Bioactive Glass in Spinal Fusion
VerifiedAdded on 2020/03/16
|17
|4142
|60
Report
AI Summary
This report analyzes the use of bioactive glass in spinal fusion interventions, highlighting its benefits, evidence-based findings, and potential to enhance surgical outcomes. It discusses the material's properties, clinical applications, and the importance of systematic management in reducing post-operative complications. The report is supported by extensive research and references, providing a comprehensive overview of bioactive glass as a promising alternative in spinal surgery.

Project-1 Report
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1 | P a g e
Table of Content
Introduction and Background…………………………………………………………………………………………………………….2
Bioactive glass based spinal interventions management – An evidence based analysis……………………..4
Data collection methods……………………………………………………………………………………………………………………7
Results……………………………………………………………………………………………………………………………………………….8
Discussion………………………………………………………………………………………………………………………………………….8
Conclusion……………………………………………………………………………………………………………………………………….10
References………………………………………………………………………………………………………………………………………11
1 | P a g e
Table of Content
Introduction and Background…………………………………………………………………………………………………………….2
Bioactive glass based spinal interventions management – An evidence based analysis……………………..4
Data collection methods……………………………………………………………………………………………………………………7
Results……………………………………………………………………………………………………………………………………………….8
Discussion………………………………………………………………………………………………………………………………………….8
Conclusion……………………………………………………………………………………………………………………………………….10
References………………………………………………………………………………………………………………………………………11
1 | P a g e

2 | P a g e
Introduction and Background
Spinal fusion interventions require the systematic utilization of various ceramic
products attributing to β TCP, hydroxypartite, healos and calcium sulphate. The lumbar
spinal fusion interventions evidentially utilize ICBG (iliac crest bone graft) while undertaking
the process of joints arthrodesis (Vaz, et al., 2010). Alternatively, TCP (tricalcium phosphate)
and HA (hydroxyapatite) are also utilized in spinal fusion process due to their proven healing
characteristics (Pugely, Petersen, DeVries-Watson, & Fredericks, 2017). Evidence-based
clinical literature advocates the influence of bone graft quality on the pattern of surgically
induced spinal fusion (McGuire, Pilcher, & Dettori, 2011). The quality and configuration of
bone graft (in spinal fusion surgeries) is of paramount importance in terms of reducing the
occurrence of surgical complications and associated adverse clinical manifestations in the
treated patients (Nouh, 2012). β TCP (beta-tricalcium phosphate) is regarded as a significant
bone substitute that facilitates the formation of bone structure through the process of
osteoclast-mediated resorption (Tanaka, et al., 2017). Therefore, β TCP substitute is utilized
in various spinal fusion interventions with the objective of facilitating the regeneration of
cortical bone. Porous titanium is a bioactive metal that is evidentially utilized in configuring
bone grafts while undertaking complex spinal fusion interventions in the context of its
elevated bone bonding capacity. The alloy of titanium undergoes thermal and chemical
surface treatment to facilitate its active transformation to a bioactive material (Fujibayashi, et
