (Lin,1989, 2000)to assess the reli-
ability of the 3D printed reproductions againstthe original
maxillary dentition.
RESULTS
Three-dimensionalprinting ofprosected specimens based on
CT data sets produced highly realistic 3D replicas in which
even smallnerves and vessels could be readily distinguished
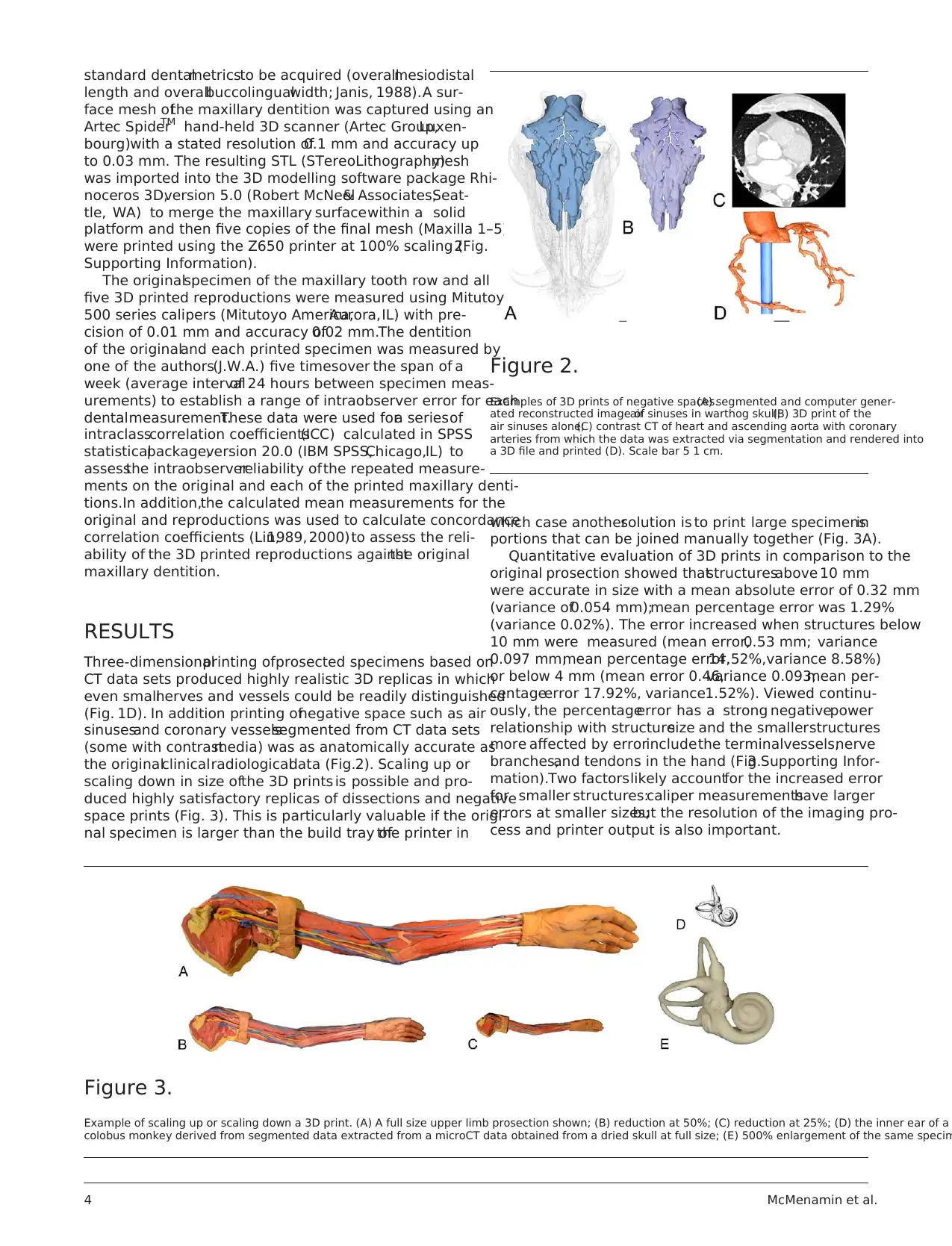
(Fig. 1D). In addition printing ofnegative space such as air
sinusesand coronary vesselssegmented from CT data sets
(some with contrastmedia) was as anatomically accurate as
the originalclinicalradiologicaldata (Fig.2). Scaling up or
scaling down in size ofthe 3D prints is possible and pro-
duced highly satisfactory replicas of dissections and negative
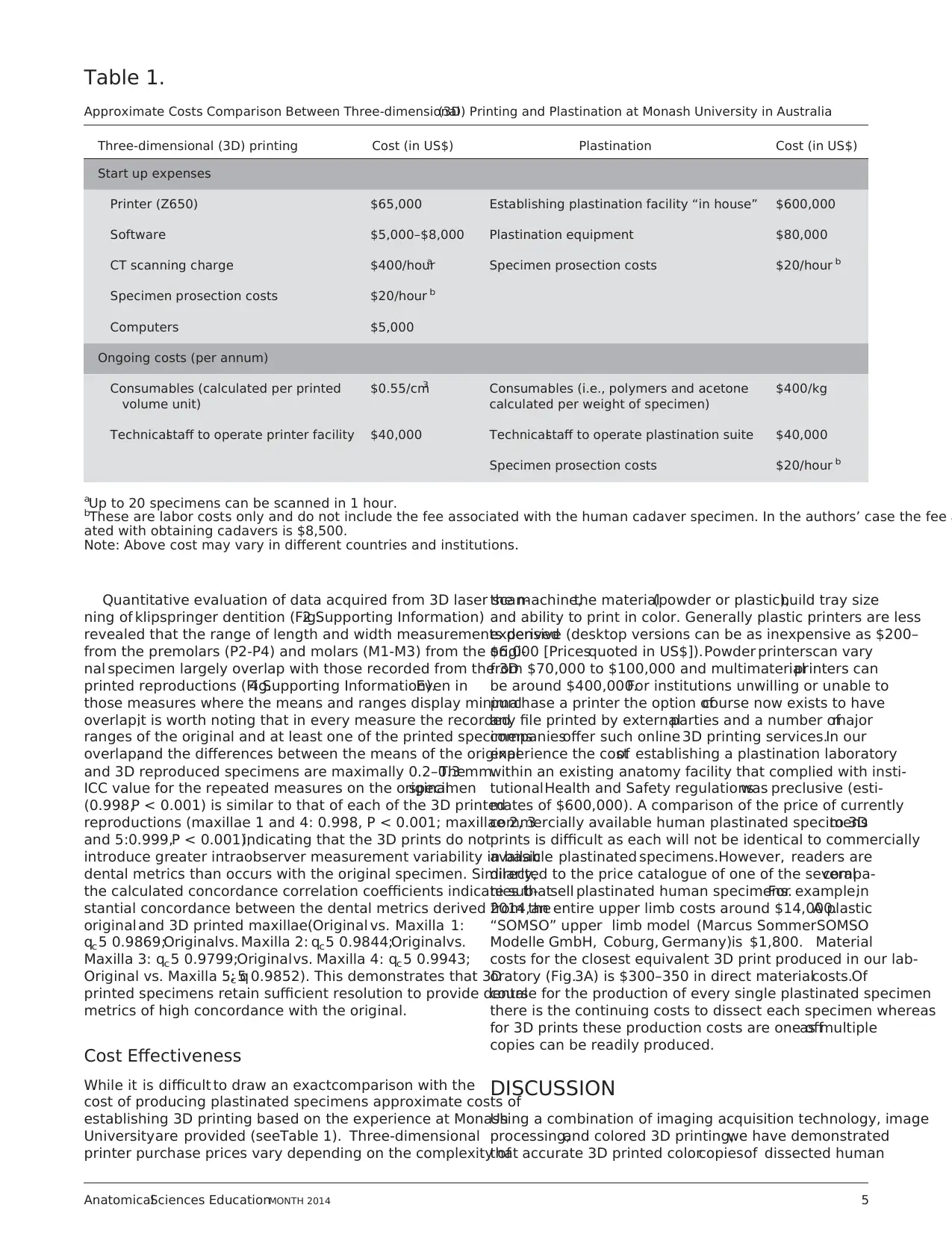
space prints (Fig. 3). This is particularly valuable if the origi-
nal specimen is larger than the build tray ofthe printer in
which case anothersolution is to print large specimensin
portions that can be joined manually together (Fig. 3A).
