MNG00723 - AUSMED: Global Expansion Strategy, Risks & Opportunities
VerifiedAdded on 2023/06/08
|16
|3354
|206
Report
AI Summary
This report assesses the potential for AUSMED, an Australian pharmaceutical company, to expand its operations into China and South Africa. It analyzes the risks and opportunities associated with each market, considering factors like market growth, regulatory environments, labor availability, and financial risks. The report highlights China's rapidly growing pharmaceutical market and the increasing demand for drugs, alongside challenges like stringent quality standards and legal approvals. South Africa presents opportunities in urbanization and drug supply but faces government restrictions and regulatory hurdles. Ultimately, the report recommends China as the more favorable market for AUSMED's expansion, citing its significant business potential and skilled labor force. The report proposes a market entry strategy, emphasizing the importance of considering the company's objectives, resource availability, and risk appetite. This analysis aims to guide AUSMED in making informed decisions about its global expansion strategy.

Global Business
8/30/2018
8/30/2018
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Global Business 1
Executive summary
The report is prepared by the operational manager of the Ausmed, an Australia Pharma company
who is willing to be considered as a global corporation. A global corporation is a corporation
who operates their business operations in two or more countries. This shows that the company is
willing to expand its business operations in other countries. The major operation of the company
is manufacturing drugs which are growing in the last 10 years. Considering the same, the
company has decided to expand its business operations in South Africa and China. The
operational manager conducts the analysis in which they evaluate the risk and opportunities for
the country where they are willing to expand the business. The country has been selected for
expanding the business in China with the licensing strategy.
Executive summary
The report is prepared by the operational manager of the Ausmed, an Australia Pharma company
who is willing to be considered as a global corporation. A global corporation is a corporation
who operates their business operations in two or more countries. This shows that the company is
willing to expand its business operations in other countries. The major operation of the company
is manufacturing drugs which are growing in the last 10 years. Considering the same, the
company has decided to expand its business operations in South Africa and China. The
operational manager conducts the analysis in which they evaluate the risk and opportunities for
the country where they are willing to expand the business. The country has been selected for
expanding the business in China with the licensing strategy.

Global Business 2
Contents
Introduction......................................................................................................................................3
Analysis of risks and opportunities..................................................................................................4
China............................................................................................................................................4
Opportunities...........................................................................................................................4
Risks........................................................................................................................................5
South Africa.................................................................................................................................7
Opportunities...........................................................................................................................7
Risks........................................................................................................................................8
Selection of the country...................................................................................................................9
Proposing and selecting Market entry strategy................................................................................9
Conclusion.....................................................................................................................................12
References......................................................................................................................................13
Contents
Introduction......................................................................................................................................3
Analysis of risks and opportunities..................................................................................................4
China............................................................................................................................................4
Opportunities...........................................................................................................................4
Risks........................................................................................................................................5
South Africa.................................................................................................................................7
Opportunities...........................................................................................................................7
Risks........................................................................................................................................8
Selection of the country...................................................................................................................9
Proposing and selecting Market entry strategy................................................................................9
Conclusion.....................................................................................................................................12
References......................................................................................................................................13
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Global Business 3
Introduction
The aim of the report is to explore the opportunity for the company so that they can meet their
objective of expanding the business and achieving the profit. This report is prepared for the case
study in which the case of Ausmed, Pharma company is explained who is now willing to expand
their business operations. The company is manufacturing drugs for the medicines from the past
10 years with this the company is able to achieve the success in the Australian market. The
company has approx. 60 employees on their staff with the turnover of AUD 30 million. This
shows that the company is able to achieve the success and growth. Considering this growth, now
the company is willing to expand their business operations in South Africa and China. The
manager of the company is performing the analysis of the market so that the company can select
one company where they can expand the business operations. In the end, the proposed market
entry strategy is discussed with the justification of the same.
