SOEE2481: Atmospheric Chemistry Assignment on Ozone and Kinetics
VerifiedAdded on 2022/08/10
|7
|1401
|297
Homework Assignment
AI Summary
This assignment solution explores various aspects of atmospheric chemistry, including the interconversion of gas concentrations, particularly ozone. It delves into kinetics and photochemistry, examining bond energies, wavelength estimations, and the order of dissociation for compounds like acetone, NO, and NO2. The solution also analyzes OH reactions, photolysis processes, and their role in ozone production, along with the formation and impact of HOx radicals. Furthermore, it investigates acetone photolysis, its effect on the HOx budget, and its removal from the atmosphere. The assignment also includes a detailed analysis of a specific chemical reaction involving CIO, BrO, and BrCl, calculating reaction rates, steady-state concentrations, and photochemical stability. Finally, it confirms the short-lived nature of BrCl and discusses its photochemical behavior at night, considering the mixing ratios of reactants and the influence of OClO and ClO.

1
Environmental chemistry
Student’s First Name, Middle Initial(s), Last Name
Institutional Affiliation
Course Number and Name
Instructor’s Name and Title
Assignment Due Date
Environmental chemistry
Student’s First Name, Middle Initial(s), Last Name
Institutional Affiliation
Course Number and Name
Instructor’s Name and Title
Assignment Due Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
2
Interconversion of Gas Concentrations
1)
n = PV/RT 1 L = 1 dm3 = (0.1 m) 3 = 0.95 atm x 1 m3 / {(0.082 L atm mole-1 K-1) x 10-3
m3 x 300 K} = 38.6 moles
Total moles of ozone = 40 x 10-9 x 38.6 = 1.544 x 10-6 moles per m3
1 cm3 = (0.01m) 3 = 10-6 m3
No moles of ozone = 1.544 x 10-6 moles m-3 /106 cm3 m-3
= 1.544 x 10-12 moles cm-3
No molecules of ozone = 1.544 x 10-12 moles cm-3 x 6.02 x1023
= 9.29 x 1011 molecules per cm3
b) Mass of ozone = 1.544 x 10-6 moles per m3 x 48 g
= 74.1 x 10-6 g = 74.1 x 10-3 mg m-3.
2) Kinetics and Photochemistry
Compound Bond energy/ kJ mol-1 ʎ / nm
CH3COCH3 + hν→ CH3CO +CH3 C-C: 353.6 339nm
NO2 (or ONO)+ hν→ NO + O. N-O: 284 422 nm
NO + hν→ N2 + O. N-O: 630 190nm
Wavelength estimation
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
284kJmol-1 x 10^3 X 1/6.023^23
ʎ = 4.22x10-7 m =422 nm
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
353.6 kJmol-1 x 10^3 X 1/6.023^23
= 339nm
Interconversion of Gas Concentrations
1)
n = PV/RT 1 L = 1 dm3 = (0.1 m) 3 = 0.95 atm x 1 m3 / {(0.082 L atm mole-1 K-1) x 10-3
m3 x 300 K} = 38.6 moles
Total moles of ozone = 40 x 10-9 x 38.6 = 1.544 x 10-6 moles per m3
1 cm3 = (0.01m) 3 = 10-6 m3
No moles of ozone = 1.544 x 10-6 moles m-3 /106 cm3 m-3
= 1.544 x 10-12 moles cm-3
No molecules of ozone = 1.544 x 10-12 moles cm-3 x 6.02 x1023
= 9.29 x 1011 molecules per cm3
b) Mass of ozone = 1.544 x 10-6 moles per m3 x 48 g
= 74.1 x 10-6 g = 74.1 x 10-3 mg m-3.