al., 2011). This bioactive intervention substantially elevates the fusion capacity of titanium
implant during spinal fusion interventions. However, the research intervention by
(Ilharreborde , et al., 2008) indicates the elevated capacity and cost effectiveness of bioactive
glass (compared to other ceramic products) in terms of facilitating spinal fusion undertaken to
treat the pattern of idiopathic scoliosis. Bioactive glass is preferred over iliac crest autograft
in terms of bone substitution for effectively treating (AIS) adolescent idiopathic scoliosis
2 | P a g e
Introduction and Background
Spinal fusion interventions require the systematic utilization of various ceramic
products attributing to β TCP, hydroxypartite, healos and calcium sulphate. The lumbar
spinal fusion interventions evidentially utilize ICBG (iliac crest bone graft) while undertaking
the process of joints arthrodesis (Vaz, et al., 2010). Alternatively, TCP (tricalcium phosphate)
and HA (hydroxyapatite) are also utilized in spinal fusion process due to their proven healing
characteristics (Pugely, Petersen, DeVries-Watson, & Fredericks, 2017). Evidence-based
clinical literature advocates the influence of bone graft quality on the pattern of surgically
induced spinal fusion (McGuire, Pilcher, & Dettori, 2011). The quality and configuration of
bone graft (in spinal fusion surgeries) is of paramount importance in terms of reducing the
occurrence of surgical complications and associated adverse clinical manifestations in the
treated patients (Nouh, 2012). β TCP (beta-tricalcium phosphate) is regarded as a significant
bone substitute that facilitates the formation of bone structure through the process of
osteoclast-mediated resorption (Tanaka, et al., 2017). Therefore, β TCP substitute is utilized
in various spinal fusion interventions with the objective of facilitating the regeneration of
cortical bone. Porous titanium is a bioactive metal that is evidentially utilized in configuring
bone grafts while undertaking complex spinal fusion interventions in the context of its
elevated bone bonding capacity. The alloy of titanium undergoes thermal and chemical
surface treatment to facilitate its active transformation to a bioactive material (Fujibayashi, et
al., 2011). This bioactive intervention substantially elevates the fusion capacity of titanium
implant during spinal fusion interventions. However, the research intervention by
(Ilharreborde , et al., 2008) indicates the elevated capacity and cost effectiveness of bioactive
glass (compared to other ceramic products) in terms of facilitating spinal fusion undertaken to
treat the pattern of idiopathic scoliosis. Bioactive glass is preferred over iliac crest autograft
in terms of bone substitution for effectively treating (AIS) adolescent idiopathic scoliosis
2 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3 | P a g e
(Ilharreborde , et al., 2008). The bioactive glass intervention leads to the long-term correction
of AIS in comparison to other ceramic interventions. Bioactive glass exhibits osteoconductive
activity that leads to the establishment of bone fusion outcomes after spinal interventions
(Miyazaki, Tsumura, Wang, & Alanay, 2009). Bioactive glass when integrated with other
osteoinductive and osteogenic agents leads to the substantial enhancement of bone fusion
activity. Bioactive glass proves to be versatile in terms of its handling and utilization while
undertaking spinal fusion interventions (Pugley, Petersen, DeVries-Watson, & Fredericks,