Introduction
The aim of the report is to explore the opportunity for the company so that they can meet their
objective of expanding the business and achieving the profit. This report is prepared for the case
study in which the case of Ausmed, Pharma company is explained who is now willing to expand
their business operations. The company is manufacturing drugs for the medicines from the past
10 years with this the company is able to achieve the success in the Australian market. The
company has approx. 60 employees on their staff with the turnover of AUD 30 million. This
shows that the company is able to achieve the success and growth. Considering this growth, now
the company is willing to expand their business operations in South Africa and China. The
manager of the company is performing the analysis of the market so that the company can select
one company where they can expand the business operations. In the end, the proposed market
entry strategy is discussed with the justification of the same.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Global Business 4
Analysis of risks and opportunities
China
China market is growing in terms of the pharmaceuticals with this it has the fastest emerging
market for the sector. Along with this, the company is moving towards the important innovator
of the Pharmaceuticals products (Zhang, 2018). According to the health-care information
company IQVIA, China is considered the world's 2nd largest national pharmaceutical market in
the year 2017 which is worth the $122.6 billion. The pharmaceuticals market of China is an
emerging market with the growth tipped to reach $145 billion to $175 billion by the year 2022
(Tan, 2018). The market for manufacturing drugs in China is rising but there are many issues that
the manufacturer might face in the market.
Opportunities
Increase in the manufacturing of drugs
According to the report, this has been found that China pharmaceuticals manufacturer sales are
increasing which is clear from the image that is given below: -
Analysis of risks and opportunities
China
China market is growing in terms of the pharmaceuticals with this it has the fastest emerging
market for the sector. Along with this, the company is moving towards the important innovator
of the Pharmaceuticals products (Zhang, 2018). According to the health-care information
company IQVIA, China is considered the world's 2nd largest national pharmaceutical market in
the year 2017 which is worth the $122.6 billion. The pharmaceuticals market of China is an
emerging market with the growth tipped to reach $145 billion to $175 billion by the year 2022
(Tan, 2018). The market for manufacturing drugs in China is rising but there are many issues that
the manufacturer might face in the market.
Opportunities
Increase in the manufacturing of drugs
According to the report, this has been found that China pharmaceuticals manufacturer sales are
increasing which is clear from the image that is given below: -
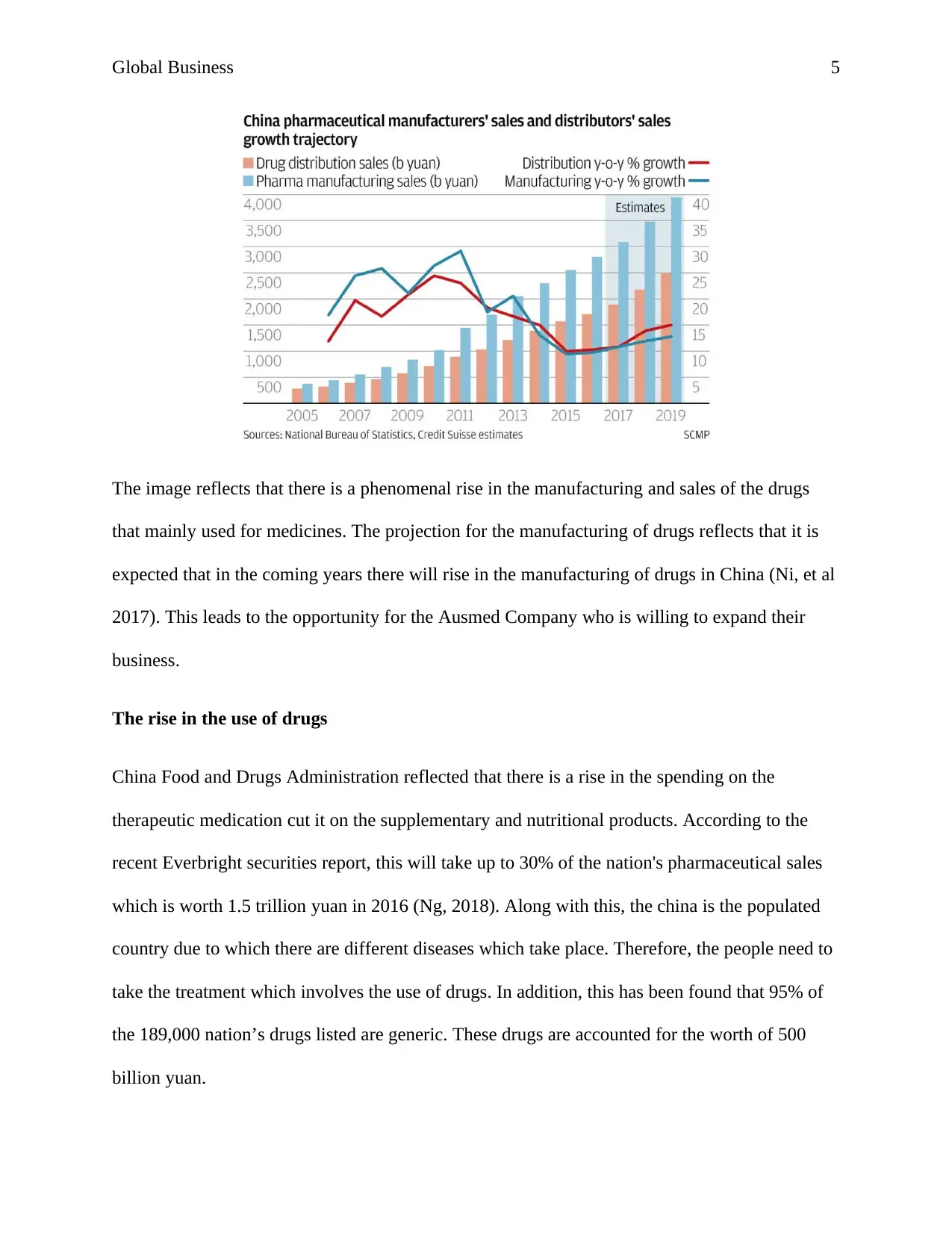
Global Business 5
The image reflects that there is a phenomenal rise in the manufacturing and sales of the drugs
that mainly used for medicines. The projection for the manufacturing of drugs reflects that it is
expected that in the coming years there will rise in the manufacturing of drugs in China (Ni, et al
2017). This leads to the opportunity for the Ausmed Company who is willing to expand their
business.
The rise in the use of drugs
China Food and Drugs Administration reflected that there is a rise in the spending on the
therapeutic medication cut it on the supplementary and nutritional products. According to the
