Comparison of Batch vs. Continuous Process in Pharmaceutical Industry
VerifiedAdded on 2022/08/23
|11
|3107
|20
Report
AI Summary
This report provides a comprehensive comparison of batch and continuous processes in the pharmaceutical industry, exploring their applications, advantages, and disadvantages. It begins with an introduction highlighting the historical dominance of batch processes and the increasing trend towards continuous processes due to demands for higher production volumes. The report then delves into the roles of process engineers, outlining the key stages of a project: process evaluation, development, design, and marketing. It uses aspirin production as a case study, detailing the batch process steps and associated cost estimations. Furthermore, the report presents two additional case studies: benzimidazole synthesis and hydrogen peroxide oxidation, comparing batch and continuous process conditions and analyzing productivity capacity. The study also covers Aspen simulation for the two processes and identifies drivers for process design improvement, including yield optimization and utility reduction, offering insights into the future of pharmaceutical manufacturing.

COMPARISON OF BATCH VS CONTINOUS PROCESS IN REVOLUTIONIZING THE
PHARMACEUTICAL INDUSTRY
1.INTRODUCTION
1.Is the process for future, present or past application
Unlike petrochemical and large chemical industries that produce bulky reagents by the use of continuous
process method of production; pharmaceutical industries still use batch process production in spite of it
being old and traditional method of production. This trend can be attributed to the complexity of the
product, the low volume needed and the availability of multi –step operation. When we factor this
constrains batch process seems more appropriate. But for the future more innovation and new design
strategies are required to increase the efficiency of production. This initiative will enable the industry to
compete favorably to sustain the competition (Ebrahimi et.al, 2009).
Nowadays process engineers are at a dilemma of which process to use. There have been an increased
demand of drugs and therefore it is necessary to devise new methods. The batch process has been seen
incompetent due to low production volume, this has made transition from batch process to continuous
process and inevitable fate for the pharmaceutical companies.
Although nowadays the industry uses batch method, the trend is to change in the near future. Various
organization have taken initiative into the research of this field and analysis has been done from the lab-
scale reaction up to how they will come up which the factory set up.
But despite this revolution in the pharmaceutical industry there are major drawbacks of continuous
process that must be addressed before fully transitioning into continuous process. One of the drawbacks is
the overall safety of the process. continuous process is highly intense and might have adverse effect to the
manner on how the drugs are produced.
In this paper assessment of different chemical engineering operations will be evaluated, and strategies
devised to make continuous process in pharmaceutical industry a suitable option (Gentile et al ,2003).
2.The role of process engineers in the project
The project will have four main elements in its structure;
(i)process evaluation. The objective of this will be to carry tasks related to economic and financial
suitability of the projects and provide the technical aspects of the process. Different tasks such as cost of
construction of preliminary chemical flow diagrams, cost evaluation and locating different fields which
require more research will be done in this stage (Jensen 2011).
(ii)process development stage
The objective of this will be to obtain data which will be used by the process engineers to do comparison
of the most economical method of production. This will be achieved by plan development program,
analysis of pilot-plant, doing economic evaluations and revision of the project flow diagram
PHARMACEUTICAL INDUSTRY
1.INTRODUCTION
1.Is the process for future, present or past application
Unlike petrochemical and large chemical industries that produce bulky reagents by the use of continuous
process method of production; pharmaceutical industries still use batch process production in spite of it
being old and traditional method of production. This trend can be attributed to the complexity of the
product, the low volume needed and the availability of multi –step operation. When we factor this
constrains batch process seems more appropriate. But for the future more innovation and new design
strategies are required to increase the efficiency of production. This initiative will enable the industry to
compete favorably to sustain the competition (Ebrahimi et.al, 2009).
Nowadays process engineers are at a dilemma of which process to use. There have been an increased
demand of drugs and therefore it is necessary to devise new methods. The batch process has been seen
incompetent due to low production volume, this has made transition from batch process to continuous
process and inevitable fate for the pharmaceutical companies.
Although nowadays the industry uses batch method, the trend is to change in the near future. Various
organization have taken initiative into the research of this field and analysis has been done from the lab-
scale reaction up to how they will come up which the factory set up.
But despite this revolution in the pharmaceutical industry there are major drawbacks of continuous
process that must be addressed before fully transitioning into continuous process. One of the drawbacks is
the overall safety of the process. continuous process is highly intense and might have adverse effect to the
manner on how the drugs are produced.
In this paper assessment of different chemical engineering operations will be evaluated, and strategies
devised to make continuous process in pharmaceutical industry a suitable option (Gentile et al ,2003).
2.The role of process engineers in the project
The project will have four main elements in its structure;
(i)process evaluation. The objective of this will be to carry tasks related to economic and financial
suitability of the projects and provide the technical aspects of the process. Different tasks such as cost of
construction of preliminary chemical flow diagrams, cost evaluation and locating different fields which
require more research will be done in this stage (Jensen 2011).