Quantitative evaluation of 3D prints in comparison to the
original prosection showed thatstructuresabove 10 mm
were accurate in size with a mean absolute error of 0.32 mm
(variance of0.054 mm);mean percentage error was 1.29%
(variance 0.02%). The error increased when structures below
10 mm were measured (mean error,0.53 mm; variance
0.097 mm;mean percentage error,14.52%,variance 8.58%)
or below 4 mm (mean error 0.46,variance 0.093;mean per-
centageerror 17.92%, variance1.52%). Viewed continu-
ously, the percentageerror has a strong negativepower
relationship with structuresize and the smallerstructures
more affected by errorincludethe terminalvessels,nerve
branches,and tendons in the hand (Fig.3 Supporting Infor-
mation).Two factorslikely accountfor the increased error
for smaller structures:caliper measurementshave larger
errors at smaller sizes;but the resolution of the imaging pro-
cess and printer output is also important.
Figure 2.
Examples of 3D prints of negative spaces.(A) segmented and computer gener-
ated reconstructed image ofair sinuses in warthog skull;(B) 3D print of the
air sinuses alone;(C) contrast CT of heart and ascending aorta with coronary
arteries from which the data was extracted via segmentation and rendered into
a 3D file and printed (D). Scale bar 5 1 cm.
Figure 3.
Example of scaling up or scaling down a 3D print. (A) A full size upper limb prosection shown; (B) reduction at 50%; (C) reduction at 25%; (D) the inner ear of a
colobus monkey derived from segmented data extracted from a microCT data obtained from a dried skull at full size; (E) 500% enlargement of the same specim
4 McMenamin et al.
length and overallbuccolingualwidth; Janis, 1988).A sur-
face mesh ofthe maxillary dentition was captured using an
Artec SpiderTM hand-held 3D scanner (Artec Group,Luxen-
bourg)with a stated resolution of0.1 mm and accuracy up
to 0.03 mm. The resulting STL (STereoLithography)mesh
was imported into the 3D modelling software package Rhi-
noceros 3D,version 5.0 (Robert McNeel& Associates,Seat-
tle, WA) to merge the maxillary surfacewithin a solid
platform and then five copies of the final mesh (Maxilla 1–5)
were printed using the Z650 printer at 100% scaling (Fig.2
Supporting Information).
The originalspecimen of the maxillary tooth row and all
five 3D printed reproductions were measured using Mitutoyo
500 series calipers (Mitutoyo America,Aurora,IL) with pre-
cision of 0.01 mm and accuracy of0.02 mm.The dentition
of the originaland each printed specimen was measured by
one of the authors(J.W.A.) five timesover the span of a
week (average intervalof 24 hours between specimen meas-
urements) to establish a range of intraobserver error for each
dentalmeasurement.These data were used fora seriesof
intraclasscorrelation coefficients(ICC) calculated in SPSS
statisticalpackage,version 20.0 (IBM SPSS,Chicago,IL) to
assessthe intraobserverreliability ofthe repeated measure-
ments on the original and each of the printed maxillary denti-
tions.In addition,the calculated mean measurements for the
original and reproductions was used to calculate concordance
correlation coefficients (Lin,1989, 2000)to assess the reli-
ability of the 3D printed reproductions againstthe original
maxillary dentition.
RESULTS
Three-dimensionalprinting ofprosected specimens based on
CT data sets produced highly realistic 3D replicas in which
even smallnerves and vessels could be readily distinguished
(Fig. 1D). In addition printing ofnegative space such as air
sinusesand coronary vesselssegmented from CT data sets
(some with contrastmedia) was as anatomically accurate as
the originalclinicalradiologicaldata (Fig.2). Scaling up or
scaling down in size ofthe 3D prints is possible and pro-
duced highly satisfactory replicas of dissections and negative
space prints (Fig. 3). This is particularly valuable if the origi-
nal specimen is larger than the build tray ofthe printer in
which case anothersolution is to print large specimensin
portions that can be joined manually together (Fig. 3A).
Quantitative evaluation of 3D prints in comparison to the
original prosection showed thatstructuresabove 10 mm
were accurate in size with a mean absolute error of 0.32 mm
(variance of0.054 mm);mean percentage error was 1.29%
(variance 0.02%). The error increased when structures below
10 mm were measured (mean error,0.53 mm; variance
0.097 mm;mean percentage error,14.52%,variance 8.58%)
or below 4 mm (mean error 0.46,variance 0.093;mean per-
centageerror 17.92%, variance1.52%). Viewed continu-
ously, the percentageerror has a strong negativepower
relationship with structuresize and the smallerstructures
more affected by errorincludethe terminalvessels,nerve
branches,and tendons in the hand (Fig.3 Supporting Infor-
mation).Two factorslikely accountfor the increased error
for smaller structures:caliper measurementshave larger
errors at smaller sizes;but the resolution of the imaging pro-
cess and printer output is also important.
Figure 2.
Examples of 3D prints of negative spaces.(A) segmented and computer gener-
ated reconstructed image ofair sinuses in warthog skull;(B) 3D print of the
air sinuses alone;(C) contrast CT of heart and ascending aorta with coronary
arteries from which the data was extracted via segmentation and rendered into
a 3D file and printed (D). Scale bar 5 1 cm.
Figure 3.