recent Everbright securities report, this will take up to 30% of the nation's pharmaceutical sales
which is worth 1.5 trillion yuan in 2016 (Ng, 2018). Along with this, the china is the populated
country due to which there are different diseases which take place. Therefore, the people need to
take the treatment which involves the use of drugs. In addition, this has been found that 95% of
the 189,000 nation’s drugs listed are generic. These drugs are accounted for the worth of 500
billion yuan.
The image reflects that there is a phenomenal rise in the manufacturing and sales of the drugs
that mainly used for medicines. The projection for the manufacturing of drugs reflects that it is
expected that in the coming years there will rise in the manufacturing of drugs in China (Ni, et al
2017). This leads to the opportunity for the Ausmed Company who is willing to expand their
business.
The rise in the use of drugs
China Food and Drugs Administration reflected that there is a rise in the spending on the
therapeutic medication cut it on the supplementary and nutritional products. According to the
recent Everbright securities report, this will take up to 30% of the nation's pharmaceutical sales
which is worth 1.5 trillion yuan in 2016 (Ng, 2018). Along with this, the china is the populated
country due to which there are different diseases which take place. Therefore, the people need to
take the treatment which involves the use of drugs. In addition, this has been found that 95% of
the 189,000 nation’s drugs listed are generic. These drugs are accounted for the worth of 500
billion yuan.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Global Business 6
Labour for manufacturing
In the China market, there is the presence of the large number manufacturer and this is the only
reason due to which people of China are aware of the work that they are supposed to perform.
Ausmed Company will not find any issues related to the labour when they expand their business
in the market. This is the opportunities for the company because it is hard to find the labours who
can actually manufacture the drugs (Torrey, 2018).
Risks
The rise in standards for the quality
China’s fragmented Pharmaceuticals industry is predicted to undergo a wave of consolidation
over the next 5 years due to the rise in the strictness the drug quality regulations. It is essential
for the manufacturer to test the drug that they are willing to distribute in the market of China
considering the new standard. This shows that Ausmed will face the risk related to the checking
of drugs that they are willing to distribute in the market of China. China food and drugs
administration has brought the need of test required to ensure the safety and efficacy of the off-
patent generic drugs as a part of a national strategy which is essential to building a strong
pharmaceutical industry through reform.
Decrease in distribution
In the China market, the quality standards have removed the small competitors from the market.
This has brought reduce in the layers of the distribution intermediaries who distribute the
products in the market of China due to which the distribution of the products has been reduced in
the market. Along with this, the existing companies who are performing their operations in China
are facing the competitive pressure because Beijing implements a raft of measures announced
Labour for manufacturing
In the China market, there is the presence of the large number manufacturer and this is the only
reason due to which people of China are aware of the work that they are supposed to perform.