(ii)process development stage
The objective of this will be to obtain data which will be used by the process engineers to do comparison
of the most economical method of production. This will be achieved by plan development program,
analysis of pilot-plant, doing economic evaluations and revision of the project flow diagram
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
(iii) process design stage
The main focus of engineering is mainly here, chemical and mechanical engineers will size the
equipment, design control systems and do evaluation of safety according to the regulatory bodies of the
area. Mass and energy balance of the system will then be calculated by the process engineer (Heikilah,
1999).
(iv)Marketing stage
Since the product is for commercial purposes, a good rapport is supposed to be established between
consumers and the producers of the commodity. The marketing department advertises and provides the
general public on information about the product. The objective of the marketing will be to do market
research and product development.
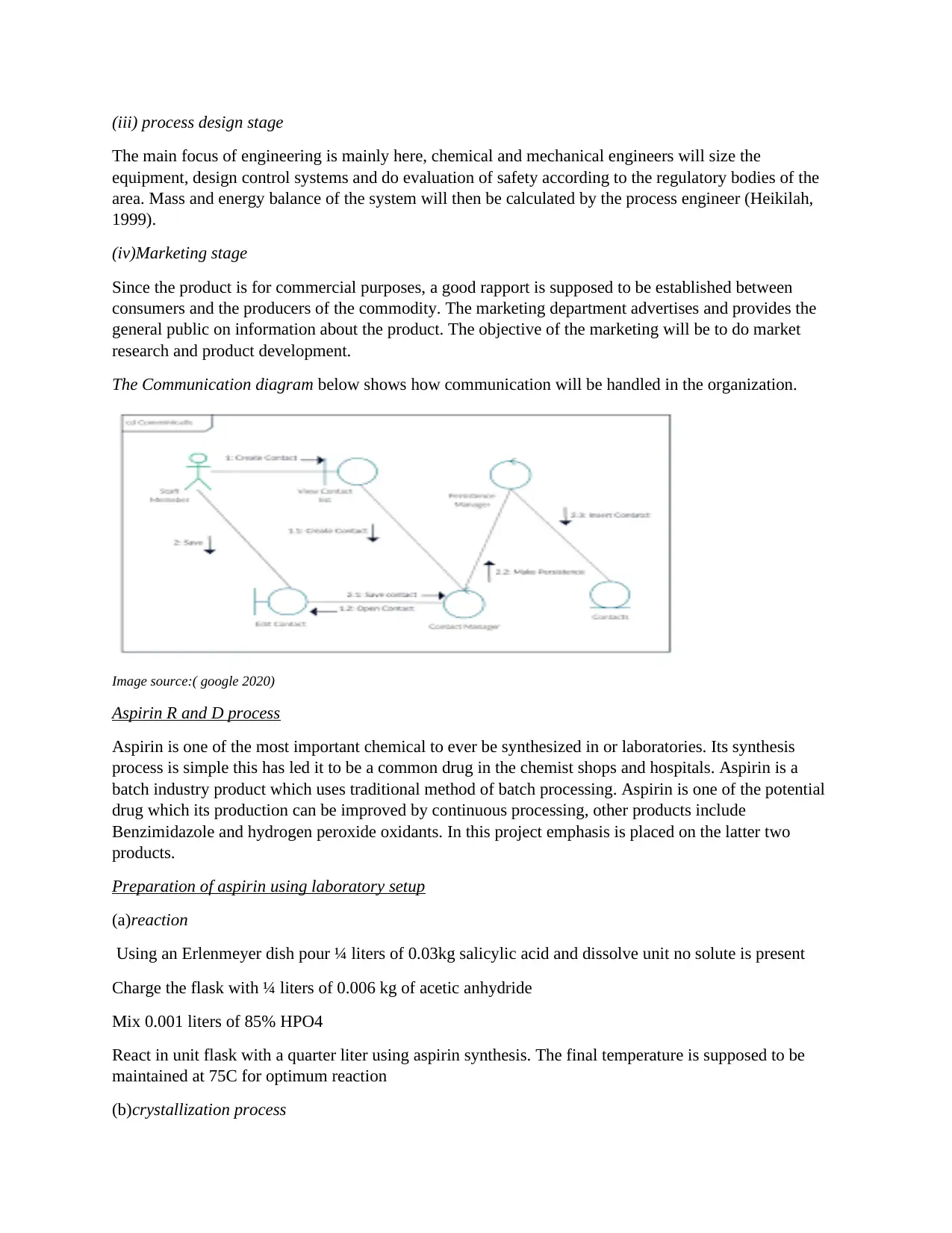
The Communication diagram below shows how communication will be handled in the organization.
Image source:( google 2020)
Aspirin R and D process
Aspirin is one of the most important chemical to ever be synthesized in or laboratories. Its synthesis
process is simple this has led it to be a common drug in the chemist shops and hospitals. Aspirin is a
batch industry product which uses traditional method of batch processing. Aspirin is one of the potential
drug which its production can be improved by continuous processing, other products include
Benzimidazole and hydrogen peroxide oxidants. In this project emphasis is placed on the latter two
products.
