BA Management & Leadership Research Ethics
VerifiedAdded on 2020/02/05
|10
|2376
|57
Report
AI Summary
The document outlines the Stage 1 Research Ethics Application Form for a BA (Hons) Management & Leadership project at Anglia Ruskin University. It includes details about the researcher, research methodology, sampling population, data collection methods, and ethical considerations. The research aims to identify key factors influencing foreign investment in the UK economy, emphasizing the importance of ethical compliance and risk management in research involving human participants.

Stage 1 Research Ethics ApplicationForm
Section 1: Details of the Researcher and theirResearch
N.B. If you are conducting research that
involves‘animalsandsignificanthabitats’,pleaseuse the
Stage1ResearchEthicsApplicationForm involvingAnimalsandHabitats
(www.anglia.ac.uk/researchethics).
Applicants carrying out research with children or vulnerable adults may also
need to carry out an online Safeguarding course and submit the pass
certificate with their ethics application. Please refer to the Question
Specific Advice for the Stage 1 Research Ethics Application Form at the
above weblink.
Researcherdetails
Firstname ABC
Familyname ABC H
Department/Faculty Management and leadership
Emailaddress Abc@gmail.com
NameofInstitutionwhereyoustudy or work
(if not Anglia Ruskin)
Anglia Ruskin
Areyou:
Pleasetick
Undergraduate(UG)Student
PostgraduateTaught(PGT)Student
Postgraduate Research (PGR) Student
MemberofARUStaff
MemberofARUstaffcarryingoutMasters/Doctorateresearch
Students (including staff proposing researchon a course/programme)
Your SID
Your course/programme title BA (Hons) Management & Leadership
NameofyourFirstSupervisor(for
PGR)orSupervisor(forUGandPGT)
XYZ
Research details
1
Section 1: Details of the Researcher and theirResearch
N.B. If you are conducting research that
involves‘animalsandsignificanthabitats’,pleaseuse the
Stage1ResearchEthicsApplicationForm involvingAnimalsandHabitats
(www.anglia.ac.uk/researchethics).
Applicants carrying out research with children or vulnerable adults may also
need to carry out an online Safeguarding course and submit the pass
certificate with their ethics application. Please refer to the Question
Specific Advice for the Stage 1 Research Ethics Application Form at the
above weblink.
Researcherdetails
Firstname ABC
Familyname ABC H
Department/Faculty Management and leadership
Emailaddress Abc@gmail.com
NameofInstitutionwhereyoustudy or work
(if not Anglia Ruskin)
Anglia Ruskin
Areyou:
Pleasetick
Undergraduate(UG)Student
PostgraduateTaught(PGT)Student
Postgraduate Research (PGR) Student
MemberofARUStaff
MemberofARUstaffcarryingoutMasters/Doctorateresearch
Students (including staff proposing researchon a course/programme)
Your SID
Your course/programme title BA (Hons) Management & Leadership
NameofyourFirstSupervisor(for
PGR)orSupervisor(forUGandPGT)
XYZ
Research details
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
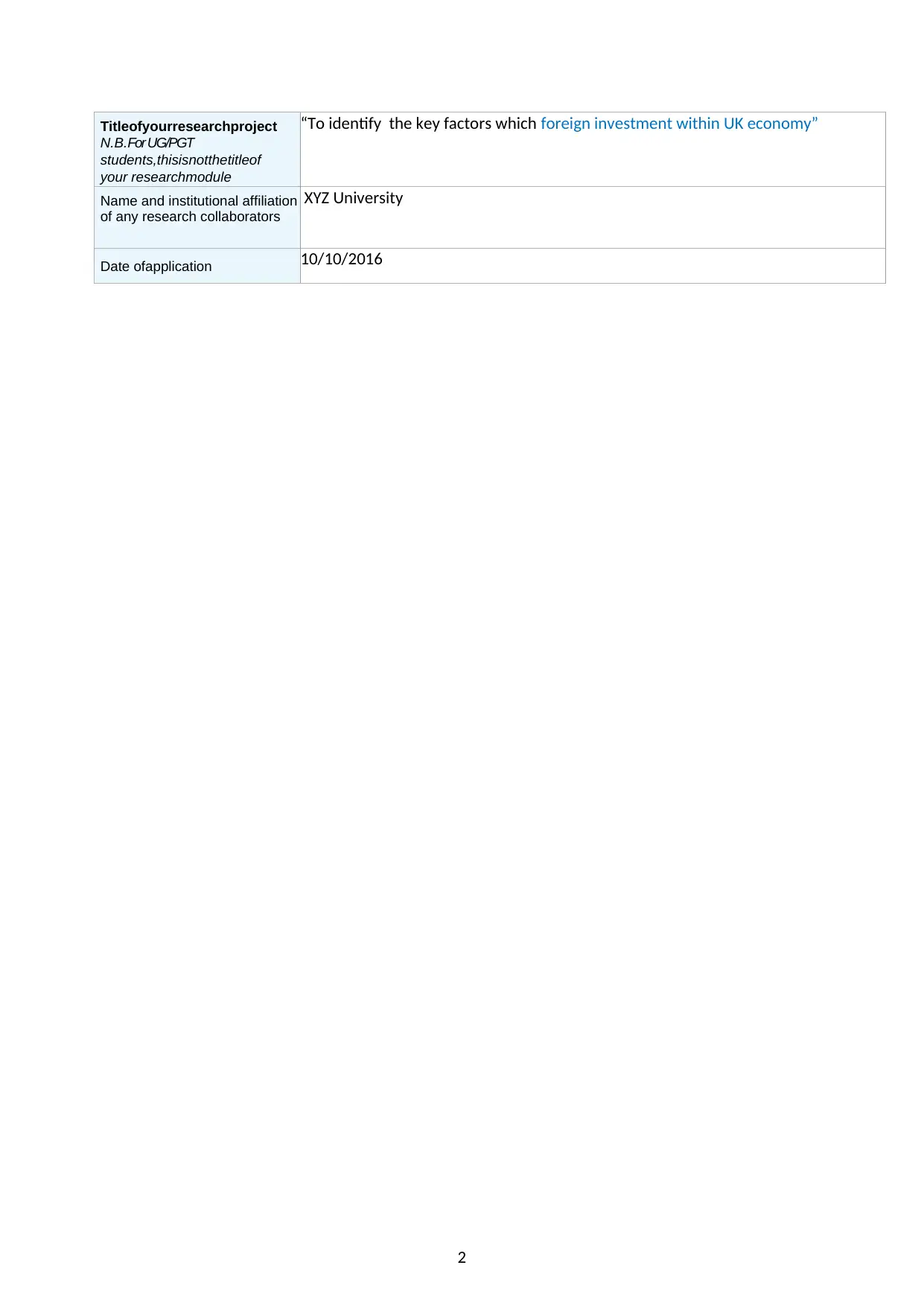
Titleofyourresearchproject
N.B.For UG/PGT
students,thisisnotthetitleof
your researchmodule
“To identify the key factors which foreign investment within UK economy”
Name and institutional affiliation
of any research collaborators
XYZ University
Date ofapplication 10/10/2016
2
N.B.For UG/PGT
students,thisisnotthetitleof
your researchmodule
“To identify the key factors which foreign investment within UK economy”
Name and institutional affiliation
of any research collaborators
XYZ University
Date ofapplication 10/10/2016
2
Brief
ProjectSummary(upto7
00words) Please
summarise your research in
non-specialist language.