Ausmed Company will not find any issues related to the labour when they expand their business
in the market. This is the opportunities for the company because it is hard to find the labours who
can actually manufacture the drugs (Torrey, 2018).
Risks
The rise in standards for the quality
China’s fragmented Pharmaceuticals industry is predicted to undergo a wave of consolidation
over the next 5 years due to the rise in the strictness the drug quality regulations. It is essential
for the manufacturer to test the drug that they are willing to distribute in the market of China
considering the new standard. This shows that Ausmed will face the risk related to the checking
of drugs that they are willing to distribute in the market of China. China food and drugs
administration has brought the need of test required to ensure the safety and efficacy of the off-
patent generic drugs as a part of a national strategy which is essential to building a strong
pharmaceutical industry through reform.
Decrease in distribution
In the China market, the quality standards have removed the small competitors from the market.
This has brought reduce in the layers of the distribution intermediaries who distribute the
products in the market of China due to which the distribution of the products has been reduced in
the market. Along with this, the existing companies who are performing their operations in China
are facing the competitive pressure because Beijing implements a raft of measures announced
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Global Business 7
from the past two years to weed out the practices that had brought to mediocre drug
inefficiencies and quality.
Legal approval
The legal approval for manufacturing the drug is one of the major risks that are faced by the
Ausmed Company in the market of China. CFDA is responsible for the approval and ensuring
the quality of the drugs. The company who is willing to manufacture the drugs in the markets
need to take the approval from the CFDA and after that only they will be able to set-up their
business. This is found in the analysis that it is difficult for the company to take the approval
related to the drug that they are manufacturing (China Pharma Industry, 2018).
South Africa
South Africa market has registered a growth for the pharmaceutical products and this is expected
that in the near future this will reach to $40 billion to $ 65 billion by 2020. This shows the
opportunity for the company who is willing to expand their business operations. In addition, this
has been found that medical drugs are predicted to rise in the year 2013-2020 (Hassen, 2017).
This is evident from the data which shows that the rise will get multiple annual growth rates of
approx. 6% generics at 9% (Finance 24, 2017). Though, the regulations of the legal and political
elements can affect the working.
Opportunities
Urbanisation leads to a rise in the use of drugs
In South Africa, there is rapid urbanization of South Africa which includes the improvement in
the lifestyles of the people and drastic increment in the dietary trends. The awareness for the
dietary products has made the people demand the long-term pharmaceuticals mainly chronic
from the past two years to weed out the practices that had brought to mediocre drug
inefficiencies and quality.
Legal approval
The legal approval for manufacturing the drug is one of the major risks that are faced by the
Ausmed Company in the market of China. CFDA is responsible for the approval and ensuring
the quality of the drugs. The company who is willing to manufacture the drugs in the markets
need to take the approval from the CFDA and after that only they will be able to set-up their
business. This is found in the analysis that it is difficult for the company to take the approval
related to the drug that they are manufacturing (China Pharma Industry, 2018).
South Africa
South Africa market has registered a growth for the pharmaceutical products and this is expected
that in the near future this will reach to $40 billion to $ 65 billion by 2020. This shows the
opportunity for the company who is willing to expand their business operations. In addition, this
has been found that medical drugs are predicted to rise in the year 2013-2020 (Hassen, 2017).
This is evident from the data which shows that the rise will get multiple annual growth rates of
approx. 6% generics at 9% (Finance 24, 2017). Though, the regulations of the legal and political
elements can affect the working.
Opportunities
Urbanisation leads to a rise in the use of drugs
In South Africa, there is rapid urbanization of South Africa which includes the improvement in
the lifestyles of the people and drastic increment in the dietary trends. The awareness for the
dietary products has made the people demand the long-term pharmaceuticals mainly chronic

Global Business 8
related diseases which increase the major use of the drugs (Holt, Lahrichi and Seliva, 2015). The
improvement in the healthcare capabilities is also making the people use the more of products
that can make them fit and fresh.
Supply of drugs
In the current market of South Africa, this has been found that the supply of the drugs is not as
effective as most of the people are not able to access some of the medicines involving drugs
easily. The improvement in the capacities of the health care is an increase in the supply for the
drugs which is one of the major opportunities for the company (Pharmaceuticals and medical
devices sector, 2018). Ausmed Company can involve in manufacturing the products and
supplying it to the customers that can improve the supply of the Pharma drugs in South Africa
Risks
Government Restriction
In the market of South Africa, the government regulations affect the entry of the company into
the market. this has been found that the government has restricted the imports of the medical
drugs to control the availability of the drugs in the market as this the effective way through
which they can motivate the domestic manufacturer (Saidi and Douglas, 2018). The chains of
Pharma companies are consolidating, horizontal and vertical integration are improving in the
South Africa which is one of the biggest threat for the manufacturer.