2017). The hydration of the bioactive glass strips through bone marrow aspirate and
subsequent integration with the autograft enhances autoconduction, facilitates the process of
cell attachment and induces the generation of osseous tissue for correcting the bony
deformities of the spine (Pugely , Petersen , DeVries-Watson , & Fredericks , 2017). These
findings confirm the potential of bioactive glass in terms of facilitating the extension of bone
graft warranted for inducing posterolateral spinal fusion. Furthermore, bioactive glass
intervention accelerates the rate of spinal fusion and promotes the pattern of 100% healing of
the quality and quantity of bone that any autograft group could generate after spinal fusion
surgery (Pugely, Petersen, DeVries-Watson , & Fredericks , 2017). These evidence-based
facts indicate the elevated potential of bioactive glass ceramic product in terms of enhancing
the quality, pace and long-term outcomes of spinal fusion intervention. The osteostimulative
property of bioactive glass makes it a promising candidate requiring utilization in terms of a
bone graft substitute for treating the pattern of spinal osteomyelitis. Bioactive glass does not
produce ectopic bone while facilitating spinal fusion and facilitates the generation of new
orthotopic bone (Gestel, et al., 2015). The systematic implantation of bioactive glass leads to
the formation of the layer of calcium phosphate over its surface (after the sustained exposure
to the body fluid). Eventually, the glass surface layer releases phosphate, calcium, silica and
sodium ions that substantially increases osmotic pressure and localized pH (Gestel, et al.,
3 | P a g e
(Ilharreborde , et al., 2008). The bioactive glass intervention leads to the long-term correction
of AIS in comparison to other ceramic interventions. Bioactive glass exhibits osteoconductive
activity that leads to the establishment of bone fusion outcomes after spinal interventions
(Miyazaki, Tsumura, Wang, & Alanay, 2009). Bioactive glass when integrated with other
osteoinductive and osteogenic agents leads to the substantial enhancement of bone fusion
activity. Bioactive glass proves to be versatile in terms of its handling and utilization while
undertaking spinal fusion interventions (Pugley, Petersen, DeVries-Watson, & Fredericks,
2017). The hydration of the bioactive glass strips through bone marrow aspirate and
subsequent integration with the autograft enhances autoconduction, facilitates the process of
cell attachment and induces the generation of osseous tissue for correcting the bony
deformities of the spine (Pugely , Petersen , DeVries-Watson , & Fredericks , 2017). These
findings confirm the potential of bioactive glass in terms of facilitating the extension of bone
graft warranted for inducing posterolateral spinal fusion. Furthermore, bioactive glass
intervention accelerates the rate of spinal fusion and promotes the pattern of 100% healing of
the quality and quantity of bone that any autograft group could generate after spinal fusion
surgery (Pugely, Petersen, DeVries-Watson , & Fredericks , 2017). These evidence-based
facts indicate the elevated potential of bioactive glass ceramic product in terms of enhancing
the quality, pace and long-term outcomes of spinal fusion intervention. The osteostimulative
property of bioactive glass makes it a promising candidate requiring utilization in terms of a