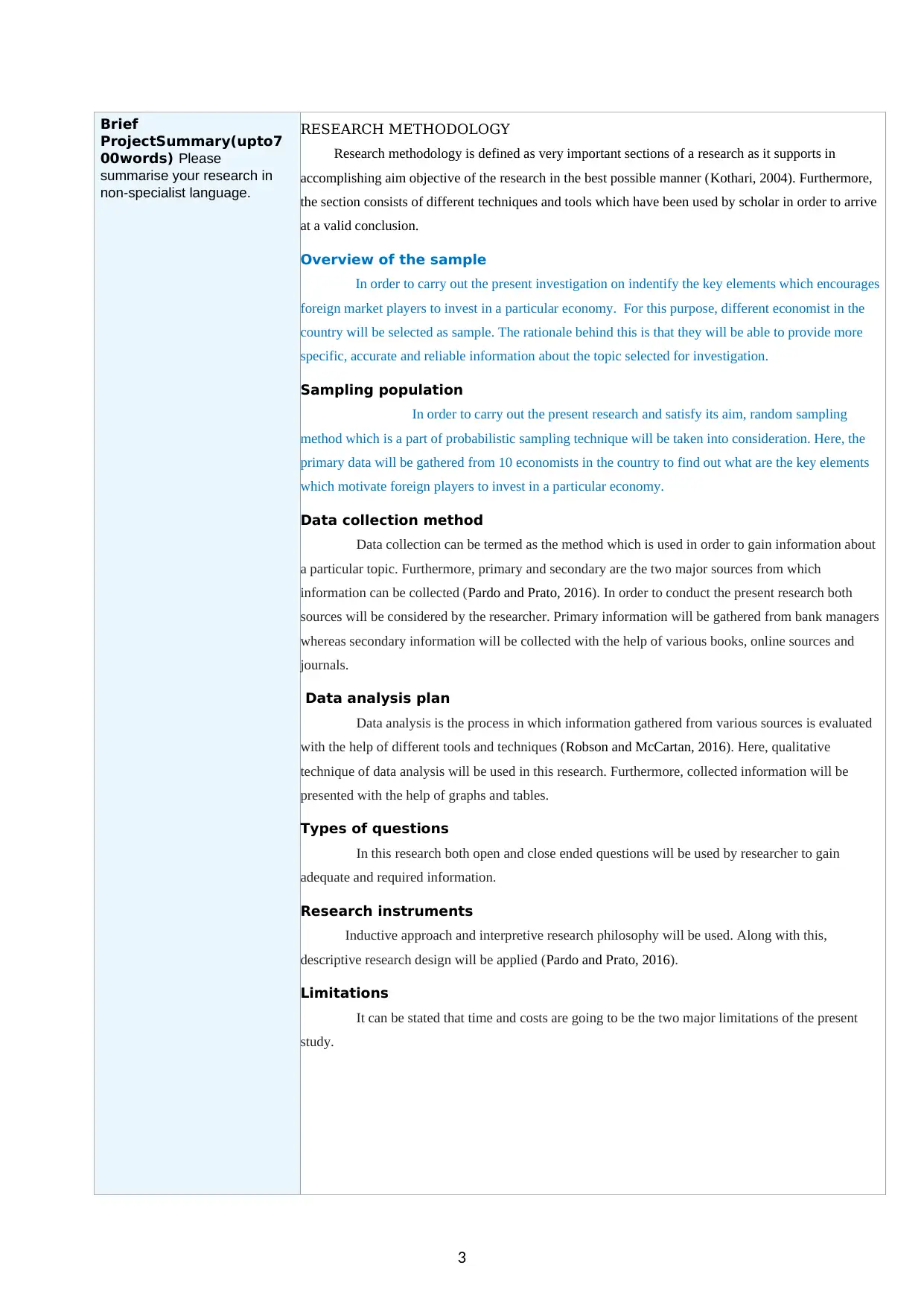
RESEARCH METHODOLOGY
Research methodology is defined as very important sections of a research as it supports in
accomplishing aim objective of the research in the best possible manner (Kothari, 2004). Furthermore,
the section consists of different techniques and tools which have been used by scholar in order to arrive
at a valid conclusion.
Overview of the sample
In order to carry out the present investigation on indentify the key elements which encourages
foreign market players to invest in a particular economy. For this purpose, different economist in the
country will be selected as sample. The rationale behind this is that they will be able to provide more
specific, accurate and reliable information about the topic selected for investigation.
Sampling population
In order to carry out the present research and satisfy its aim, random sampling
method which is a part of probabilistic sampling technique will be taken into consideration. Here, the
primary data will be gathered from 10 economists in the country to find out what are the key elements
which motivate foreign players to invest in a particular economy.
Data collection method
Data collection can be termed as the method which is used in order to gain information about
a particular topic. Furthermore, primary and secondary are the two major sources from which
information can be collected (Pardo and Prato, 2016). In order to conduct the present research both
sources will be considered by the researcher. Primary information will be gathered from bank managers
whereas secondary information will be collected with the help of various books, online sources and
journals.
Data analysis plan
Data analysis is the process in which information gathered from various sources is evaluated
with the help of different tools and techniques (Robson and McCartan, 2016). Here, qualitative
technique of data analysis will be used in this research. Furthermore, collected information will be
presented with the help of graphs and tables.
Types of questions
In this research both open and close ended questions will be used by researcher to gain
adequate and required information.