Regulator of Pharmaceuticals
MCC (Medicines Control Council) is the main body that regulated the medicine in South Africa.
The regulator has increased the regulations to maintain the quality in the drugs that they offer to
related diseases which increase the major use of the drugs (Holt, Lahrichi and Seliva, 2015). The
improvement in the healthcare capabilities is also making the people use the more of products
that can make them fit and fresh.
Supply of drugs
In the current market of South Africa, this has been found that the supply of the drugs is not as
effective as most of the people are not able to access some of the medicines involving drugs
easily. The improvement in the capacities of the health care is an increase in the supply for the
drugs which is one of the major opportunities for the company (Pharmaceuticals and medical
devices sector, 2018). Ausmed Company can involve in manufacturing the products and
supplying it to the customers that can improve the supply of the Pharma drugs in South Africa
Risks
Government Restriction
In the market of South Africa, the government regulations affect the entry of the company into
the market. this has been found that the government has restricted the imports of the medical
drugs to control the availability of the drugs in the market as this the effective way through
which they can motivate the domestic manufacturer (Saidi and Douglas, 2018). The chains of
Pharma companies are consolidating, horizontal and vertical integration are improving in the
South Africa which is one of the biggest threat for the manufacturer.
Regulator of Pharmaceuticals
MCC (Medicines Control Council) is the main body that regulated the medicine in South Africa.
The regulator has increased the regulations to maintain the quality in the drugs that they offer to
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Global Business 9
their customers (Newton, Hanson and Goodman, 2017). MCC has brought an agreement of the
medicines which include the details related to the increase in the clinical trial in the South Africa
up to four times comparing it with the international best practice. In addition, MCC has
appointed a team which will perform the activity of reviewing the institution and to offer the
recommendation for the new regulations. Ausmed Company might face the high threat due to the
regulations of the regulator in the market.
Financial risk
The financial risk is also there which is faced by the company as when they enter the market they
need to carry out the medical trials which need huge capital which is difficult for the business to
source. The clinical trials cost to the company to the value of R1m to R5.5m on the clinical trials
which are hard for the company to invest. Along with this, if in case the company will get fail in
the clinical trials then the amount that was invested also gets failed.
Selection of the country
The analysis related to the risk and opportunities which might be faced by the company in South
Africa and China reflects that the need of the healthcare facilities is increasing which indirectly
leads to the rise in the opportunities for the Ausmed Company. Considering the analysis, the
country that will be best for the Ausmed Pharma Company is China. The company should enter
into the market of China. The company will find the huge business opportunity as there are many
manufacturers who are producing drugs. Along with this, the market includes the rise in the
demand of the medicines or therapy that involves drugs. In addition to this, the company will not
find any issues related to the human resources as there is the presence of skilled and experienced
labours. Though, this is the fact that the company will also face some risk in which the
their customers (Newton, Hanson and Goodman, 2017). MCC has brought an agreement of the
medicines which include the details related to the increase in the clinical trial in the South Africa
up to four times comparing it with the international best practice. In addition, MCC has
appointed a team which will perform the activity of reviewing the institution and to offer the
recommendation for the new regulations. Ausmed Company might face the high threat due to the
regulations of the regulator in the market.
Financial risk
The financial risk is also there which is faced by the company as when they enter the market they
need to carry out the medical trials which need huge capital which is difficult for the business to
source. The clinical trials cost to the company to the value of R1m to R5.5m on the clinical trials
which are hard for the company to invest. Along with this, if in case the company will get fail in
the clinical trials then the amount that was invested also gets failed.
Selection of the country
The analysis related to the risk and opportunities which might be faced by the company in South
Africa and China reflects that the need of the healthcare facilities is increasing which indirectly
leads to the rise in the opportunities for the Ausmed Company. Considering the analysis, the
country that will be best for the Ausmed Pharma Company is China. The company should enter
into the market of China. The company will find the huge business opportunity as there are many
manufacturers who are producing drugs. Along with this, the market includes the rise in the
demand of the medicines or therapy that involves drugs. In addition to this, the company will not
find any issues related to the human resources as there is the presence of skilled and experienced
labours. Though, this is the fact that the company will also face some risk in which the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Global Business 10
applicability of the standards related to the quality is included. Ausmed Company needs to take
the approval from the government for performing the operations in China (Yao, et al 2017).