bone graft substitute for treating the pattern of spinal osteomyelitis. Bioactive glass does not
produce ectopic bone while facilitating spinal fusion and facilitates the generation of new
orthotopic bone (Gestel, et al., 2015). The systematic implantation of bioactive glass leads to
the formation of the layer of calcium phosphate over its surface (after the sustained exposure
to the body fluid). Eventually, the glass surface layer releases phosphate, calcium, silica and
sodium ions that substantially increases osmotic pressure and localized pH (Gestel, et al.,
3 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4 | P a g e
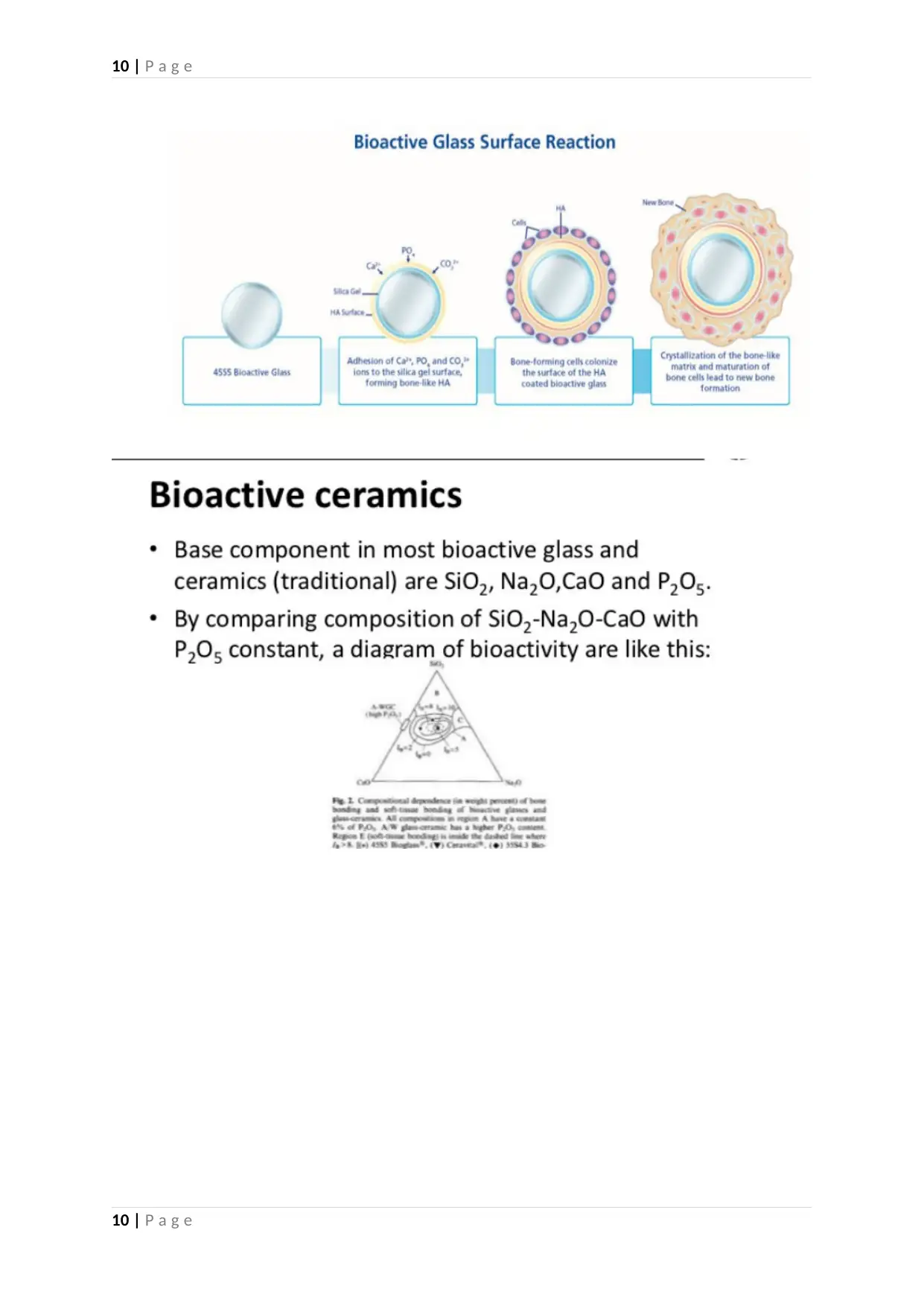
2015). The layer of silica gel then gradually deposits on the glass surface layer and
precipitated under the influence of amorphous calcium phosphate. The eventual
crystallization of these amorphous substitutes to the pattern of natural hydroxyapatite leads to
the development of osteoblasts that facilitate the generation of the new bone (Gestel, et al.,
2015). The antibacterial and angiogenetic properties of the bioactive glass make this
biomaterial a suitable choice for undertaking spinal interventions.
Bioactive glass based spinal interventions management – An evidence based analysis
The pattern of post-operative spinal infections potentially hinders the after-
management of spinal fusion interventions in the clinical setting (Hedge, Meredith, Kepler, &
Huang, 2012). The invasion of the spinal fusion site by MRSA (methicillin-resistant
Staphylococcus aureus) leads to the development of clinical complications that eventually
facilitate the development of spinal stability and substantial reversal of the deformity
correction (Hedge, Meredith, Kepler, & Huang, 2012). However, the utilization of bioactive
glass (S53P4 model) leads to the reduced predisposition of the spinal surgery candidate in
terms of developing the infectious processes at the site of surgery in the post-operative tenure
(Lindfors , et al., 2017). The bone bonding and antibacterial properties of bioactive glass
elevate its potential in terms of reducing the scope of infection development after spinal
fusion. The angiogenetic potential of the bioactive glass attributes to its property of
generating fibrous tissue that subsequently facilitates the formation of vasculature at the
desirable bone locations (Drago, et al., 2013). The osteoproductive property of bioactive glass
assists in the generation of bone matrix, osteogenic cells differentiation, replication and
migration. The utilization of the isolated autograft bone for undertaking anterior cervical
fusion leads to the sustained reduction in the rate of spinal fusion during the initial 3-6
months (Fischer, et al., 2013). This leads to the establishment of spinal instability until the
development of 100% fusion at the site of spinal intervention. Contrarily, bioactive glass
4 | P a g e
2015). The layer of silica gel then gradually deposits on the glass surface layer and
precipitated under the influence of amorphous calcium phosphate. The eventual
crystallization of these amorphous substitutes to the pattern of natural hydroxyapatite leads to
the development of osteoblasts that facilitate the generation of the new bone (Gestel, et al.,
2015). The antibacterial and angiogenetic properties of the bioactive glass make this
biomaterial a suitable choice for undertaking spinal interventions.