Research instruments
Inductive approach and interpretive research philosophy will be used. Along with this,
descriptive research design will be applied (Pardo and Prato, 2016).
Limitations
It can be stated that time and costs are going to be the two major limitations of the present
study.
3
ProjectSummary(upto7
00words) Please
summarise your research in
non-specialist language.
RESEARCH METHODOLOGY
Research methodology is defined as very important sections of a research as it supports in
accomplishing aim objective of the research in the best possible manner (Kothari, 2004). Furthermore,
the section consists of different techniques and tools which have been used by scholar in order to arrive
at a valid conclusion.
Overview of the sample
In order to carry out the present investigation on indentify the key elements which encourages
foreign market players to invest in a particular economy. For this purpose, different economist in the
country will be selected as sample. The rationale behind this is that they will be able to provide more
specific, accurate and reliable information about the topic selected for investigation.
Sampling population
In order to carry out the present research and satisfy its aim, random sampling
method which is a part of probabilistic sampling technique will be taken into consideration. Here, the
primary data will be gathered from 10 economists in the country to find out what are the key elements
which motivate foreign players to invest in a particular economy.
Data collection method
Data collection can be termed as the method which is used in order to gain information about
a particular topic. Furthermore, primary and secondary are the two major sources from which
information can be collected (Pardo and Prato, 2016). In order to conduct the present research both
sources will be considered by the researcher. Primary information will be gathered from bank managers
whereas secondary information will be collected with the help of various books, online sources and
journals.
Data analysis plan
Data analysis is the process in which information gathered from various sources is evaluated
with the help of different tools and techniques (Robson and McCartan, 2016). Here, qualitative
technique of data analysis will be used in this research. Furthermore, collected information will be
presented with the help of graphs and tables.
Types of questions
In this research both open and close ended questions will be used by researcher to gain
adequate and required information.
Research instruments
Inductive approach and interpretive research philosophy will be used. Along with this,
descriptive research design will be applied (Pardo and Prato, 2016).
Limitations
It can be stated that time and costs are going to be the two major limitations of the present
study.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
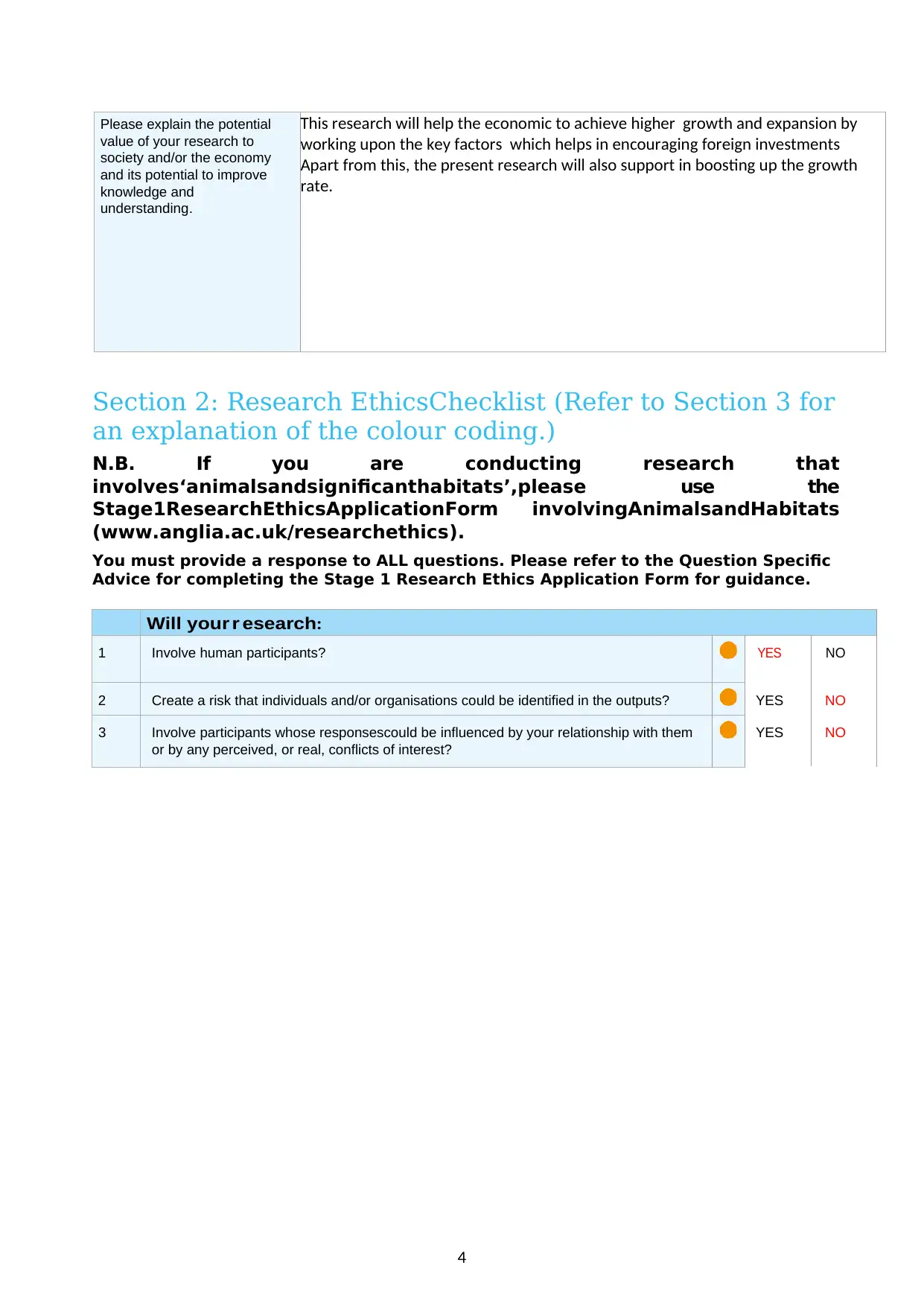
Please explain the potential
value of your research to
society and/or the economy
and its potential to improve
knowledge and
understanding.
This research will help the economic to achieve higher growth and expansion by
working upon the key factors which helps in encouraging foreign investments
Apart from this, the present research will also support in boosting up the growth
rate.
Section 2: Research EthicsChecklist (Refer to Section 3 for
an explanation of the colour coding.)