2) Kinetics and Photochemistry
Compound Bond energy/ kJ mol-1 ʎ / nm
CH3COCH3 + hν→ CH3CO +CH3 C-C: 353.6 339nm
NO2 (or ONO)+ hν→ NO + O. N-O: 284 422 nm
NO + hν→ N2 + O. N-O: 630 190nm
Wavelength estimation
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
284kJmol-1 x 10^3 X 1/6.023^23
ʎ = 4.22x10-7 m =422 nm
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
353.6 kJmol-1 x 10^3 X 1/6.023^23
= 339nm

3
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
630kJmol-1 x 10^3 X 1/6.023^23
= 190nm
Order of Dissociation
A molecule must always be excited above its dissociation energy (Do) for it to photo
dissociate at given wavelength. Besides, the molecules can only be photochemical active
when their dissociation energy corresponds to >290nm
NO is chemically and thermally stable in the troposphere against photo dissociation. As
evident in the table above, NO will require a higher bond energy of 630 kJ mol-1 to photo-
dissociate to N2 + O. Hence will be the last to photo dissociate.
The covalent bond between C-C (353.6) is stronger compared to the N-O (284) covalent
bond. The C-C bond in acetone requires a bond energy of 353.6kjm-1 to fully photo-
dissociate which is lower than bond energy required to break the N-O bond. Acetone is,
therefore, both chemically and thermally stable as compared to NO2 hence NO2 will
dissociate faster as compared to acetone. The three compounds will, therefore, dissociate an
increasing order of NO→ CH3COCH3→NO2
3)
a) OH reactions and photolysis are critical for the production of O3. OH forms from ozone and act as
an initiator agent in process of photolysis in the troposphere. Ozone dissociate into oxygen radical
and Oxygen molecule. The highly reactive oxygen radical reacts with atmospheric vapor to form
hydroxyl ions that propagate other atmospheric reactions. The active hydroxyl radicles then react
with carbon (II) Oxide to form hydrogen atom that further with other atmospheric gases forming
ozone.
O3 + hν →O* + O2
O* + H2O → 2 OH
CO + OH → CO2 + H
hν = hc/ʎ→ ʎ = hc/E = 6.63x 10-34 Js x 3.0X10^8 MS-1
630kJmol-1 x 10^3 X 1/6.023^23
= 190nm
Order of Dissociation
A molecule must always be excited above its dissociation energy (Do) for it to photo
dissociate at given wavelength. Besides, the molecules can only be photochemical active
when their dissociation energy corresponds to >290nm
NO is chemically and thermally stable in the troposphere against photo dissociation. As
evident in the table above, NO will require a higher bond energy of 630 kJ mol-1 to photo-
dissociate to N2 + O. Hence will be the last to photo dissociate.
The covalent bond between C-C (353.6) is stronger compared to the N-O (284) covalent
bond. The C-C bond in acetone requires a bond energy of 353.6kjm-1 to fully photo-
dissociate which is lower than bond energy required to break the N-O bond. Acetone is,
therefore, both chemically and thermally stable as compared to NO2 hence NO2 will
dissociate faster as compared to acetone. The three compounds will, therefore, dissociate an
increasing order of NO→ CH3COCH3→NO2
3)
a) OH reactions and photolysis are critical for the production of O3. OH forms from ozone and act as
an initiator agent in process of photolysis in the troposphere. Ozone dissociate into oxygen radical
and Oxygen molecule. The highly reactive oxygen radical reacts with atmospheric vapor to form
hydroxyl ions that propagate other atmospheric reactions. The active hydroxyl radicles then react
with carbon (II) Oxide to form hydrogen atom that further with other atmospheric gases forming
ozone.
O3 + hν →O* + O2
O* + H2O → 2 OH
CO + OH → CO2 + H
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
4
H + O2 + M → HO2 + M (fast)
HO2 + NO → OH + NO2
NO2 + hv → NO + O
O + O2 + M → O3 + M
HOx, alkoxy and peroxy are mainly produced through acetone photolysis. Acetone forms through
reaction with OH and through surface deposition
CH3COCH3 + OH + O2 →CH3COCH2 O2 + H2O
CH3COCH3 + hν + 2 O2→ CH3O2 + CH3C (O) O2
The amount of alkoxy and peroxy radicals formed through acetone photolysis varies with altitude hence
causing change in the odd-nitrogen hence change in the HOx balance.