Example of scaling up or scaling down a 3D print. (A) A full size upper limb prosection shown; (B) reduction at 50%; (C) reduction at 25%; (D) the inner ear of a
colobus monkey derived from segmented data extracted from a microCT data obtained from a dried skull at full size; (E) 500% enlargement of the same specim
4 McMenamin et al.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Quantitative evaluation of data acquired from 3D laser scan-
ning of klipspringer dentition (Fig.2 Supporting Information)
revealed that the range of length and width measurements derived
from the premolars (P2-P4) and molars (M1-M3) from the origi-
nal specimen largely overlap with those recorded from the 3D
printed reproductions (Fig.4 Supporting Information).Even in
those measures where the means and ranges display minimal
overlap,it is worth noting that in every measure the recorded
ranges of the original and at least one of the printed specimens
overlap;and the differences between the means of the original
and 3D reproduced specimens are maximally 0.2–0.3 mm.The
ICC value for the repeated measures on the originalspecimen
(0.998,P < 0.001) is similar to that of each of the 3D printed
reproductions (maxillae 1 and 4: 0.998, P < 0.001; maxillae 2, 3
and 5:0.999,P < 0.001),indicating that the 3D prints do not
introduce greater intraobserver measurement variability in basic
dental metrics than occurs with the original specimen. Similarly,
the calculated concordance correlation coefficients indicate sub-
stantial concordance between the dental metrics derived from the
original and 3D printed maxillae(Original vs. Maxilla 1:
qc5 0.9869;Originalvs. Maxilla 2: qc5 0.9844;Originalvs.
Maxilla 3: qc5 0.9799;Originalvs. Maxilla 4: qc5 0.9943;
Original vs. Maxilla 5: qc5 0.9852). This demonstrates that 3D
printed specimens retain sufficient resolution to provide dental
metrics of high concordance with the original.
Cost Effectiveness
While it is difficult to draw an exactcomparison with the
cost of producing plastinated specimens approximate costs of
establishing 3D printing based on the experience at Monash
Universityare provided (seeTable 1). Three-dimensional
printer purchase prices vary depending on the complexity of
the machine,the material(powder or plastic),build tray size
and ability to print in color. Generally plastic printers are less
expensive (desktop versions can be as inexpensive as $200–
$6,000 [Pricesquoted in US$]).Powder printerscan vary
from $70,000 to $100,000 and multimaterialprinters can
be around $400,000.For institutions unwilling or unable to
purchase a printer the option ofcourse now exists to have
any file printed by externalparties and a number ofmajor
companiesoffer such online 3D printing services.In our
experience the costof establishing a plastination laboratory
within an existing anatomy facility that complied with insti-
tutionalHealth and Safety regulationswas preclusive (esti-
mates of $600,000). A comparison of the price of currently
commercially available human plastinated specimensto 3D
prints is difficult as each will not be identical to commercially
available plastinated specimens.However, readers are
directed to the price catalogue of one of the severalcompa-
nies thatsell plastinated human specimens.For example,in
2014,an entire upper limb costs around $14,000.A plastic
“SOMSO” upper limb model (Marcus SommerSOMSO
Modelle GmbH, Coburg, Germany)is $1,800. Material
costs for the closest equivalent 3D print produced in our lab-
oratory (Fig.3A) is $300–350 in direct materialcosts.Of
course for the production of every single plastinated specimen
there is the continuing costs to dissect each specimen whereas
for 3D prints these production costs are one offas multiple
copies can be readily produced.
DISCUSSION
Using a combination of imaging acquisition technology, image
processing,and colored 3D printing,we have demonstrated
that accurate 3D printed colorcopiesof dissected human
Table 1.
Approximate Costs Comparison Between Three-dimensional(3D) Printing and Plastination at Monash University in Australia
Three-dimensional (3D) printing Cost (in US$) Plastination Cost (in US$)
Start up expenses
Printer (Z650) $65,000 Establishing plastination facility “in house” $600,000
Software $5,000–$8,000 Plastination equipment $80,000
CT scanning charge $400/houra Specimen prosection costs $20/hour b
Specimen prosection costs $20/hour b
Computers $5,000
Ongoing costs (per annum)
Consumables (calculated per printed
volume unit)
$0.55/cm3 Consumables (i.e., polymers and acetone
calculated per weight of specimen)
$400/kg
Technicalstaff to operate printer facility $40,000 Technicalstaff to operate plastination suite $40,000
Specimen prosection costs $20/hour b
aUp to 20 specimens can be scanned in 1 hour.
bThese are labor costs only and do not include the fee associated with the human cadaver specimen. In the authors’ case the fee a
ated with obtaining cadavers is $8,500.
Note: Above cost may vary in different countries and institutions.
AnatomicalSciences EducationMONTH 2014 5
ning of klipspringer dentition (Fig.2 Supporting Information)
revealed that the range of length and width measurements derived
from the premolars (P2-P4) and molars (M1-M3) from the origi-
nal specimen largely overlap with those recorded from the 3D
printed reproductions (Fig.4 Supporting Information).Even in
those measures where the means and ranges display minimal
overlap,it is worth noting that in every measure the recorded
ranges of the original and at least one of the printed specimens
overlap;and the differences between the means of the original
and 3D reproduced specimens are maximally 0.2–0.3 mm.The
ICC value for the repeated measures on the originalspecimen
(0.998,P < 0.001) is similar to that of each of the 3D printed
reproductions (maxillae 1 and 4: 0.998, P < 0.001; maxillae 2, 3
and 5:0.999,P < 0.001),indicating that the 3D prints do not
introduce greater intraobserver measurement variability in basic
dental metrics than occurs with the original specimen. Similarly,
the calculated concordance correlation coefficients indicate sub-
stantial concordance between the dental metrics derived from the
original and 3D printed maxillae(Original vs. Maxilla 1:
qc5 0.9869;Originalvs. Maxilla 2: qc5 0.9844;Originalvs.
Maxilla 3: qc5 0.9799;Originalvs. Maxilla 4: qc5 0.9943;
Original vs. Maxilla 5: qc5 0.9852). This demonstrates that 3D
printed specimens retain sufficient resolution to provide dental
metrics of high concordance with the original.
Cost Effectiveness
While it is difficult to draw an exactcomparison with the
cost of producing plastinated specimens approximate costs of
establishing 3D printing based on the experience at Monash
Universityare provided (seeTable 1). Three-dimensional
printer purchase prices vary depending on the complexity of
the machine,the material(powder or plastic),build tray size
and ability to print in color. Generally plastic printers are less
expensive (desktop versions can be as inexpensive as $200–
$6,000 [Pricesquoted in US$]).Powder printerscan vary
from $70,000 to $100,000 and multimaterialprinters can
be around $400,000.For institutions unwilling or unable to
purchase a printer the option ofcourse now exists to have
any file printed by externalparties and a number ofmajor
companiesoffer such online 3D printing services.In our
experience the costof establishing a plastination laboratory
within an existing anatomy facility that complied with insti-
tutionalHealth and Safety regulationswas preclusive (esti-
mates of $600,000). A comparison of the price of currently
commercially available human plastinated specimensto 3D
prints is difficult as each will not be identical to commercially
available plastinated specimens.However, readers are
directed to the price catalogue of one of the severalcompa-
nies thatsell plastinated human specimens.For example,in
2014,an entire upper limb costs around $14,000.A plastic
“SOMSO” upper limb model (Marcus SommerSOMSO
Modelle GmbH, Coburg, Germany)is $1,800. Material
costs for the closest equivalent 3D print produced in our lab-
oratory (Fig.3A) is $300–350 in direct materialcosts.Of
course for the production of every single plastinated specimen
there is the continuing costs to dissect each specimen whereas
for 3D prints these production costs are one offas multiple
copies can be readily produced.