N.B. If you are conducting research that
involves‘animalsandsignificanthabitats’,please use the
Stage1ResearchEthicsApplicationForm involvingAnimalsandHabitats
(www.anglia.ac.uk/researchethics).
You must provide a response to ALL questions. Please refer to the Question Specific
Advice for completing the Stage 1 Research Ethics Application Form for guidance.
Will your r esearch:
1 Involve human participants? YES NO
2 Create a risk that individuals and/or organisations could be identified in the outputs? YES NO
3 Involve participants whose responsescould be influenced by your relationship with them
or by any perceived, or real, conflicts of interest?
YES NO
4
value of your research to
society and/or the economy
and its potential to improve
knowledge and
understanding.
This research will help the economic to achieve higher growth and expansion by
working upon the key factors which helps in encouraging foreign investments
Apart from this, the present research will also support in boosting up the growth
rate.
Section 2: Research EthicsChecklist (Refer to Section 3 for
an explanation of the colour coding.)
N.B. If you are conducting research that
involves‘animalsandsignificanthabitats’,please use the
Stage1ResearchEthicsApplicationForm involvingAnimalsandHabitats
(www.anglia.ac.uk/researchethics).
You must provide a response to ALL questions. Please refer to the Question Specific
Advice for completing the Stage 1 Research Ethics Application Form for guidance.
Will your r esearch:
1 Involve human participants? YES NO
2 Create a risk that individuals and/or organisations could be identified in the outputs? YES NO
3 Involve participants whose responsescould be influenced by your relationship with them
or by any perceived, or real, conflicts of interest?
YES NO
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
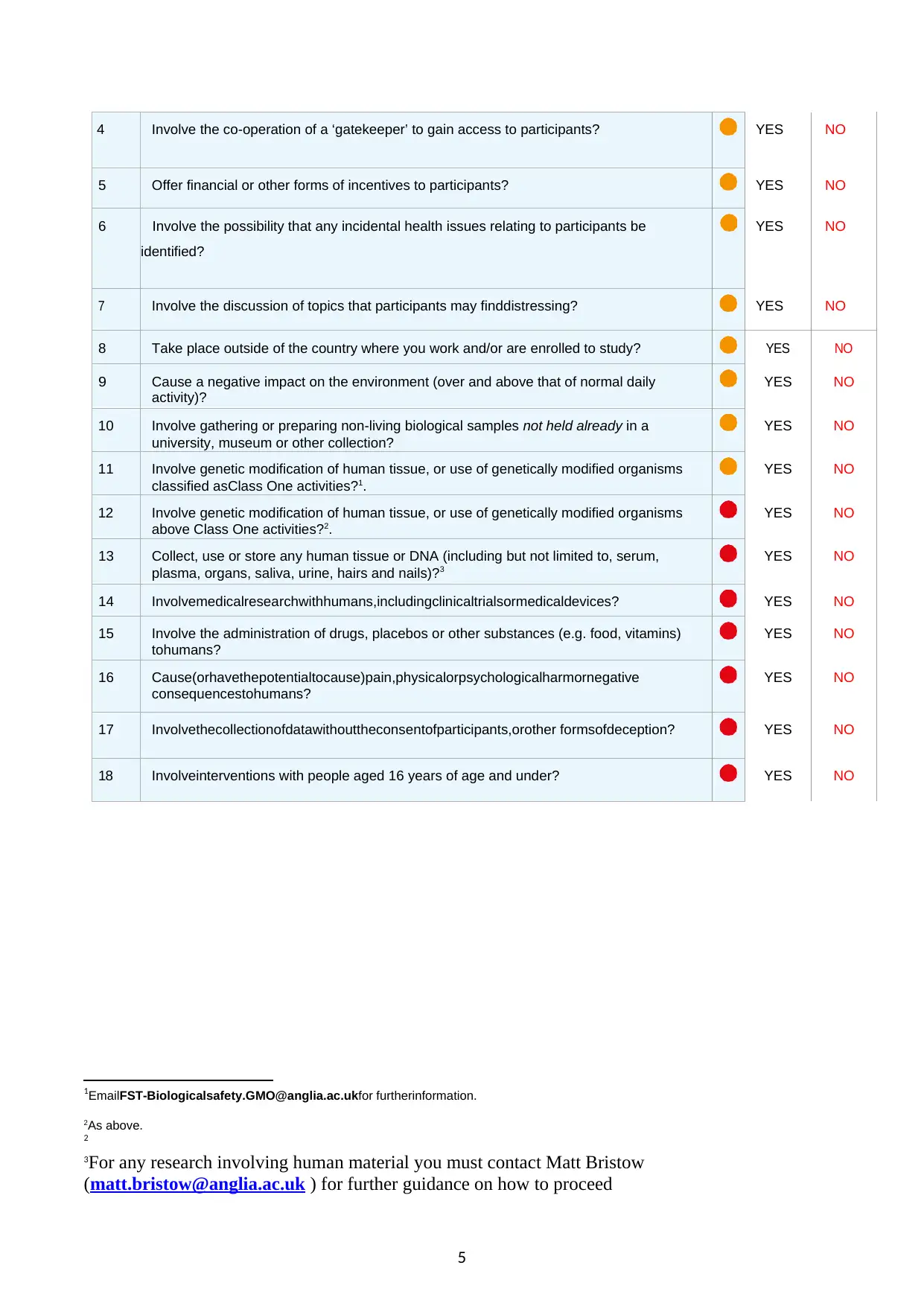
4 Involve the co-operation of a ‘gatekeeper’ to gain access to participants? YES NO
5 Offer financial or other forms of incentives to participants? YES NO
6 Involve the possibility that any incidental health issues relating to participants be
identified?
YES NO
7 Involve the discussion of topics that participants may finddistressing? YES NO
8 Take place outside of the country where you work and/or are enrolled to study? YES NO
9 Cause a negative impact on the environment (over and above that of normal daily
activity)?
YES NO
10 Involve gathering or preparing non-living biological samples not held already in a
university, museum or other collection?
YES NO
11 Involve genetic modification of human tissue, or use of genetically modified organisms
classified asClass One activities?1.
YES NO
12 Involve genetic modification of human tissue, or use of genetically modified organisms
above Class One activities?2.
YES NO
13 Collect, use or store any human tissue or DNA (including but not limited to, serum,
plasma, organs, saliva, urine, hairs and nails)?3
YES NO
14 Involvemedicalresearchwithhumans,includingclinicaltrialsormedicaldevices? YES NO
15 Involve the administration of drugs, placebos or other substances (e.g. food, vitamins)
tohumans?