Preparation of aspirin using laboratory setup
(a)reaction
Using an Erlenmeyer dish pour ¼ liters of 0.03kg salicylic acid and dissolve unit no solute is present
Charge the flask with ¼ liters of 0.006 kg of acetic anhydride
Mix 0.001 liters of 85% HPO4
React in unit flask with a quarter liter using aspirin synthesis. The final temperature is supposed to be
maintained at 75C for optimum reaction
(b)crystallization process
The main focus of engineering is mainly here, chemical and mechanical engineers will size the
equipment, design control systems and do evaluation of safety according to the regulatory bodies of the
area. Mass and energy balance of the system will then be calculated by the process engineer (Heikilah,
1999).
(iv)Marketing stage
Since the product is for commercial purposes, a good rapport is supposed to be established between
consumers and the producers of the commodity. The marketing department advertises and provides the
general public on information about the product. The objective of the marketing will be to do market
research and product development.
The Communication diagram below shows how communication will be handled in the organization.
Image source:( google 2020)
Aspirin R and D process
Aspirin is one of the most important chemical to ever be synthesized in or laboratories. Its synthesis
process is simple this has led it to be a common drug in the chemist shops and hospitals. Aspirin is a
batch industry product which uses traditional method of batch processing. Aspirin is one of the potential
drug which its production can be improved by continuous processing, other products include
Benzimidazole and hydrogen peroxide oxidants. In this project emphasis is placed on the latter two
products.
Preparation of aspirin using laboratory setup
(a)reaction
Using an Erlenmeyer dish pour ¼ liters of 0.03kg salicylic acid and dissolve unit no solute is present
Charge the flask with ¼ liters of 0.006 kg of acetic anhydride
Mix 0.001 liters of 85% HPO4
React in unit flask with a quarter liter using aspirin synthesis. The final temperature is supposed to be
maintained at 75C for optimum reaction
(b)crystallization process
Charge the Erlenmeyer dish with a quarter liter of 0.002 kg of water crystallize the mixture until we
obtain 100% crystal phase
(c)Filtration
The batch should be filtered from 250 ml flask after this wash the cake in the unit filter. The stream
obtained should belong to solvent recovery.
(d)Drying. This is the final stage after which agglomeration of the product is done.
Estimating production cost of aspirin using batch process
Operation labor
Operating labor is obtained by calculation of the number of hours which are sufficient to produce just one
kg of aspirin. This can be obtained by determining the total amount of process units used in the
manufacturing process, this value can be obtained by evaluating the flow diagram of the overall
manufacturing process. According to the data used, the following values were obtained. In the large scale
manufacturing reactants and the recycled gases such as ammonia and hydrogen are allowed to mix into
the packed catalytic reactor. After the reaction the gases condense cooling the condensable parts of the
reactor. The stream leaves as a separate phase. These gases are then compressed by the compressor and
then recycled back into the system. Therefore, to obtain the labor cost of this process we will have to
make the following assumptions (Gursen &Lang ,2012)
(i)The purification section separates into two or one section
(ii)If the purification section separates into two section then we have a complex situation
(iii) The process is highly automated.
Capital cost estimation
Before establishing this it will be necessary to do calculations on utility requirements, energy and the
mass flow rates of the system in order to size the process equipment. As for this case it is large scale
production and the initial capital cost will be extremely high. But using the building information
modelling (BIM). The optimum sizing of the plant can be generated (Gentile et al, 2003).
Image source:( Kevin ,2020)
obtain 100% crystal phase
(c)Filtration
The batch should be filtered from 250 ml flask after this wash the cake in the unit filter. The stream
obtained should belong to solvent recovery.
(d)Drying. This is the final stage after which agglomeration of the product is done.
Estimating production cost of aspirin using batch process
Operation labor
Operating labor is obtained by calculation of the number of hours which are sufficient to produce just one
kg of aspirin. This can be obtained by determining the total amount of process units used in the
manufacturing process, this value can be obtained by evaluating the flow diagram of the overall
manufacturing process. According to the data used, the following values were obtained. In the large scale
manufacturing reactants and the recycled gases such as ammonia and hydrogen are allowed to mix into
the packed catalytic reactor. After the reaction the gases condense cooling the condensable parts of the
reactor. The stream leaves as a separate phase. These gases are then compressed by the compressor and
then recycled back into the system. Therefore, to obtain the labor cost of this process we will have to
make the following assumptions (Gursen &Lang ,2012)
(i)The purification section separates into two or one section
(ii)If the purification section separates into two section then we have a complex situation
(iii) The process is highly automated.
Capital cost estimation
Before establishing this it will be necessary to do calculations on utility requirements, energy and the
mass flow rates of the system in order to size the process equipment. As for this case it is large scale
production and the initial capital cost will be extremely high. But using the building information
modelling (BIM). The optimum sizing of the plant can be generated (Gentile et al, 2003).
Image source:( Kevin ,2020)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Fig 3.2 plant sizing using BIM
3.The Process classification
In the process we will use different analogies to come up with the suitable process of production
(i)Case study one Benzimidazole synthesis
Benzimazadole is one of the primary compounds contained in most on the pharmaceutical. This chemical
is contained in pain relievers such as aspirin anti-cancer, anti-depressants and antimicrobial therefore
devising an efficient method of producing this compound is vital to every process engineer that is in the
industry (Laird, 2006).