DISCUSSION
Using a combination of imaging acquisition technology, image
processing,and colored 3D printing,we have demonstrated
that accurate 3D printed colorcopiesof dissected human
Table 1.
Approximate Costs Comparison Between Three-dimensional(3D) Printing and Plastination at Monash University in Australia
Three-dimensional (3D) printing Cost (in US$) Plastination Cost (in US$)
Start up expenses
Printer (Z650) $65,000 Establishing plastination facility “in house” $600,000
Software $5,000–$8,000 Plastination equipment $80,000
CT scanning charge $400/houra Specimen prosection costs $20/hour b
Specimen prosection costs $20/hour b
Computers $5,000
Ongoing costs (per annum)
Consumables (calculated per printed
volume unit)
$0.55/cm3 Consumables (i.e., polymers and acetone
calculated per weight of specimen)
$400/kg
Technicalstaff to operate printer facility $40,000 Technicalstaff to operate plastination suite $40,000
Specimen prosection costs $20/hour b
aUp to 20 specimens can be scanned in 1 hour.
bThese are labor costs only and do not include the fee associated with the human cadaver specimen. In the authors’ case the fee a
ated with obtaining cadavers is $8,500.
Note: Above cost may vary in different countries and institutions.
AnatomicalSciences EducationMONTH 2014 5

anatomical specimens can be rapidly and economically repro-
duced.In addition,we have shown that it is possible to seg-
ment negative spaces such as air sinuses and vascular spaces
from radiologicaldata and print them in a form suitable for
teaching.
To date there have been limited publications examining the
potentialrole of 3D printing in medicaleducation.A recent
investigation examined the value of3D prints of the distal
equine limb in the teaching of anatomy to veterinary students
(Preece etal., 2013).This model,which was based on high
resolution MRI scanning data,reproduced the bones in hard
plastic and the soft tissues (ligaments, tendons) in a more flexi-
ble material.Students were able to physically reconstruct the
horse foot modeldue to the aid of magnets embedded in the
printed elements.In an evaluation of their modelthe authors
found a significant improvement in students’ overall scores in
an MRI based assessment of equine foot anatomy and a raised
confidencelevelsin dealing with visuospatialinformation.
Unlike the prints produced in the present investigation the sin-
gle equine foot modelwas largely monochrome and did not
attempt to capture neural or vascular structures. Another study
utilized 3D printing technology to copy a rare corrosion cast of
the human lungs and airways Liet al.(2012).Thus the data
was not obtained from soft-tissue human anatomical material
or indeed medical imaging, but rather employed 3D printing to
reproduce a complex castthat would have been otherwise
impossible to copy by conventional manufacturing methods.
The advantages of the 3D printed copies of cadaver pro-
sectionsor anatomicalspecimensillustrated in the present
report include durability,accuracy,ease of reproduction,cost
effectiveness,and the avoidance ofhealth and safety issues
associated with wetfixed cadaverspecimensor plastinated
specimens.The most suitableprinter types for producing
teaching specimens are powder infiltration,plastic extrusion,
and multi-property.Each has strengths and limitations.Pow-
der infiltration printers, which essentiallyutilize a glue
(binder) to print over a layer of fine plaster powder,layer by
layer into a 3D shape,are relatively economicalto run and
have fast build times. Plastic extrusion printersproduce
slightly higherresolutionsin a stronger materialbut have
higher consumable costs, longer print times and limited color
options,although rapid technologicalchanges may soon neg-
ate some ofthese issues.It is likely that in the very near
future plastic printers will be able to print in the same sort of
color fidelity that currentlypowder printerscan and we
believe these will be more robust for teaching purposes while
powder printers may remain critical for prototyping.
Multiproperty printersare able to print complex struc-
tures in a variety of materials, including rubbers and plastics,
but are commensurately more expensive,and until recently
have generally not been capable of producing a palette of col-
ors as used in the present study.The quantitative evaluation
we performed also showed the close correlation between the
real prosection and the 3D printed reproduction,allowing
the printing of even the smallest digital nerves with only rela-
tively minor variations in size when compared to the original
specimen.A previous study by Smith etal (2013)found an
accuracy of0.1 mm in printing ofradiographic data ofhip
and shoulder joint surfaces and highlighted that segmentation
is actually a greater source of variance that the printing itself.
When 3D printing from DICOM data is being performed it
is vitalthat the scan resolution (slice thickness) is as close to
the layer thickness of the chosen 3D printer,a point recently
made by Houtilainen etal. (2014)and also evidentin our
experience. A further advantage of the present method of 3D
printing wasthe relative ease with which we could rescale
the data files to reproduce accurate larger or smaller repro-
ductions of prosected material or negative spaces (Fig. 3).
Perhaps the most notable advantages of 3D printing of repro-
ductions of anatomical dissections are its multiple benefits over
plastic models and plastinated cadaver specimens. Plastic mod-
els are in common use in high schools,doctors’ surgeries and
medical schools. They are mass produced copies or molds of a
“hypothetical” or“caricatured” anatomicalspecimensthat
often lack importantspecific details.While suitable for some
teaching purposes they are not ideal for teaching detailed anat-
omy typically required in medical and other allied health profes-
sionalcourses.While plastinated specimens can be produced
that display detailed anatomicalinformation a noteworthy
advantage of 3D printed reproductions is that they are reason-
ably inexpensive, easy to reproduce multiple copies and thus are
an attractive alternative forteaching facilitiesor universities
which may have no access to cadavers for teaching for logistical
reasons (rural sites,developing countries) or due to socialand
culturalbarriers.In addition,anatomicalvariations can easily
be demonstrated by printing multiple data sets; indeed we have
already begun to do this by printing left dominant, right domi-
nant and codominant variants of coronary artery distribution.
One of the alternatives to 3D printing, plastination, is a method
of substitution or infiltration ofdehydrated cadaver material
with an inflexible polymer silicon compound. It was developed
by Gunther von Hagensand madepopularby the “Body
Worlds” and other similar exhibitions (von Hagens, 1979; Bick-
ley et al., 1981). However, ethical issues have been raised about
the source of cadavers and the trading in human remains in one
country to another part of the world for commercial gain (Jones
and Whitaker,2009;Collier,2010). Preparation of plastinated
specimens from cadavers sourced from local bequest programs
is one possible solution but this involves considerable costs and
health and safety issues due to the large volumes of flammable
solvents involved.In addition,teaching large classes requires
multiple prosections of each body region,and preparing these
requires a localpool of skilled prosectors to create multiple
specimens for plastination.By contrast,a modern 3D printer
can be housed in a conventional office.