YES NO
16 Cause(orhavethepotentialtocause)pain,physicalorpsychologicalharmornegative
consequencestohumans?
YES NO
17 Involvethecollectionofdatawithouttheconsentofparticipants,orother formsofdeception? YES NO
18 Involveinterventions with people aged 16 years of age and under? YES NO
1EmailFST-Biologicalsafety.GMO@anglia.ac.ukfor furtherinformation.
2As above.
2
3For any research involving human material you must contact Matt Bristow
(matt.bristow@anglia.ac.uk ) for further guidance on how to proceed
5
5 Offer financial or other forms of incentives to participants? YES NO
6 Involve the possibility that any incidental health issues relating to participants be
identified?
YES NO
7 Involve the discussion of topics that participants may finddistressing? YES NO
8 Take place outside of the country where you work and/or are enrolled to study? YES NO
9 Cause a negative impact on the environment (over and above that of normal daily
activity)?
YES NO
10 Involve gathering or preparing non-living biological samples not held already in a
university, museum or other collection?
YES NO
11 Involve genetic modification of human tissue, or use of genetically modified organisms
classified asClass One activities?1.
YES NO
12 Involve genetic modification of human tissue, or use of genetically modified organisms
above Class One activities?2.
YES NO
13 Collect, use or store any human tissue or DNA (including but not limited to, serum,
plasma, organs, saliva, urine, hairs and nails)?3
YES NO
14 Involvemedicalresearchwithhumans,includingclinicaltrialsormedicaldevices? YES NO
15 Involve the administration of drugs, placebos or other substances (e.g. food, vitamins)
tohumans?
YES NO
16 Cause(orhavethepotentialtocause)pain,physicalorpsychologicalharmornegative
consequencestohumans?
YES NO
17 Involvethecollectionofdatawithouttheconsentofparticipants,orother formsofdeception? YES NO
18 Involveinterventions with people aged 16 years of age and under? YES NO
1EmailFST-Biologicalsafety.GMO@anglia.ac.ukfor furtherinformation.
2As above.
2
3For any research involving human material you must contact Matt Bristow
(matt.bristow@anglia.ac.uk ) for further guidance on how to proceed
5
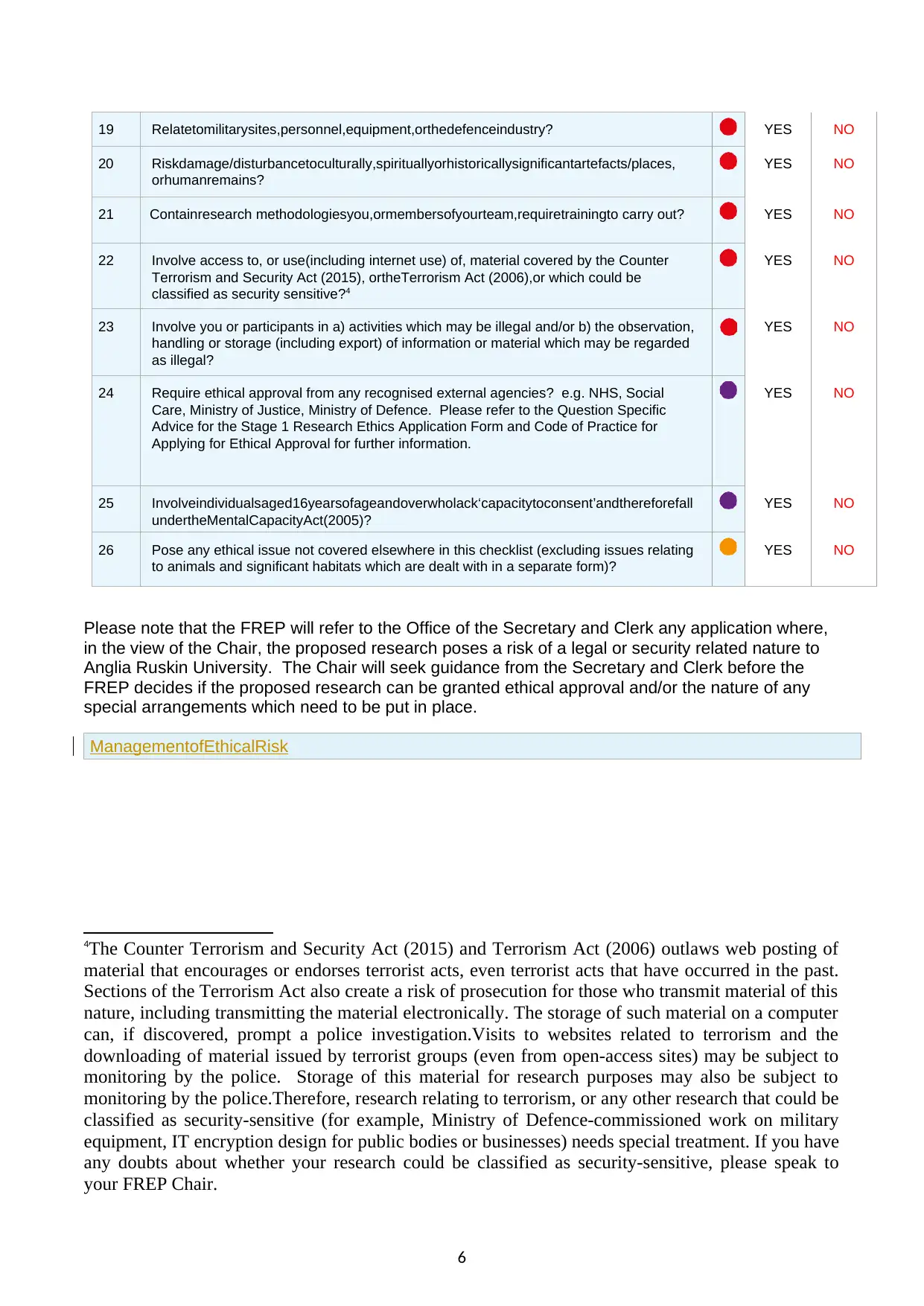
19 Relatetomilitarysites,personnel,equipment,orthedefenceindustry? YES NO
20 Riskdamage/disturbancetoculturally,spirituallyorhistoricallysignificantartefacts/places,
orhumanremains?