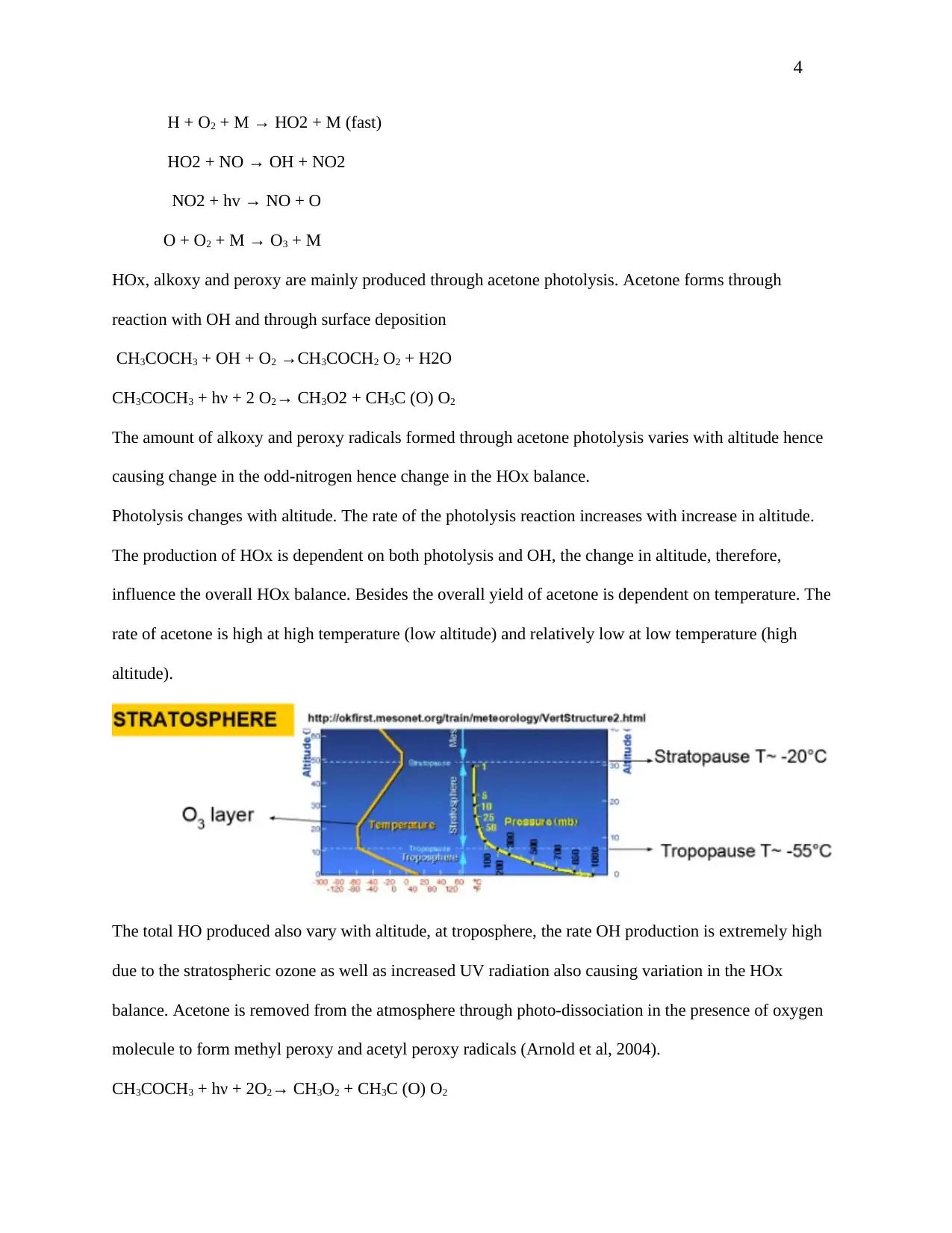
Photolysis changes with altitude. The rate of the photolysis reaction increases with increase in altitude.
The production of HOx is dependent on both photolysis and OH, the change in altitude, therefore,
influence the overall HOx balance. Besides the overall yield of acetone is dependent on temperature. The
rate of acetone is high at high temperature (low altitude) and relatively low at low temperature (high
altitude).