Under section 32 of the Human Tissue Act (1982) in Aus-
tralia the purposes for which a body can be donated are “the
use of [the] body for the study and teaching of the anatomy
of the human body.” We consider that our 3D copies of par-
ticular body parts are produced as a teaching aid and there-
fore come under the purpose of “teaching of anatomy” and
have been advised by the responsible localgovernment legis-
lative authority thatthey seeno ethical dilemma in their
reproduction.Indeed one could easily draw the analogy of
the reproduction of3D imageswith that of 2D imagesof
human cadaveric dissectionsthat are widely used in many
textbook or multimedia teaching aids.
Limitations to 3D Printing
There are of course some limitations to 3D printed copies of
human anatomicalprosections.Firstly,the outputis only as
good as the input,therefore it is imperative that high quality
prosections illustrating as many features as possible without
being overly complex are produced and selected forimage
acquisition and processing.The dissected specimens have to
be amenable to scanning and reproduction by 3D printing. A
6 McMenamin et al.
duced.In addition,we have shown that it is possible to seg-
ment negative spaces such as air sinuses and vascular spaces
from radiologicaldata and print them in a form suitable for
teaching.
To date there have been limited publications examining the
potentialrole of 3D printing in medicaleducation.A recent
investigation examined the value of3D prints of the distal
equine limb in the teaching of anatomy to veterinary students
(Preece etal., 2013).This model,which was based on high
resolution MRI scanning data,reproduced the bones in hard
plastic and the soft tissues (ligaments, tendons) in a more flexi-
ble material.Students were able to physically reconstruct the
horse foot modeldue to the aid of magnets embedded in the
printed elements.In an evaluation of their modelthe authors
found a significant improvement in students’ overall scores in
an MRI based assessment of equine foot anatomy and a raised
confidencelevelsin dealing with visuospatialinformation.
Unlike the prints produced in the present investigation the sin-
gle equine foot modelwas largely monochrome and did not
attempt to capture neural or vascular structures. Another study
utilized 3D printing technology to copy a rare corrosion cast of
the human lungs and airways Liet al.(2012).Thus the data
was not obtained from soft-tissue human anatomical material
or indeed medical imaging, but rather employed 3D printing to
reproduce a complex castthat would have been otherwise
impossible to copy by conventional manufacturing methods.
The advantages of the 3D printed copies of cadaver pro-
sectionsor anatomicalspecimensillustrated in the present
report include durability,accuracy,ease of reproduction,cost
effectiveness,and the avoidance ofhealth and safety issues
associated with wetfixed cadaverspecimensor plastinated
specimens.The most suitableprinter types for producing
teaching specimens are powder infiltration,plastic extrusion,
and multi-property.Each has strengths and limitations.Pow-
der infiltration printers, which essentiallyutilize a glue
(binder) to print over a layer of fine plaster powder,layer by
layer into a 3D shape,are relatively economicalto run and
have fast build times. Plastic extrusion printersproduce
slightly higherresolutionsin a stronger materialbut have
higher consumable costs, longer print times and limited color
options,although rapid technologicalchanges may soon neg-
ate some ofthese issues.It is likely that in the very near
future plastic printers will be able to print in the same sort of
color fidelity that currentlypowder printerscan and we
believe these will be more robust for teaching purposes while
powder printers may remain critical for prototyping.
Multiproperty printersare able to print complex struc-
tures in a variety of materials, including rubbers and plastics,
but are commensurately more expensive,and until recently
have generally not been capable of producing a palette of col-
ors as used in the present study.The quantitative evaluation
we performed also showed the close correlation between the
real prosection and the 3D printed reproduction,allowing
the printing of even the smallest digital nerves with only rela-
tively minor variations in size when compared to the original
specimen.A previous study by Smith etal (2013)found an
accuracy of0.1 mm in printing ofradiographic data ofhip
and shoulder joint surfaces and highlighted that segmentation
is actually a greater source of variance that the printing itself.
When 3D printing from DICOM data is being performed it
is vitalthat the scan resolution (slice thickness) is as close to
the layer thickness of the chosen 3D printer,a point recently
made by Houtilainen etal. (2014)and also evidentin our
experience. A further advantage of the present method of 3D
printing wasthe relative ease with which we could rescale
the data files to reproduce accurate larger or smaller repro-
ductions of prosected material or negative spaces (Fig. 3).
Perhaps the most notable advantages of 3D printing of repro-
ductions of anatomical dissections are its multiple benefits over
plastic models and plastinated cadaver specimens. Plastic mod-
els are in common use in high schools,doctors’ surgeries and
medical schools. They are mass produced copies or molds of a
“hypothetical” or“caricatured” anatomicalspecimensthat
often lack importantspecific details.While suitable for some
teaching purposes they are not ideal for teaching detailed anat-
omy typically required in medical and other allied health profes-
sionalcourses.While plastinated specimens can be produced
that display detailed anatomicalinformation a noteworthy
advantage of 3D printed reproductions is that they are reason-
ably inexpensive, easy to reproduce multiple copies and thus are
an attractive alternative forteaching facilitiesor universities
which may have no access to cadavers for teaching for logistical
reasons (rural sites,developing countries) or due to socialand
culturalbarriers.In addition,anatomicalvariations can easily
be demonstrated by printing multiple data sets; indeed we have
already begun to do this by printing left dominant, right domi-
nant and codominant variants of coronary artery distribution.
One of the alternatives to 3D printing, plastination, is a method
of substitution or infiltration ofdehydrated cadaver material
with an inflexible polymer silicon compound. It was developed
by Gunther von Hagensand madepopularby the “Body
Worlds” and other similar exhibitions (von Hagens, 1979; Bick-
ley et al., 1981). However, ethical issues have been raised about
the source of cadavers and the trading in human remains in one
country to another part of the world for commercial gain (Jones
and Whitaker,2009;Collier,2010). Preparation of plastinated
specimens from cadavers sourced from local bequest programs
is one possible solution but this involves considerable costs and
health and safety issues due to the large volumes of flammable
solvents involved.In addition,teaching large classes requires
multiple prosections of each body region,and preparing these
requires a localpool of skilled prosectors to create multiple
specimens for plastination.By contrast,a modern 3D printer
can be housed in a conventional office.
Under section 32 of the Human Tissue Act (1982) in Aus-
tralia the purposes for which a body can be donated are “the
use of [the] body for the study and teaching of the anatomy
of the human body.” We consider that our 3D copies of par-
ticular body parts are produced as a teaching aid and there-
fore come under the purpose of “teaching of anatomy” and
have been advised by the responsible localgovernment legis-
lative authority thatthey seeno ethical dilemma in their
reproduction.Indeed one could easily draw the analogy of
the reproduction of3D imageswith that of 2D imagesof
human cadaveric dissectionsthat are widely used in many
textbook or multimedia teaching aids.