YES NO
21 Containresearch methodologiesyou,ormembersofyourteam,requiretrainingto carry out? YES NO
22 Involve access to, or use(including internet use) of, material covered by the Counter
Terrorism and Security Act (2015), ortheTerrorism Act (2006),or which could be
classified as security sensitive?4
YES NO
23 Involve you or participants in a) activities which may be illegal and/or b) the observation,
handling or storage (including export) of information or material which may be regarded
as illegal?
YES NO
24 Require ethical approval from any recognised external agencies? e.g. NHS, Social
Care, Ministry of Justice, Ministry of Defence. Please refer to the Question Specific
Advice for the Stage 1 Research Ethics Application Form and Code of Practice for
Applying for Ethical Approval for further information.
YES NO
25 Involveindividualsaged16yearsofageandoverwholack‘capacitytoconsent’andthereforefall
undertheMentalCapacityAct(2005)?
YES NO
26 Pose any ethical issue not covered elsewhere in this checklist (excluding issues relating
to animals and significant habitats which are dealt with in a separate form)?
YES NO
Please note that the FREP will refer to the Office of the Secretary and Clerk any application where,
in the view of the Chair, the proposed research poses a risk of a legal or security related nature to
Anglia Ruskin University. The Chair will seek guidance from the Secretary and Clerk before the
FREP decides if the proposed research can be granted ethical approval and/or the nature of any
special arrangements which need to be put in place.
ManagementofEthicalRisk
4The Counter Terrorism and Security Act (2015) and Terrorism Act (2006) outlaws web posting of
material that encourages or endorses terrorist acts, even terrorist acts that have occurred in the past.
Sections of the Terrorism Act also create a risk of prosecution for those who transmit material of this
nature, including transmitting the material electronically. The storage of such material on a computer
can, if discovered, prompt a police investigation.Visits to websites related to terrorism and the
downloading of material issued by terrorist groups (even from open-access sites) may be subject to
monitoring by the police. Storage of this material for research purposes may also be subject to
monitoring by the police.Therefore, research relating to terrorism, or any other research that could be
classified as security-sensitive (for example, Ministry of Defence-commissioned work on military
equipment, IT encryption design for public bodies or businesses) needs special treatment. If you have
any doubts about whether your research could be classified as security-sensitive, please speak to
your FREP Chair.
6
20 Riskdamage/disturbancetoculturally,spirituallyorhistoricallysignificantartefacts/places,
orhumanremains?
YES NO
21 Containresearch methodologiesyou,ormembersofyourteam,requiretrainingto carry out? YES NO
22 Involve access to, or use(including internet use) of, material covered by the Counter
Terrorism and Security Act (2015), ortheTerrorism Act (2006),or which could be
classified as security sensitive?4
YES NO
23 Involve you or participants in a) activities which may be illegal and/or b) the observation,
handling or storage (including export) of information or material which may be regarded
as illegal?
YES NO
24 Require ethical approval from any recognised external agencies? e.g. NHS, Social
Care, Ministry of Justice, Ministry of Defence. Please refer to the Question Specific
Advice for the Stage 1 Research Ethics Application Form and Code of Practice for
Applying for Ethical Approval for further information.
YES NO
25 Involveindividualsaged16yearsofageandoverwholack‘capacitytoconsent’andthereforefall
undertheMentalCapacityAct(2005)?
YES NO
26 Pose any ethical issue not covered elsewhere in this checklist (excluding issues relating
to animals and significant habitats which are dealt with in a separate form)?
YES NO
Please note that the FREP will refer to the Office of the Secretary and Clerk any application where,
in the view of the Chair, the proposed research poses a risk of a legal or security related nature to
Anglia Ruskin University. The Chair will seek guidance from the Secretary and Clerk before the
FREP decides if the proposed research can be granted ethical approval and/or the nature of any
special arrangements which need to be put in place.
ManagementofEthicalRisk
4The Counter Terrorism and Security Act (2015) and Terrorism Act (2006) outlaws web posting of
material that encourages or endorses terrorist acts, even terrorist acts that have occurred in the past.
Sections of the Terrorism Act also create a risk of prosecution for those who transmit material of this
nature, including transmitting the material electronically. The storage of such material on a computer
can, if discovered, prompt a police investigation.Visits to websites related to terrorism and the
downloading of material issued by terrorist groups (even from open-access sites) may be subject to
monitoring by the police. Storage of this material for research purposes may also be subject to
monitoring by the police.Therefore, research relating to terrorism, or any other research that could be
classified as security-sensitive (for example, Ministry of Defence-commissioned work on military
equipment, IT encryption design for public bodies or businesses) needs special treatment. If you have
any doubts about whether your research could be classified as security-sensitive, please speak to
your FREP Chair.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

For each of Questions 1-11and Question 26, where you have responded ‘Yes’,pleaseexplain for the committee
howyoujustify andwillmanagetheethicalrisk created.Your research is in the Yellow risk category.
1. Ethical risk in human participants will be managed through compliance with ethical rules and principles.
2. Manipulation in generated data is a risk that can be identified in the output.
3. If investor will involve those respondents which have either any direct or indirect relationship with any
member then, lack of confidentiality in information about their responses, and conflicts of interest will be
the ethical issues. Data protection Act has been complied to maintain such ethical issue.
4. Investigator will take prior permission from the gatekeeper so as to maintain ethnicity and gain acces to
the participants.
5. Financial or other forms of incentives will be offer to participants so as to generate reliable data.
6. It is also essential for the proposed investigation to identify the possibility of participants health issues so
that research can be conducted easily.
7. Participants may find stress if they don’t have sufficient times to give their responses and also there is
lack of confidentiality of information.
8. Research will be conducted only inside UK thus; outside data will not be gathered.
9. Negative impact to the environment comprises like disposal of hard copies, wastage, pollution etc.
10. Not involved.
11. Compliance with Control of Substance hazardous to health and Regulations and Management of Health
and Safety at work will enable researcher to manage genetic modification of human tissues.
26. All the ethical issues have been covered in the research.
7
howyoujustify andwillmanagetheethicalrisk created.Your research is in the Yellow risk category.
1. Ethical risk in human participants will be managed through compliance with ethical rules and principles.
2. Manipulation in generated data is a risk that can be identified in the output.
3. If investor will involve those respondents which have either any direct or indirect relationship with any
member then, lack of confidentiality in information about their responses, and conflicts of interest will be
the ethical issues. Data protection Act has been complied to maintain such ethical issue.