Fig show molecular structure on Benzimidazole
Image source:( yahoo 2020)
The following table shows the difference in production condition of batch and continuous process
Batch continuous
Temperature(c) 200 270
Pressure(psi) 145 1885
Reaction time(min) 5 0.5
Yield(%) 98% 94%
Reaction volume 1000 4
For any significant difference between the two process we are forced to make the following assumptions
3.The Process classification
In the process we will use different analogies to come up with the suitable process of production
(i)Case study one Benzimidazole synthesis
Benzimazadole is one of the primary compounds contained in most on the pharmaceutical. This chemical
is contained in pain relievers such as aspirin anti-cancer, anti-depressants and antimicrobial therefore
devising an efficient method of producing this compound is vital to every process engineer that is in the
industry (Laird, 2006).
Fig show molecular structure on Benzimidazole
Image source:( yahoo 2020)
The following table shows the difference in production condition of batch and continuous process
Batch continuous
Temperature(c) 200 270
Pressure(psi) 145 1885
Reaction time(min) 5 0.5
Yield(%) 98% 94%
Reaction volume 1000 4
For any significant difference between the two process we are forced to make the following assumptions
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

(i) There is perfect missing of reagents in the reactor
(ii)In both systems the cooling conditions are not faulty
(iii)The reaction condition for the process are constant
From the table it can be seen that reaction volume for continuous process is much lower than that of batch
process. This difference will have a major economic feasibility of large scale production using batch
product. This is because a larger reactor volume will be needed for efficient function hence increasing the
project capital costs. By observing the chemical reaction conditions, it can be noted that continuous
process operates at higher pressure and temperature and therefore safety installations must be made to the
reactor to minimize engineering failure.
Productivity capacity for this case
The following equations can be used in estimation of productivity of production of benzimidazodole
Annual product = operation time x yield
Yield = flow rates of reagents X percentage of conversion of benzimadazole
Normally the mass flow rate is supposed to be directly proportional to what was obtained as the average
reactor volume. Doing this calculation, it is possible to obtain an annual production limit of 1000 tons per
year
Calculating the product yield the annual operation time of batch reactor = 24 hours’ /day x 300 day /year
And the annual same rate for continuous reactor is
Time = 8000hr/ day
Since there is only one production line in pharmaceutical industries this means that Benzimidazole cannot
occupy the line for one year, therefore an estimate of 3 months /year is used. Also it is important to note
that it is vital to get the ratio of reaction to total time for the batch reactor, factors such as filling cleaning
and other additional work have been ignored in this case. By the use of the above assumptions the time
ratio is calculated as
Reaction time /total time = 0.185
Case study two: Oxidation of hydrogen peroxide
Hydrogen peroxide as an oxidizing agent has numerous application in the pharmaceutical industry
example, synthesis of lactone and alkylpridine oxidants. It is also used in the synthesis of peracetic acid.
The figure below shows the chemical reaction for oxidation of hydrogen peroxide (Mannan ,2012).
Fig 3.1 peracetic acid
(ii)In both systems the cooling conditions are not faulty
(iii)The reaction condition for the process are constant
From the table it can be seen that reaction volume for continuous process is much lower than that of batch
process. This difference will have a major economic feasibility of large scale production using batch
product. This is because a larger reactor volume will be needed for efficient function hence increasing the
project capital costs. By observing the chemical reaction conditions, it can be noted that continuous
process operates at higher pressure and temperature and therefore safety installations must be made to the
reactor to minimize engineering failure.
Productivity capacity for this case
The following equations can be used in estimation of productivity of production of benzimidazodole
Annual product = operation time x yield
Yield = flow rates of reagents X percentage of conversion of benzimadazole
Normally the mass flow rate is supposed to be directly proportional to what was obtained as the average
reactor volume. Doing this calculation, it is possible to obtain an annual production limit of 1000 tons per
year
Calculating the product yield the annual operation time of batch reactor = 24 hours’ /day x 300 day /year
And the annual same rate for continuous reactor is
Time = 8000hr/ day
Since there is only one production line in pharmaceutical industries this means that Benzimidazole cannot
occupy the line for one year, therefore an estimate of 3 months /year is used. Also it is important to note
that it is vital to get the ratio of reaction to total time for the batch reactor, factors such as filling cleaning
and other additional work have been ignored in this case. By the use of the above assumptions the time
ratio is calculated as
Reaction time /total time = 0.185
Case study two: Oxidation of hydrogen peroxide
Hydrogen peroxide as an oxidizing agent has numerous application in the pharmaceutical industry
example, synthesis of lactone and alkylpridine oxidants. It is also used in the synthesis of peracetic acid.
The figure below shows the chemical reaction for oxidation of hydrogen peroxide (Mannan ,2012).