The total HO produced also vary with altitude, at troposphere, the rate OH production is extremely high
due to the stratospheric ozone as well as increased UV radiation also causing variation in the HOx
balance. Acetone is removed from the atmosphere through photo-dissociation in the presence of oxygen
molecule to form methyl peroxy and acetyl peroxy radicals (Arnold et al, 2004).
CH3COCH3 + hν + 2O2→ CH3O2 + CH3C (O) O2
H + O2 + M → HO2 + M (fast)
HO2 + NO → OH + NO2
NO2 + hv → NO + O
O + O2 + M → O3 + M
HOx, alkoxy and peroxy are mainly produced through acetone photolysis. Acetone forms through
reaction with OH and through surface deposition
CH3COCH3 + OH + O2 →CH3COCH2 O2 + H2O
CH3COCH3 + hν + 2 O2→ CH3O2 + CH3C (O) O2
The amount of alkoxy and peroxy radicals formed through acetone photolysis varies with altitude hence
causing change in the odd-nitrogen hence change in the HOx balance.
Photolysis changes with altitude. The rate of the photolysis reaction increases with increase in altitude.
The production of HOx is dependent on both photolysis and OH, the change in altitude, therefore,
influence the overall HOx balance. Besides the overall yield of acetone is dependent on temperature. The
rate of acetone is high at high temperature (low altitude) and relatively low at low temperature (high
altitude).
The total HO produced also vary with altitude, at troposphere, the rate OH production is extremely high
due to the stratospheric ozone as well as increased UV radiation also causing variation in the HOx
balance. Acetone is removed from the atmosphere through photo-dissociation in the presence of oxygen
molecule to form methyl peroxy and acetyl peroxy radicals (Arnold et al, 2004).
CH3COCH3 + hν + 2O2→ CH3O2 + CH3C (O) O2
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
CH3COCH3 + hν→ CH3CO +CH3
b)
Based on the new HOx budget, the rate loss of HOx through self-reaction of HOx increases with altitude.
It can also be deduced that the quantum yield of HOx significantly affected by temperature in the
troposphere. The contribution of acetone to the overall HOx budget is also reduced by a factor of 4
through the reduced rate (J) of acetone photolysis (Blitz et al, 2004). The overall lifetime of acetone in the
troposphere is also increased.
4)
a)
CIO + BrO → O2 + BrCl
Rate = k [CIO][BrO]
BrCl + hν → Br + CI
Rate = K [BrCl][hν]
b)
CIO + BrO → O2 + BrCl
Rate= kQ [CIO][BrO]
d[BrCI] = kQ [CIO][BrO] → d[BrCI] = KQ [BrO]k1f[O2][CIO]
dt dt K1r + kQ [BrO]
d[BrCI] = KQ[BrO]k1f [O2][CIO] → d[BrCI] = ktotal[O2][CIO]
dt k1r + KQ[BrO] dt
c]
Confirm that BrCl is indeed short-lived
t = 1/J =
J= 0.08s-1
t = 1/0.08s-1 = 12.5s hence short-lived
CH3COCH3 + hν→ CH3CO +CH3
b)
Based on the new HOx budget, the rate loss of HOx through self-reaction of HOx increases with altitude.
It can also be deduced that the quantum yield of HOx significantly affected by temperature in the
troposphere. The contribution of acetone to the overall HOx budget is also reduced by a factor of 4
through the reduced rate (J) of acetone photolysis (Blitz et al, 2004). The overall lifetime of acetone in the
troposphere is also increased.
4)
a)
CIO + BrO → O2 + BrCl
Rate = k [CIO][BrO]
BrCl + hν → Br + CI
Rate = K [BrCl][hν]
b)
CIO + BrO → O2 + BrCl
Rate= kQ [CIO][BrO]
d[BrCI] = kQ [CIO][BrO] → d[BrCI] = KQ [BrO]k1f[O2][CIO]
dt dt K1r + kQ [BrO]
d[BrCI] = KQ[BrO]k1f [O2][CIO] → d[BrCI] = ktotal[O2][CIO]
dt k1r + KQ[BrO] dt
c]
Confirm that BrCl is indeed short-lived
t = 1/J =
J= 0.08s-1
t = 1/0.08s-1 = 12.5s hence short-lived

6
CIO + BrO → O2 + BrCl
[BrCl] ss = kBrO [CIO][BrO]
kCIO [CIO] + K O2 [O2]
Mixing ratio for CIO and BrO = 2ppbv:10pptv
k= 2x10-11cm3s-1
1ppb = 1000000pptv
2ppb=?