Limitations to 3D Printing
There are of course some limitations to 3D printed copies of
human anatomicalprosections.Firstly,the outputis only as
good as the input,therefore it is imperative that high quality
prosections illustrating as many features as possible without
being overly complex are produced and selected forimage
acquisition and processing.The dissected specimens have to
be amenable to scanning and reproduction by 3D printing. A
6 McMenamin et al.

furtherlimitation isthe lack of pliability compared to real
dissections,however,this is also a limitation ofplastinated
specimens.Thus we advocate 3D printed anatomicalreplicas
not as a replacementbut an adjunctto actualdissection.If
access to cadaver materialis not an option or unavailable to
students we maintain that 3D prints may offer a novel, accu-
rate and effective substitute.Evaluation studies are planned
to gather direct evidence of their value in teaching.
CONCLUSIONS
The range of possible usesof 3D printing for reproducing
accurate replicas of human anatomical material presented are
different from previous methods of producing teaching mate-
rials. They are only made possible by the application of tech-
nological advancesthat allow the physical printing of
computer generated three-dimensionaldata.While this tech-
nology has been available to engineers for severaldecades it
is only now thatits biomedicalapplications are being real-
ized. Three-dimensional printing is likely to play a significant
role in pathology teaching, veterinary anatomy teaching, zoo-
logicalspecimen reproduction,reproduction of rare museum
specimens,to name a few potential applications.We are
actively exploring the use ofmultiple materialprinting and
the printing of cell and tissue data from confocal microscopic
studies which willintroduce yet another entirely new dimen-
sion to this science education revolution.
NOTES ON CONTRIBUTORS
PAUL G. MCMENAMIN, D.Sc. (Med), is a professorand
Director of the Centre for Human Anatomy Education in the
Department of Anatomy and DevelopmentalBiology,Faculty
of Medicine, Nursing and Health Sciences at Monash Univer-
sity,Clayton,Australia.He has been teaching human anat-
omy to undergraduate and postgraduate medicaland science
students for over 30 years.
MICHELLE R. QUAYLE, B.Env.Sc.Mgt.(Hons), is a
research and technicalassistantfor the Centre of Human
Anatomy Education in the Departmentof Anatomy and
DevelopmentalBiology,Faculty of Medicine,Nursing and
Health Sciencesat Monash University,Clayton, Australia.
She researches 3D modeling techniques and runs the Centre
of Human Anatomy Education’s 3D printer.
COLIN R. MCHENRY, Ph.D., is a lecturer in the Centre
for Human Anatomy Education in the Department of Anat-
omy and DevelopmentalBiology,Faculty of Medicine,Nurs-
ing and Health Sciencesat Monash University,Clayton,
Australia.He teacheshuman and comparative anatomy to
medical and science undergraduate and postgraduate students
and utilizes 3D modeling in his research.
JUSTIN W. ADAMS, Ph.D., is a senior lecturerin the
Centre for Human Anatomy Education in the Department of
Anatomy and DevelopmentalBiology,Faculty of Medicine,
Nursing and Health Sciences at Monash University,Clayton,
Australia.He teacheshuman and comparative anatomy to
medical and science undergraduate and postgraduate students
and uses 3D printing in his paleontology research.
LITERATURE CITED
AAA. 2012.American Association of Anatomists.Gross Anatomy Laboratory
Design. AAA, Bethesda,MD. URL: http://www.anatomy.org/content/gross-
anatomy-laboratory-design [accessed 25 March 2014].
Bickley HC, von Hagens G,Townsend FM.1981.An improved method for
the preservation of teaching specimens. Arch Pathol Lab Med 105:674–676.
Chambers J,Emlyn-Jones D.2009. Keeping dissection alive for medicalstu-
dents. Anat Sci Educ 2:302–303.
Cohen A,Laviv A, Berman P,Nashef R,Abu-Tair J. 2009.Mandibular recon-
struction using stereolithographic 3-dimensionalprinting modeling technology.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:661–666.
Collier R. 2010. Cadaver shows stir controversy. CMAJ 182:687–688.
Drake RL, McBride JM, Lachman N, Pawlina W. 2009. Medical education in the ana-
tomical sciences: The winds of change continue to blow. Anat Sci Educ 2:253–259.
Ebert LC, Thali MJ, Ross S. 2011. Getting in touch-3D printing in forensic
imaging. Forensic Sci Int 10:e1–e6.
Esses SJ,Berman P,Bloom AI, Sosna J.2011.Clinical applications of physical
3D models derived from MDCT data and created by rapid prototyping.AJR
Am J Roentgenol 196:W683–W688.
Estevez ME, Lindgren KA, Bergethon PR.2010. A novel three-dimensional
tool for teaching human anatomy. Anat Sci Educ 3:309–317.
Heetun M. 2009.Anatomy dissection:A valuable surgicaltraining tool.Br J
Hosp Med (Lond) 70:540.
Human Tissue Act. 1982. Version No. 036. Human Tissue Act 1982 No. 9860
of 1982. Version incorporating amendmentsas at 1 January 2010. Public
Health Branch, Rural and Regional Health and Aged Care Services Division of
the Victorian State Government,Department ofHealth,Melbourne,Victoria,
Australia. URL: http://www.health.vic.gov.au/humantissue/htact[accessed 31
May 2014].
Huotilainen E,Paloheimo M,SalmiM, Paloheimo KS,Bj€orkstrand R,Tuomi
J, Markkola A, M €akitie A. 2014. Imaging requirements for medicalapplica-
tions of additive manufacturing. Acta Radiol 55:78–85.
Isolan GR, Rowe R, Al-Mefty O. 2007. Microanatomy and surgical
approaches to the infratemporal fossa: An anaglyphic three-dimensional stereo-
scopic printing study. Skull Base 17:285–302.
Janis CM. 1988. An estimation oftooth volume and hypsodonty indicies in
ungulate mammals,and the correlation ofthese factorswith dietary prefer-
ence.In: RusselDE, Santoro JP,Sigogneau-RussellD (Editors). Teeth Revis-
ited: Proceedingsof the VIIth International Symposium on Dental
Morphology:Memoires Du Museum NationalD’Histoire Naturelle Series C
53. 1st Ed. Paris, France: Museum National D’Histoire Naturelle. p 367–387.
Johnson EO, Charchanti AV, Troupis TG. 2012. Modernization of an anatomy
class:From conceptualization to implementation.A case for integrated multi-
modal–multidisciplinary teaching. Anat Sci Educ 5:354–366.
Jones DG, Whitaker MI. 2009. Engaging with plastination and theBody
Worlds phenomenon:A culturaland intellectual challenge for anatomists.Clin
Anat 22:770–776.
Kalender WA. 2006. X-ray computed tomography. Phys Med Biol 51:R29–R43.