Fig 3.1 peracetic acid
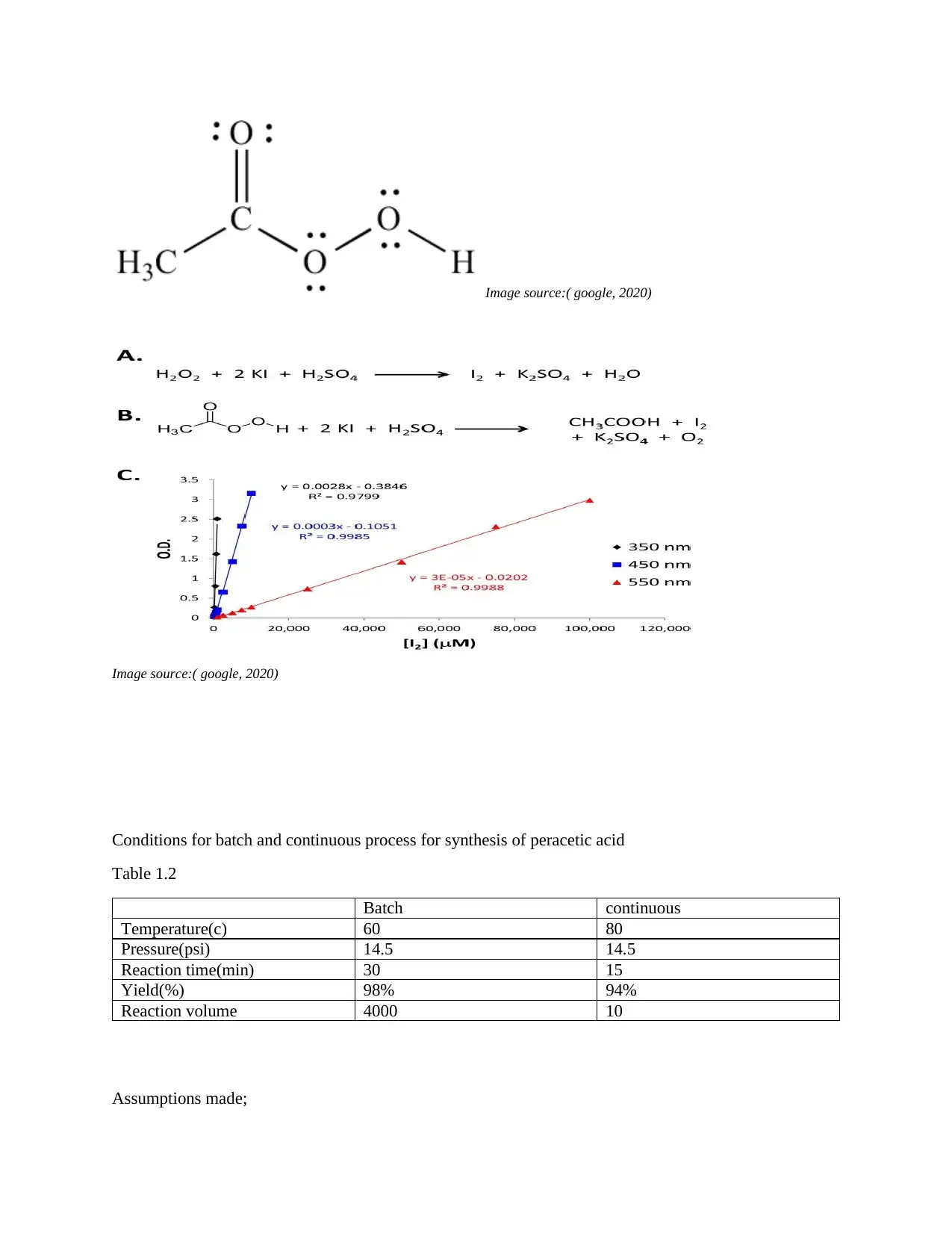
Image source:( google, 2020)
Image source:( google, 2020)
Conditions for batch and continuous process for synthesis of peracetic acid
Table 1.2
Batch continuous
Temperature(c) 60 80
Pressure(psi) 14.5 14.5
Reaction time(min) 30 15
Yield(%) 98% 94%
Reaction volume 4000 10
Assumptions made;
Image source:( google, 2020)
Conditions for batch and continuous process for synthesis of peracetic acid
Table 1.2
Batch continuous
Temperature(c) 60 80
Pressure(psi) 14.5 14.5
Reaction time(min) 30 15
Yield(%) 98% 94%
Reaction volume 4000 10
Assumptions made;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(i)There is perfect mixing of the reagents
(ii)No decomposition of hydrogen peroxide occurs
(iii)There is complete dissociation of suphuric acid ions
(iv)The cooling systems for the process are operating at perfect conditions
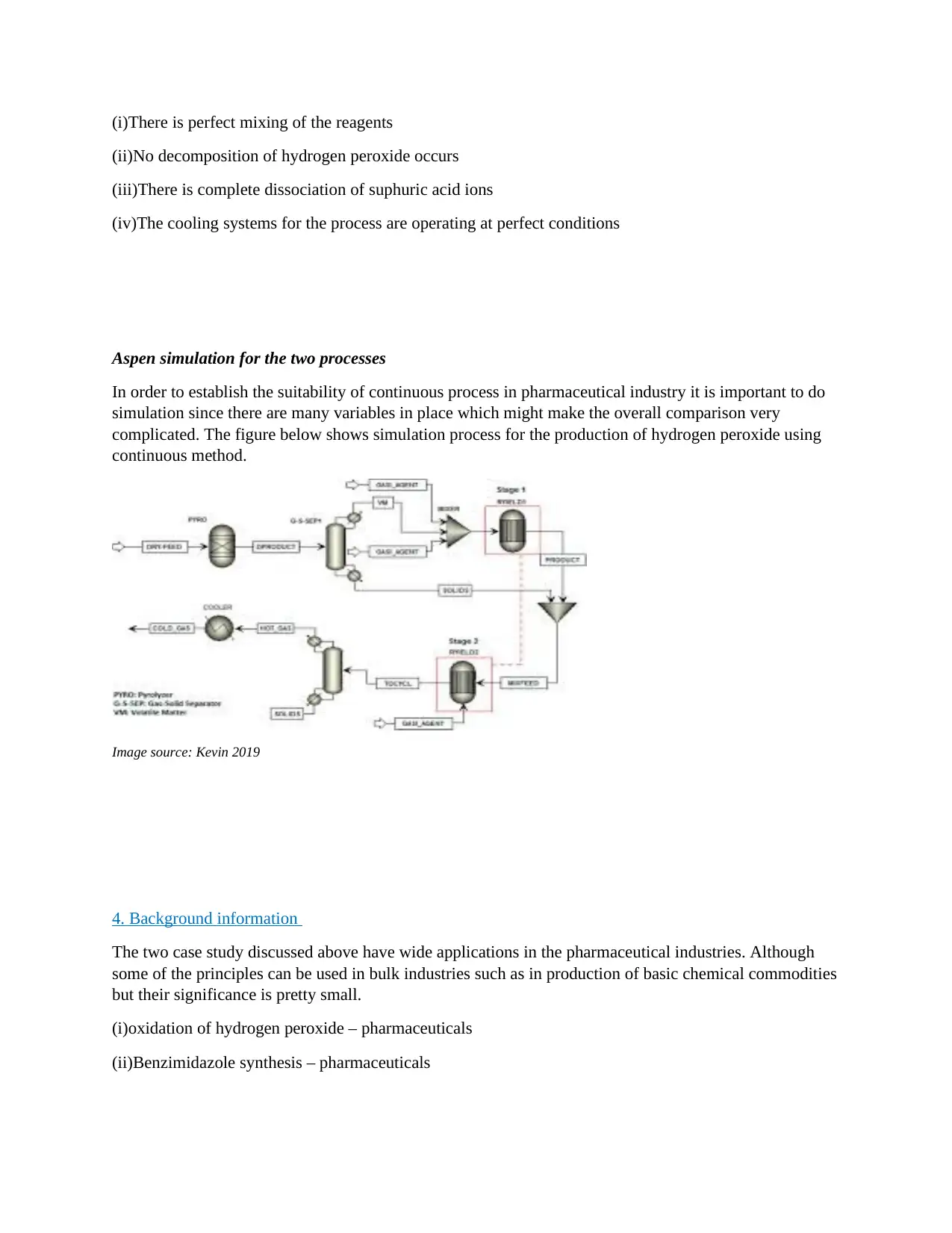
Aspen simulation for the two processes
In order to establish the suitability of continuous process in pharmaceutical industry it is important to do
simulation since there are many variables in place which might make the overall comparison very
complicated. The figure below shows simulation process for the production of hydrogen peroxide using
continuous method.
Image source: Kevin 2019
4. Background information
The two case study discussed above have wide applications in the pharmaceutical industries. Although
some of the principles can be used in bulk industries such as in production of basic chemical commodities
but their significance is pretty small.
(i)oxidation of hydrogen peroxide – pharmaceuticals
(ii)Benzimidazole synthesis – pharmaceuticals
(ii)No decomposition of hydrogen peroxide occurs
(iii)There is complete dissociation of suphuric acid ions
(iv)The cooling systems for the process are operating at perfect conditions
Aspen simulation for the two processes
In order to establish the suitability of continuous process in pharmaceutical industry it is important to do
simulation since there are many variables in place which might make the overall comparison very
complicated. The figure below shows simulation process for the production of hydrogen peroxide using
continuous method.
Image source: Kevin 2019
4. Background information
The two case study discussed above have wide applications in the pharmaceutical industries. Although
some of the principles can be used in bulk industries such as in production of basic chemical commodities
but their significance is pretty small.