.
Proposing and selecting Market entry strategy
Market entry strategy is the strategy that is selected by the company to enter into the market for
offering the effective goods and services to their targeted customer across the market. The entry
mode is directly depended on the nature and business of the company. Along with this, while
selecting the mode of entry into the market the company need to consider some elements which
include the goals and objectives of the company, resources availability, risk and opportunities for
the company. In addition, they need to ensure the profit sharing and distribution of the capital
elements. There are different types of market strategies which are used by the company which
include Joint Venture, licensing, franchising and many others (Collinson, 2015). These strategies
vary a lot from each other and according to that, the company need to select the best strategy for
them. The method which is suitable for the manufacturing company is Exporting and licensing.
Out of which, it is essential for the business to select one for which the comparison between both
the methods has been done.
Licensing is a strategy in which the company takes the approval from the government for
operating the business. On the other hand, exporting is the market entry in which the
manufacturer sent their products in the foreign market. In both, the method, the degree of control
remains with the single owner. Though, the major difference takes place when it comes to the
cost of entering the market (Gillespie and Riddle, 2015). In licensing, the company is supposed
to pay the one-time amount to the government for entering in the market. The agreement on
applicability of the standards related to the quality is included. Ausmed Company needs to take
the approval from the government for performing the operations in China (Yao, et al 2017).
.
Proposing and selecting Market entry strategy
Market entry strategy is the strategy that is selected by the company to enter into the market for
offering the effective goods and services to their targeted customer across the market. The entry
mode is directly depended on the nature and business of the company. Along with this, while
selecting the mode of entry into the market the company need to consider some elements which
include the goals and objectives of the company, resources availability, risk and opportunities for
the company. In addition, they need to ensure the profit sharing and distribution of the capital
elements. There are different types of market strategies which are used by the company which
include Joint Venture, licensing, franchising and many others (Collinson, 2015). These strategies
vary a lot from each other and according to that, the company need to select the best strategy for
them. The method which is suitable for the manufacturing company is Exporting and licensing.
Out of which, it is essential for the business to select one for which the comparison between both
the methods has been done.
Licensing is a strategy in which the company takes the approval from the government for
operating the business. On the other hand, exporting is the market entry in which the
manufacturer sent their products in the foreign market. In both, the method, the degree of control
remains with the single owner. Though, the major difference takes place when it comes to the
cost of entering the market (Gillespie and Riddle, 2015). In licensing, the company is supposed
to pay the one-time amount to the government for entering in the market. The agreement on

Global Business 11
which the government will take the approval is supported by the contract. On the other hand,
exporting is a strategy in which the company need to pay the tariff when they will send the
products to the other countries. Along with this, the company will make use of the resources in
the home country for producing the products (Irwin, 2012). This will cost high for the company
because the resources are cheaply available in the market of China.
The analysis reflects that licensing will be a suitable mode of entry for the Ausmed, an
Australian Pharma company. This mode of entry will offer the numerous advantages to the
company which include: -
Legal, easy and quick entry of the business into the market of China
High potential for the better return on investment
Low level of risk with the low expenses
Single control with the single owner of the profit (Laufs and Schwens, 2014)
All these benefits will help the company in successful setting up of the operations in the market
of China. Licensing will allow the company to avail the benefit of resources at the low cost in
China.
which the government will take the approval is supported by the contract. On the other hand,
exporting is a strategy in which the company need to pay the tariff when they will send the
products to the other countries. Along with this, the company will make use of the resources in
the home country for producing the products (Irwin, 2012). This will cost high for the company
because the resources are cheaply available in the market of China.
The analysis reflects that licensing will be a suitable mode of entry for the Ausmed, an
Australian Pharma company. This mode of entry will offer the numerous advantages to the
company which include: -
Legal, easy and quick entry of the business into the market of China
High potential for the better return on investment
Low level of risk with the low expenses
Single control with the single owner of the profit (Laufs and Schwens, 2014)
All these benefits will help the company in successful setting up of the operations in the market
of China. Licensing will allow the company to avail the benefit of resources at the low cost in
China.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