4. Investigator will take prior permission from the gatekeeper so as to maintain ethnicity and gain acces to
the participants.
5. Financial or other forms of incentives will be offer to participants so as to generate reliable data.
6. It is also essential for the proposed investigation to identify the possibility of participants health issues so
that research can be conducted easily.
7. Participants may find stress if they don’t have sufficient times to give their responses and also there is
lack of confidentiality of information.
8. Research will be conducted only inside UK thus; outside data will not be gathered.
9. Negative impact to the environment comprises like disposal of hard copies, wastage, pollution etc.
10. Not involved.
11. Compliance with Control of Substance hazardous to health and Regulations and Management of Health
and Safety at work will enable researcher to manage genetic modification of human tissues.
26. All the ethical issues have been covered in the research.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Section 3: Approvalprocess
AllstudentapplicationsmustbesenttoyourSupervisorforchecking.
YourSupervisormustthenforwardtheapplicationtothe DREP/FREP(asappropriate)
FREP=FacultyResearchEthicsPanel
DREP=DepartmentalResearchEthicsPanel
N
Oansweredtoallquestions
RiskcategoryGreen
CompleteSection5 of this form and
thensendittoyourDREP(orFREPfor
the FacultyofMedicalScienceonly).
Youdonotrequireethicalapproval
from a committee.
Youcanstartyourresearchimmediately.
YES to any of Questions
1-11and/or26but NO to
all other questions
Risk categoryYellow
Complete Section 4 and 5 of this form and
submit it, and the Participant Information
Sheet (PIS) and Participant Consent Form
(PCF), to your DREP (or FREP for Faculty of
Medical Science only). Your faculty may
require further documents.
Yo
uneedtowaitforethicalapprovalbeforeyo
u start your research.
YEStoanyofQuestions12-
23
RiskCategoryRed
Complete Section 5 of this form and
complete the Stage 2 Approval form. Submit
both, and any other documents required, to
your FREP.
If you answered YES to Question 22
you must also complete and submit
for consideration by the committee
the Stage 3 Approval form.
Yo
uneedtowaitforethicalapprovalbeforeyo
u start your research.
YEStoeitherorbotho
f Questions24-25
Ris
kCategoryPurple
You need external approval(s) which, if
granted, maybe regarded as equivalent to
approval from an Anglia Ruskin ethics
committee.
Refer to the Question Specific Advice for the
Stage 1 Research Ethics Application Form
and Code of Practice for Applying for Ethical
Approval for further information
Youneedtowaitforethical and/or
governanceapprovalbefore
8
AllstudentapplicationsmustbesenttoyourSupervisorforchecking.
YourSupervisormustthenforwardtheapplicationtothe DREP/FREP(asappropriate)
FREP=FacultyResearchEthicsPanel
DREP=DepartmentalResearchEthicsPanel
N
Oansweredtoallquestions
RiskcategoryGreen
CompleteSection5 of this form and
thensendittoyourDREP(orFREPfor
the FacultyofMedicalScienceonly).
Youdonotrequireethicalapproval
from a committee.
Youcanstartyourresearchimmediately.
YES to any of Questions
1-11and/or26but NO to
all other questions
Risk categoryYellow
Complete Section 4 and 5 of this form and
submit it, and the Participant Information
Sheet (PIS) and Participant Consent Form
(PCF), to your DREP (or FREP for Faculty of
Medical Science only). Your faculty may
require further documents.
Yo
uneedtowaitforethicalapprovalbeforeyo
u start your research.
YEStoanyofQuestions12-
23
RiskCategoryRed
Complete Section 5 of this form and
complete the Stage 2 Approval form. Submit
both, and any other documents required, to
your FREP.
If you answered YES to Question 22
you must also complete and submit
for consideration by the committee
the Stage 3 Approval form.
Yo
uneedtowaitforethicalapprovalbeforeyo
u start your research.
YEStoeitherorbotho
f Questions24-25
Ris
kCategoryPurple
You need external approval(s) which, if
granted, maybe regarded as equivalent to
approval from an Anglia Ruskin ethics
committee.
Refer to the Question Specific Advice for the
Stage 1 Research Ethics Application Form
and Code of Practice for Applying for Ethical
Approval for further information
Youneedtowaitforethical and/or
governanceapprovalbefore
8

yo
ustartyourrese
arch.
9
ustartyourrese
arch.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
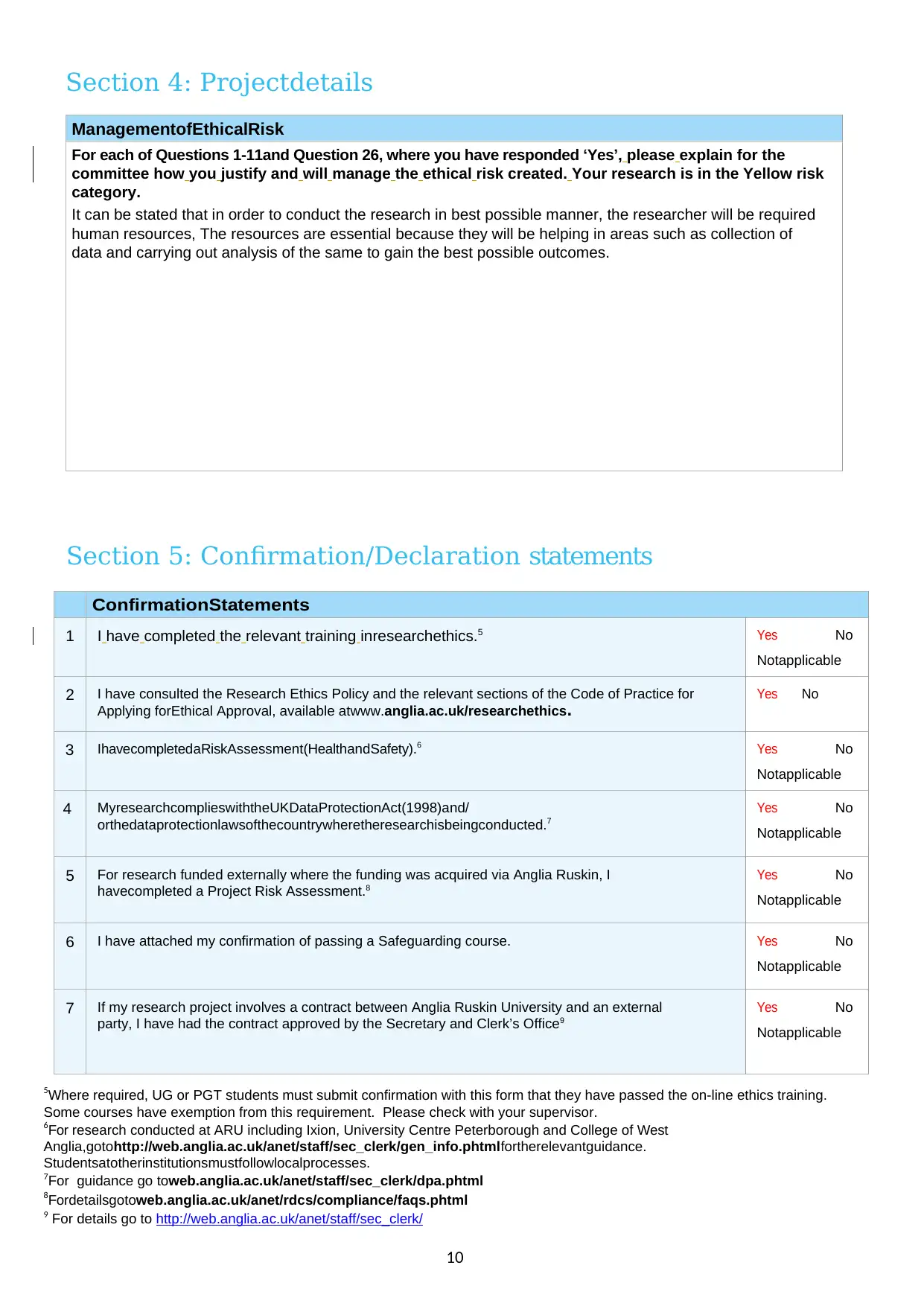
Section 4: Projectdetails
ManagementofEthicalRisk
For each of Questions 1-11and Question 26, where you have responded ‘Yes’, please explain for the