(i)oxidation of hydrogen peroxide – pharmaceuticals
(ii)Benzimidazole synthesis – pharmaceuticals
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5.Drivers to improve process design
Process design improvement
(i)improving yields by waste minimization
(a)proper sizing of continuous stirred tank reactor(CSTR)
To minimize wastage of material and energy losses the CSTR should be properly sized. This will be done
by doing analysis and calculations of the optimum reaction volume, the power of the mixer on the heat
transfer area. For production of Benzimidazole the CSTR should be connected in series and therefore, the
following assumptions are made
(i) There is perfect mixing
(ii)constant volume
(iii)constant density
(iv)constant temperature
(v)constant heat capacity
(ii)Reduction of utility (energy and water usage)
As for this case different methods of conveying materials from one place to another should be used. Three
methods will be used in production of hydrogen peroxide i.e. compression pumping and the use of
conveyor belt. This means that the operation plant will have a lot of energy needs in running of the
machinery (compressors and pumps). To minimize energy wastage process streams should be used in
separation of components to obtain favorable operation temperature of 360K and 14.5 bars for batch
process and 380K and 14.5bar for continuous operation. For this optimum conditions to be achieved it is
necessary to compress the gaseous mixture so as to have a reasonable conversion rate of 90%.the
compression work done on the gases can then later be recovered by the use of turbines. This is achieved
by conducting isentropic expansion in turbines to produce energy which will then be transferred to the
process unit in the reactor, this requires the transfer operation to be able to overcome frictional losses and
minor losses that will be experienced in the expansion valves and the pipe fittings (Chan et al ,2014).
(iii)Compliance with legislation
When conducting the difference between continuous and batch process in pharmaceuticals industry using
the Dow’s F and EI the following can be noted on the hazards of each of the process
(i)It can be seen that the comparison process is very complex because many factors are to be considered
before coming with the safest production method. It is therefore important to examine the production
efficiency, scale of production and the overall structure of equipment used (Surana et al, 2008).
(ii)By doing API synthesis and evaluation of the reactor it is noted that the continuous process is very
sensitive to an inventory compared to batch process.
(iii) Since pharmaceutical industry production is normally small scale, massive and hazardous reactors are
not allowed by legislation (Patt ,2002). In addition, it can be seen that continuous method has a higher
yield capacity which make it safer compared to batch process.
Process design improvement
(i)improving yields by waste minimization
(a)proper sizing of continuous stirred tank reactor(CSTR)
To minimize wastage of material and energy losses the CSTR should be properly sized. This will be done
by doing analysis and calculations of the optimum reaction volume, the power of the mixer on the heat
transfer area. For production of Benzimidazole the CSTR should be connected in series and therefore, the
following assumptions are made
(i) There is perfect mixing
(ii)constant volume
(iii)constant density
(iv)constant temperature
(v)constant heat capacity
(ii)Reduction of utility (energy and water usage)
As for this case different methods of conveying materials from one place to another should be used. Three
methods will be used in production of hydrogen peroxide i.e. compression pumping and the use of
conveyor belt. This means that the operation plant will have a lot of energy needs in running of the
machinery (compressors and pumps). To minimize energy wastage process streams should be used in
separation of components to obtain favorable operation temperature of 360K and 14.5 bars for batch
process and 380K and 14.5bar for continuous operation. For this optimum conditions to be achieved it is
necessary to compress the gaseous mixture so as to have a reasonable conversion rate of 90%.the
compression work done on the gases can then later be recovered by the use of turbines. This is achieved
by conducting isentropic expansion in turbines to produce energy which will then be transferred to the
process unit in the reactor, this requires the transfer operation to be able to overcome frictional losses and
minor losses that will be experienced in the expansion valves and the pipe fittings (Chan et al ,2014).
(iii)Compliance with legislation
When conducting the difference between continuous and batch process in pharmaceuticals industry using
the Dow’s F and EI the following can be noted on the hazards of each of the process
(i)It can be seen that the comparison process is very complex because many factors are to be considered
before coming with the safest production method. It is therefore important to examine the production
efficiency, scale of production and the overall structure of equipment used (Surana et al, 2008).
(ii)By doing API synthesis and evaluation of the reactor it is noted that the continuous process is very
sensitive to an inventory compared to batch process.
(iii) Since pharmaceutical industry production is normally small scale, massive and hazardous reactors are
not allowed by legislation (Patt ,2002). In addition, it can be seen that continuous method has a higher
yield capacity which make it safer compared to batch process.
6.Apects of process design to improve
separator design
The degree of freedom of the design
Process unit Unit variable Vu Unit equations Ru
Mixer 12 7
Converter 14 9
Condenser 15 10
Separator
Splitter 18 11
Total 59 37
line number Repeated variables Vr Repeated equations Rr
2 6 0
3 5 1
4 7 1
7 6 0
The degree of freedom can be calculated using the formulae below
FP=(Vu-Vr)-(Ru-Rr)
=59-24-37-2=35-35=0
Since the problem is already formulated above it is important to focus our attention in solving the
following linear equation so as to evaluate the sections that can be easily be improved in the separator.
The following 32 simultaneous equations are solved by POLYMATH after several algebraic
manipulations. The solution obtained requires that the mole ratio must be limited to range from zero to
one, the temperature of the should be limited to melting point to make the fluid (hydrogen peroxide) be
stable at higher temperatures (Noval et al ,2012).
2.CONCLUSION
In this project comparison of batch and continuous process in pharmaceutical industry was conducted.