Korf HW, Wicht H, Snipes RL, TimmermansJP, Paulsen F, Rune G,
Baumgart-VogtE. 2008. The dissection course—Necessary and indispensable
for teaching anatomy to medical students. Ann Anat 190:16–22.
Li J, Nie L, Li Z, Lin L, Tang L, Ouyang J.2012.Maximizing modern distri-
bution of complex anatomicalspatial information:3D reconstruction and
rapid prototype production of anatomical corrosion casts of human specimens.
Anat Sci Educ 5:330–339.
Lin LI. 1989. A concordance correlation coeffecient to evaluate reproducibility.
Biometrics 45:255–268.
Lin LI. 2000. A note on the concordance correlation coefficient.Biometrics
56:324–325.
McGurk M, Amis AA, Potamianos P, Goodger NM. 1997. Rapid prototyping tech-
niques for anatomical modelling in medicine. Ann R Coll Surg Engl 79:169–174.
McLachlan JC, Patten D. 2006. Anatomy teaching: Ghosts of the past, present
and future. Med Educ 40:243–253.
McMenamin PG.2008.Body painting as a toolin clinicalanatomy teaching.
Anat Sci Educ 1:139–144.
Monfared A,Mitteramskogler G,Gruber S,Salisbury JK Jr,StampflJ, Blevins
NH. 2012.High-fidelity,inexpensive surgicalmiddle ear simulator.Otol Neu-
rotol 33:1573–1577.
Parker LM. 2002.Anatomicaldissection:Why are we cutting itout? Dissec-
tion in undergraduate teaching. ANZ J Surg 72:910–912.
Pham DT, Dimov SS. 2001. Rapid Manufacturing:The Technologiesand
Applications ofRapid Prototyping and Rapid Tooling.1st Ed. London, UK:
Springer-Verlag. 234 p.
Preece D,Williams SB,Lam R, Weller R. 2013.“Let’s get physical”:Advan-
tages of a physicalmodel over 3D computer models and textbooks in learning
imaging anatomy. Anat Sci Educ 6:216–224.
Raja DS, Sultana B.2012. Potentialhealth hazards forstudentsexposed to
formaldehyde in the gross anatomy laboratory. J Environ Health 74:36–40.
Ramsey-Stewart G,Burgess AW,Hill DA. 2010.Back to the future:Teaching
anatomy by whole-body dissection. Med J Aust 193:668–671.
Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM,
Unterhinninghofen R,Kauczor HU, GieselFL. 2010. 3D printing based on
imaging data: Review of medical applications. Int J Comput Assist Radiol Surg
5:335–341.
AnatomicalSciences EducationMONTH 2014 7
dissections,however,this is also a limitation ofplastinated
specimens.Thus we advocate 3D printed anatomicalreplicas
not as a replacementbut an adjunctto actualdissection.If
access to cadaver materialis not an option or unavailable to
students we maintain that 3D prints may offer a novel, accu-
rate and effective substitute.Evaluation studies are planned
to gather direct evidence of their value in teaching.
CONCLUSIONS
The range of possible usesof 3D printing for reproducing
accurate replicas of human anatomical material presented are
different from previous methods of producing teaching mate-
rials. They are only made possible by the application of tech-
nological advancesthat allow the physical printing of
computer generated three-dimensionaldata.While this tech-
nology has been available to engineers for severaldecades it
is only now thatits biomedicalapplications are being real-
ized. Three-dimensional printing is likely to play a significant
role in pathology teaching, veterinary anatomy teaching, zoo-
logicalspecimen reproduction,reproduction of rare museum
specimens,to name a few potential applications.We are
actively exploring the use ofmultiple materialprinting and
the printing of cell and tissue data from confocal microscopic
studies which willintroduce yet another entirely new dimen-
sion to this science education revolution.
NOTES ON CONTRIBUTORS
PAUL G. MCMENAMIN, D.Sc. (Med), is a professorand
Director of the Centre for Human Anatomy Education in the
Department of Anatomy and DevelopmentalBiology,Faculty
of Medicine, Nursing and Health Sciences at Monash Univer-
sity,Clayton,Australia.He has been teaching human anat-
omy to undergraduate and postgraduate medicaland science
students for over 30 years.
MICHELLE R. QUAYLE, B.Env.Sc.Mgt.(Hons), is a
research and technicalassistantfor the Centre of Human
Anatomy Education in the Departmentof Anatomy and
DevelopmentalBiology,Faculty of Medicine,Nursing and
Health Sciencesat Monash University,Clayton, Australia.
She researches 3D modeling techniques and runs the Centre
of Human Anatomy Education’s 3D printer.
COLIN R. MCHENRY, Ph.D., is a lecturer in the Centre
for Human Anatomy Education in the Department of Anat-
omy and DevelopmentalBiology,Faculty of Medicine,Nurs-
ing and Health Sciencesat Monash University,Clayton,
Australia.He teacheshuman and comparative anatomy to
medical and science undergraduate and postgraduate students
and utilizes 3D modeling in his research.
JUSTIN W. ADAMS, Ph.D., is a senior lecturerin the
Centre for Human Anatomy Education in the Department of
Anatomy and DevelopmentalBiology,Faculty of Medicine,
Nursing and Health Sciences at Monash University,Clayton,
Australia.He teacheshuman and comparative anatomy to
medical and science undergraduate and postgraduate students
and uses 3D printing in his paleontology research.
LITERATURE CITED
AAA. 2012.American Association of Anatomists.Gross Anatomy Laboratory
Design. AAA, Bethesda,MD. URL: http://www.anatomy.org/content/gross-
anatomy-laboratory-design [accessed 25 March 2014].
Bickley HC, von Hagens G,Townsend FM.1981.An improved method for
the preservation of teaching specimens. Arch Pathol Lab Med 105:674–676.
Chambers J,Emlyn-Jones D.2009. Keeping dissection alive for medicalstu-
dents. Anat Sci Educ 2:302–303.
Cohen A,Laviv A, Berman P,Nashef R,Abu-Tair J. 2009.Mandibular recon-
struction using stereolithographic 3-dimensionalprinting modeling technology.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:661–666.
Collier R. 2010. Cadaver shows stir controversy. CMAJ 182:687–688.
Drake RL, McBride JM, Lachman N, Pawlina W. 2009. Medical education in the ana-
tomical sciences: The winds of change continue to blow. Anat Sci Educ 2:253–259.
Ebert LC, Thali MJ, Ross S. 2011. Getting in touch-3D printing in forensic
imaging. Forensic Sci Int 10:e1–e6.
Esses SJ,Berman P,Bloom AI, Sosna J.2011.Clinical applications of physical
3D models derived from MDCT data and created by rapid prototyping.AJR
Am J Roentgenol 196:W683–W688.
Estevez ME, Lindgren KA, Bergethon PR.2010. A novel three-dimensional
tool for teaching human anatomy. Anat Sci Educ 3:309–317.
Heetun M. 2009.Anatomy dissection:A valuable surgicaltraining tool.Br J
Hosp Med (Lond) 70:540.