2000000pptv
Mixing ratio of CIO: BrO = 200000:1
Kf = ko[M]. K∞
ko[M] + K∞
M= total air density [M]= 10^18cm-3
KO = 1.6. 10-32(T/300)-4.5
k∞= 2.10-12(T/300)-2.4
kf= 1.6x10^-32 x [10^18cm-3]x 2.10-12(T/300)^-4.5
1.6 x 10^-32 [10^18cm-3] + 2.10^-12(T/300)-2.4
From the ss expression the steady-state concentrate
= [BrCI] ss = kfBrO [CIO][BrO]
[CIO] + K O2 [O2]
6x10^-32 x [10^18cm-3]x 2.10-12(T/300)^-4.5 X 2x 10-11
1.6 x 10^-32 [10^18cm-3] + 2.10^-12(T/300)-2.4 2000000
[BrCI] = 1.0 x10-14
K O2 [O2] is = 0 since the ss concentration is only dependent on the reactants.
d]
BrCl is photo chemically stable at night. The photochemical steady state of BrCl at sunset is dependent on
the formation of OClO. It is also dependent on the abundance of ClO during nighttime. The mixing ratio
between ClO and BrO at night reduces thereby reducing the quantum yield of BrCl.
CIO + BrO → O2 + BrCl
[BrCl] ss = kBrO [CIO][BrO]
kCIO [CIO] + K O2 [O2]
Mixing ratio for CIO and BrO = 2ppbv:10pptv
k= 2x10-11cm3s-1
1ppb = 1000000pptv
2ppb=?
2000000pptv
Mixing ratio of CIO: BrO = 200000:1
Kf = ko[M]. K∞
ko[M] + K∞
M= total air density [M]= 10^18cm-3
KO = 1.6. 10-32(T/300)-4.5
k∞= 2.10-12(T/300)-2.4
kf= 1.6x10^-32 x [10^18cm-3]x 2.10-12(T/300)^-4.5
1.6 x 10^-32 [10^18cm-3] + 2.10^-12(T/300)-2.4
From the ss expression the steady-state concentrate
= [BrCI] ss = kfBrO [CIO][BrO]
[CIO] + K O2 [O2]
6x10^-32 x [10^18cm-3]x 2.10-12(T/300)^-4.5 X 2x 10-11
1.6 x 10^-32 [10^18cm-3] + 2.10^-12(T/300)-2.4 2000000
[BrCI] = 1.0 x10-14
K O2 [O2] is = 0 since the ss concentration is only dependent on the reactants.
d]
BrCl is photo chemically stable at night. The photochemical steady state of BrCl at sunset is dependent on
the formation of OClO. It is also dependent on the abundance of ClO during nighttime. The mixing ratio
between ClO and BrO at night reduces thereby reducing the quantum yield of BrCl.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
Reference
Arnold, S.R., Chipperfield, M.P., Blitz, M.A., Heard, D.E. and Pilling, M.J., 2004.
Photodissociation of acetone: Atmospheric implications of temperature‐dependent quantum
yields. Geophysical research letters, 31(7).
Blitz, M.A., Heard, D.E., Pilling, M.J., Arnold, S.R. and Chipperfield, M.P., 2004. Pressure and
temperature‐dependent quantum yields for the photodissociation of acetone between 279 and
327.5 nm. Geophysical research letters, 31(6).
Reference
Arnold, S.R., Chipperfield, M.P., Blitz, M.A., Heard, D.E. and Pilling, M.J., 2004.
Photodissociation of acetone: Atmospheric implications of temperature‐dependent quantum
yields. Geophysical research letters, 31(7).
Blitz, M.A., Heard, D.E., Pilling, M.J., Arnold, S.R. and Chipperfield, M.P., 2004. Pressure and
temperature‐dependent quantum yields for the photodissociation of acetone between 279 and
327.5 nm. Geophysical research letters, 31(6).
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.