The project was a success since new methods were found on how continuous method could be used to
replace the old and traditional method of batch process. If the continuous process is to be used in this
industry, it will revolutionize the industry and make manufacturing process easy and efficient. This in
turn will lower the cost of producing drugs in the developing nations, hence enabling the poor to get
access to proper medication services.
separator design
The degree of freedom of the design
Process unit Unit variable Vu Unit equations Ru
Mixer 12 7
Converter 14 9
Condenser 15 10
Separator
Splitter 18 11
Total 59 37
line number Repeated variables Vr Repeated equations Rr
2 6 0
3 5 1
4 7 1
7 6 0
The degree of freedom can be calculated using the formulae below
FP=(Vu-Vr)-(Ru-Rr)
=59-24-37-2=35-35=0
Since the problem is already formulated above it is important to focus our attention in solving the
following linear equation so as to evaluate the sections that can be easily be improved in the separator.
The following 32 simultaneous equations are solved by POLYMATH after several algebraic
manipulations. The solution obtained requires that the mole ratio must be limited to range from zero to
one, the temperature of the should be limited to melting point to make the fluid (hydrogen peroxide) be
stable at higher temperatures (Noval et al ,2012).
2.CONCLUSION
In this project comparison of batch and continuous process in pharmaceutical industry was conducted.
The project was a success since new methods were found on how continuous method could be used to
replace the old and traditional method of batch process. If the continuous process is to be used in this
industry, it will revolutionize the industry and make manufacturing process easy and efficient. This in
turn will lower the cost of producing drugs in the developing nations, hence enabling the poor to get
access to proper medication services.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3.REFERENCES
Ebrahimi, F., Kolehmainen, E., & Turunen, I. (2009). Safety advantages of on-site microprocesses.
Organic Process Research & Development, 13(5), 965-969.
Gentile, M., Rogers, W., & Mannan, M. (2003). Development of a fuzzy logic-based inherent safety
index. Process Safety and Environmental Protection, 81(6), 444- 456. Gutmann, B., Cantillo, D., & Kappe,
C. O. (2015).
Hartman, R. L., McMullen, J. P., & Jensen, K. F. (2011). Continuous-flow technology: a tool for the safe
manufacturing of active pharmaceutical ingredients. Angewandte Chemie International Edition, 54(23),
6688-6728
Heikkila, A. M. (1999). Inherent safety in process plant design: an index-based approach.Deciding
whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angewandte Chemie-
International Edition, 50(33), 7502-7519. doi:10.1002/anie.201004637
Hessel, V., Vural Gürsel, I., & Lang, J. (2012). Ph.D Dissertation, VTT Technical Research Centre of
Finland, Espoo: Finland.
Laird, T. (2006). Plant Design for Safety: a User-friendly Approach. New York: Hemisphere Publishing
Corporation.
LaPorte, T. L., Spangler, L., & Chan, S. H. (2014). What type of reactions do process chemists use on
scale? Organic Process Research & Development, 10(5), 851-852.
Patil, A., Ganguly, S., & Surana, S. (2008). Quantifying Inherent Safety of Chemical Process Routes. Ph.D
Dissertation, Loughborough University, Loughborough: England.
Patt, R., Kordsachia, O., & Hoesch, J. (2002)A systematic review of benzimidazole derivatives as an
antiulcer agent. Rasayan Journal of Chemistry, 1(3), 447-460
Wiley. 53 Pineda-Solano, A., Saenz-Noval, L., & Mannan, M. S. (2012).Ullmann’s Encyclopedia of
Industrial Chemistry. New York:
Ebrahimi, F., Kolehmainen, E., & Turunen, I. (2009). Safety advantages of on-site microprocesses.
Organic Process Research & Development, 13(5), 965-969.
Gentile, M., Rogers, W., & Mannan, M. (2003). Development of a fuzzy logic-based inherent safety
index. Process Safety and Environmental Protection, 81(6), 444- 456. Gutmann, B., Cantillo, D., & Kappe,
C. O. (2015).
Hartman, R. L., McMullen, J. P., & Jensen, K. F. (2011). Continuous-flow technology: a tool for the safe
manufacturing of active pharmaceutical ingredients. Angewandte Chemie International Edition, 54(23),
6688-6728
Heikkila, A. M. (1999). Inherent safety in process plant design: an index-based approach.Deciding
whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angewandte Chemie-
International Edition, 50(33), 7502-7519. doi:10.1002/anie.201004637
Hessel, V., Vural Gürsel, I., & Lang, J. (2012). Ph.D Dissertation, VTT Technical Research Centre of
Finland, Espoo: Finland.
Laird, T. (2006). Plant Design for Safety: a User-friendly Approach. New York: Hemisphere Publishing
Corporation.
LaPorte, T. L., Spangler, L., & Chan, S. H. (2014). What type of reactions do process chemists use on
scale? Organic Process Research & Development, 10(5), 851-852.
Patil, A., Ganguly, S., & Surana, S. (2008). Quantifying Inherent Safety of Chemical Process Routes. Ph.D
Dissertation, Loughborough University, Loughborough: England.
Patt, R., Kordsachia, O., & Hoesch, J. (2002)A systematic review of benzimidazole derivatives as an
antiulcer agent. Rasayan Journal of Chemistry, 1(3), 447-460
Wiley. 53 Pineda-Solano, A., Saenz-Noval, L., & Mannan, M. S. (2012).Ullmann’s Encyclopedia of
Industrial Chemistry. New York:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