committee how you justify and will manage the ethical risk created. Your research is in the Yellow risk
category.
It can be stated that in order to conduct the research in best possible manner, the researcher will be required
human resources, The resources are essential because they will be helping in areas such as collection of
data and carrying out analysis of the same to gain the best possible outcomes.
Section 5: Confirmation/Declaration statements
ConfirmationStatements
1 I have completed the relevant training inresearchethics.5 Yes No
Notapplicable
2 I have consulted the Research Ethics Policy and the relevant sections of the Code of Practice for
Applying forEthical Approval, available atwww.anglia.ac.uk/researchethics.
Yes No
3 IhavecompletedaRiskAssessment(HealthandSafety).6 Yes No
Notapplicable
4 MyresearchcomplieswiththeUKDataProtectionAct(1998)and/
orthedataprotectionlawsofthecountrywheretheresearchisbeingconducted.7
Yes No
Notapplicable
5 For research funded externally where the funding was acquired via Anglia Ruskin, I
havecompleted a Project Risk Assessment.8
Yes No
Notapplicable
6 I have attached my confirmation of passing a Safeguarding course. Yes No
Notapplicable
7 If my research project involves a contract between Anglia Ruskin University and an external
party, I have had the contract approved by the Secretary and Clerk’s Office9
Yes No
Notapplicable
5Where required, UG or PGT students must submit confirmation with this form that they have passed the on-line ethics training.
Some courses have exemption from this requirement. Please check with your supervisor.
6For research conducted at ARU including Ixion, University Centre Peterborough and College of West
Anglia,gotohttp://web.anglia.ac.uk/anet/staff/sec_clerk/gen_info.phtmlfortherelevantguidance.
Studentsatotherinstitutionsmustfollowlocalprocesses.
7For guidance go toweb.anglia.ac.uk/anet/staff/sec_clerk/dpa.phtml
8Fordetailsgotoweb.anglia.ac.uk/anet/rdcs/compliance/faqs.phtml
9 For details go to http://web.anglia.ac.uk/anet/staff/sec_clerk/
10
Pr oduc edb y Corpor at e Mark eting, Inter national& De v elopment Ser vic e s ,AR U 15- 16/ 136/MB
ManagementofEthicalRisk
For each of Questions 1-11and Question 26, where you have responded ‘Yes’, please explain for the
committee how you justify and will manage the ethical risk created. Your research is in the Yellow risk
category.
It can be stated that in order to conduct the research in best possible manner, the researcher will be required
human resources, The resources are essential because they will be helping in areas such as collection of
data and carrying out analysis of the same to gain the best possible outcomes.
Section 5: Confirmation/Declaration statements
ConfirmationStatements
1 I have completed the relevant training inresearchethics.5 Yes No
Notapplicable
2 I have consulted the Research Ethics Policy and the relevant sections of the Code of Practice for
Applying forEthical Approval, available atwww.anglia.ac.uk/researchethics.
Yes No
3 IhavecompletedaRiskAssessment(HealthandSafety).6 Yes No
Notapplicable
4 MyresearchcomplieswiththeUKDataProtectionAct(1998)and/
orthedataprotectionlawsofthecountrywheretheresearchisbeingconducted.7
Yes No
Notapplicable
5 For research funded externally where the funding was acquired via Anglia Ruskin, I
havecompleted a Project Risk Assessment.8
Yes No
Notapplicable
6 I have attached my confirmation of passing a Safeguarding course. Yes No
Notapplicable
7 If my research project involves a contract between Anglia Ruskin University and an external
party, I have had the contract approved by the Secretary and Clerk’s Office9
Yes No
Notapplicable
5Where required, UG or PGT students must submit confirmation with this form that they have passed the on-line ethics training.
Some courses have exemption from this requirement. Please check with your supervisor.
6For research conducted at ARU including Ixion, University Centre Peterborough and College of West
Anglia,gotohttp://web.anglia.ac.uk/anet/staff/sec_clerk/gen_info.phtmlfortherelevantguidance.
Studentsatotherinstitutionsmustfollowlocalprocesses.
7For guidance go toweb.anglia.ac.uk/anet/staff/sec_clerk/dpa.phtml
8Fordetailsgotoweb.anglia.ac.uk/anet/rdcs/compliance/faqs.phtml
9 For details go to http://web.anglia.ac.uk/anet/staff/sec_clerk/
10
Pr oduc edb y Corpor at e Mark eting, Inter national& De v elopment Ser vic e s ,AR U 15- 16/ 136/MB
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