Human Tissue Act. 1982. Version No. 036. Human Tissue Act 1982 No. 9860
of 1982. Version incorporating amendmentsas at 1 January 2010. Public
Health Branch, Rural and Regional Health and Aged Care Services Division of
the Victorian State Government,Department ofHealth,Melbourne,Victoria,
Australia. URL: http://www.health.vic.gov.au/humantissue/htact[accessed 31
May 2014].
Huotilainen E,Paloheimo M,SalmiM, Paloheimo KS,Bj€orkstrand R,Tuomi
J, Markkola A, M €akitie A. 2014. Imaging requirements for medicalapplica-
tions of additive manufacturing. Acta Radiol 55:78–85.
Isolan GR, Rowe R, Al-Mefty O. 2007. Microanatomy and surgical
approaches to the infratemporal fossa: An anaglyphic three-dimensional stereo-
scopic printing study. Skull Base 17:285–302.
Janis CM. 1988. An estimation oftooth volume and hypsodonty indicies in
ungulate mammals,and the correlation ofthese factorswith dietary prefer-
ence.In: RusselDE, Santoro JP,Sigogneau-RussellD (Editors). Teeth Revis-
ited: Proceedingsof the VIIth International Symposium on Dental
Morphology:Memoires Du Museum NationalD’Histoire Naturelle Series C
53. 1st Ed. Paris, France: Museum National D’Histoire Naturelle. p 367–387.
Johnson EO, Charchanti AV, Troupis TG. 2012. Modernization of an anatomy
class:From conceptualization to implementation.A case for integrated multi-
modal–multidisciplinary teaching. Anat Sci Educ 5:354–366.
Jones DG, Whitaker MI. 2009. Engaging with plastination and theBody
Worlds phenomenon:A culturaland intellectual challenge for anatomists.Clin
Anat 22:770–776.
Kalender WA. 2006. X-ray computed tomography. Phys Med Biol 51:R29–R43.
Korf HW, Wicht H, Snipes RL, TimmermansJP, Paulsen F, Rune G,
Baumgart-VogtE. 2008. The dissection course—Necessary and indispensable
for teaching anatomy to medical students. Ann Anat 190:16–22.
Li J, Nie L, Li Z, Lin L, Tang L, Ouyang J.2012.Maximizing modern distri-
bution of complex anatomicalspatial information:3D reconstruction and
rapid prototype production of anatomical corrosion casts of human specimens.
Anat Sci Educ 5:330–339.
Lin LI. 1989. A concordance correlation coeffecient to evaluate reproducibility.
Biometrics 45:255–268.
Lin LI. 2000. A note on the concordance correlation coefficient.Biometrics
56:324–325.
McGurk M, Amis AA, Potamianos P, Goodger NM. 1997. Rapid prototyping tech-
niques for anatomical modelling in medicine. Ann R Coll Surg Engl 79:169–174.
McLachlan JC, Patten D. 2006. Anatomy teaching: Ghosts of the past, present
and future. Med Educ 40:243–253.
McMenamin PG.2008.Body painting as a toolin clinicalanatomy teaching.
Anat Sci Educ 1:139–144.
Monfared A,Mitteramskogler G,Gruber S,Salisbury JK Jr,StampflJ, Blevins
NH. 2012.High-fidelity,inexpensive surgicalmiddle ear simulator.Otol Neu-
rotol 33:1573–1577.
Parker LM. 2002.Anatomicaldissection:Why are we cutting itout? Dissec-
tion in undergraduate teaching. ANZ J Surg 72:910–912.
Pham DT, Dimov SS. 2001. Rapid Manufacturing:The Technologiesand
Applications ofRapid Prototyping and Rapid Tooling.1st Ed. London, UK:
Springer-Verlag. 234 p.
Preece D,Williams SB,Lam R, Weller R. 2013.“Let’s get physical”:Advan-
tages of a physicalmodel over 3D computer models and textbooks in learning
imaging anatomy. Anat Sci Educ 6:216–224.
Raja DS, Sultana B.2012. Potentialhealth hazards forstudentsexposed to
formaldehyde in the gross anatomy laboratory. J Environ Health 74:36–40.
Ramsey-Stewart G,Burgess AW,Hill DA. 2010.Back to the future:Teaching
anatomy by whole-body dissection. Med J Aust 193:668–671.
Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM,
Unterhinninghofen R,Kauczor HU, GieselFL. 2010. 3D printing based on
imaging data: Review of medical applications. Int J Comput Assist Radiol Surg
5:335–341.
AnatomicalSciences EducationMONTH 2014 7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Smith EJ, Anstey JA, Venne G,Ellis RE. 2013.Using additive manufacturing
in accuracy evaluation ofreconstructionsfrom computed tomography.Proc
Inst Mech Eng H 227:551–559.
Sugand K, Abrahams P, Khurana A. 2010. The anatomy of anatomy: A review
for its modernization. Anat Sci Educ 3:83–93.
Tam MD, Laycock SD, Brown JR, Jakeways M. 2013. 3D printing of an aortic
aneurysm to facilitate decision making and device selection for endovascular
aneurysm repair in complex neck anatomy. J Endovasc Ther 20:863–867.
von Hagens G.1979.Impregnation of soft biologicalspecimens with thermo-
setting resins and elastomers. Anat Rec 194:247–255.
Waran V, Devaraj P, Hari Chandran T, MuthusamyKA, Rathinam AK,
Balakrishnan YK,Tung TS,Raman R, Rahman ZA.2012.Three-dimensional
anatomical accuracy of cranial models created by rapid prototyping techniques
validated using a neuronavigation station. J Clin Neurosci 19:574–577.
Winkelmann A.2007.Anatomicaldissection as a teaching method in medical
school: A review of the evidence. Med Educ 41:15–22.
8 McMenamin et al.
in accuracy evaluation ofreconstructionsfrom computed tomography.Proc
Inst Mech Eng H 227:551–559.
Sugand K, Abrahams P, Khurana A. 2010. The anatomy of anatomy: A review
for its modernization. Anat Sci Educ 3:83–93.
Tam MD, Laycock SD, Brown JR, Jakeways M. 2013. 3D printing of an aortic
aneurysm to facilitate decision making and device selection for endovascular
aneurysm repair in complex neck anatomy. J Endovasc Ther 20:863–867.
von Hagens G.1979.Impregnation of soft biologicalspecimens with thermo-
setting resins and elastomers. Anat Rec 194:247–255.
Waran V, Devaraj P, Hari Chandran T, MuthusamyKA, Rathinam AK,
Balakrishnan YK,Tung TS,Raman R, Rahman ZA.2012.Three-dimensional
anatomical accuracy of cranial models created by rapid prototyping techniques
validated using a neuronavigation station. J Clin Neurosci 19:574–577.
Winkelmann A.2007.Anatomicaldissection as a teaching method in medical
school: A review of the evidence. Med Educ 41:15–22.
8 McMenamin et al.
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