Bioactive glass based spinal interventions management – An evidence based analysis
The pattern of post-operative spinal infections potentially hinders the after-
management of spinal fusion interventions in the clinical setting (Hedge, Meredith, Kepler, &
Huang, 2012). The invasion of the spinal fusion site by MRSA (methicillin-resistant
Staphylococcus aureus) leads to the development of clinical complications that eventually
facilitate the development of spinal stability and substantial reversal of the deformity
correction (Hedge, Meredith, Kepler, & Huang, 2012). However, the utilization of bioactive
glass (S53P4 model) leads to the reduced predisposition of the spinal surgery candidate in
terms of developing the infectious processes at the site of surgery in the post-operative tenure
(Lindfors , et al., 2017). The bone bonding and antibacterial properties of bioactive glass
elevate its potential in terms of reducing the scope of infection development after spinal
fusion. The angiogenetic potential of the bioactive glass attributes to its property of
generating fibrous tissue that subsequently facilitates the formation of vasculature at the
desirable bone locations (Drago, et al., 2013). The osteoproductive property of bioactive glass
assists in the generation of bone matrix, osteogenic cells differentiation, replication and
migration. The utilization of the isolated autograft bone for undertaking anterior cervical
fusion leads to the sustained reduction in the rate of spinal fusion during the initial 3-6
months (Fischer, et al., 2013). This leads to the establishment of spinal instability until the
development of 100% fusion at the site of spinal intervention. Contrarily, bioactive glass
4 | P a g e

5 | P a g e
material utilization leads to the elevation in spinal fusion rate within 4-8 weeks of the surgical
intervention (Pugely , Petersen , DeVries-Watson , & Fredericks , 2017). Pre-clinical studies
indicate the high potential of Bioactive glass in terms of accomplishing the cavitary defects
through the elevated generation of osteoblasts. However, bioactive glass generates a lower
quantity of osteocytes in comparison to the autograft (Camargo, Baptista, Natalino, &
Camargo, 2015). The elevated absorbance of bioactive glass at the site of spinal fusion makes
it as a biomaterial of choice since its systematic utilization leads to the elimination of the
entire glass ceramics from the fused spine during the post-operative tenure (Lee, et al., 2014).
This elimination occurs due to high biocompatibility, resorbing property and
osteoconductivity of bioactive glass material. The elevated tensile strength of the fusion
masses attained with the utilization of bioactive glass reduces the risk of clinical
complications in the fused vertebral region during the post-surgical tenure (Lee, et al., 2014).
The spinal cord pain management theory advocates the requirement of undertaking
spinal cord stimulation with the objective of reducing the intensity of chronic spinal pain
(Jeon, 2012). This pain management intervention is also recommended in cases where spinal
implant requires removal after acquiring the desirable fusion outcome (Song, Popescu, & Bell
, 2014). The pattern of spinal pain might occur in terms of a mechanical complication of the
spinal implant. However, the elements of the biodegradable bioactive glass do not contribute
to the pain pattern because of their high dissolution property. The ceramic products
(including bioactive glass) prove to be the bone extenders under the sustained influence of a
local bone graft (Nickoli & Hsu, 2014). Bioactive glass continues to extend the lumbar spine
at the fusion area under the influence of an osteoinductive stimulus while minimizing the
scope of post-operative spinal complications. The systematic utilization of bioactive glass in
the process of posterior spondylodesis leads to the generation of new bone across the
transverse processes of the fused location. The pattern of solid spinal fusion is further
5 | P a g e
material utilization leads to the elevation in spinal fusion rate within 4-8 weeks of the surgical
intervention (Pugely , Petersen , DeVries-Watson , & Fredericks , 2017). Pre-clinical studies
indicate the high potential of Bioactive glass in terms of accomplishing the cavitary defects
through the elevated generation of osteoblasts. However, bioactive glass generates a lower
quantity of osteocytes in comparison to the autograft (Camargo, Baptista, Natalino, &
Camargo, 2015). The elevated absorbance of bioactive glass at the site of spinal fusion makes
it as a biomaterial of choice since its systematic utilization leads to the elimination of the
entire glass ceramics from the fused spine during the post-operative tenure (Lee, et al., 2014).
This elimination occurs due to high biocompatibility, resorbing property and
osteoconductivity of bioactive glass material. The elevated tensile strength of the fusion
masses attained with the utilization of bioactive glass reduces the risk of clinical
complications in the fused vertebral region during the post-surgical tenure (Lee, et al., 2014).
The spinal cord pain management theory advocates the requirement of undertaking
spinal cord stimulation with the objective of reducing the intensity of chronic spinal pain
(Jeon, 2012). This pain management intervention is also recommended in cases where spinal
implant requires removal after acquiring the desirable fusion outcome (Song, Popescu, & Bell
, 2014). The pattern of spinal pain might occur in terms of a mechanical complication of the
spinal implant. However, the elements of the biodegradable bioactive glass do not contribute
to the pain pattern because of their high dissolution property. The ceramic products
(including bioactive glass) prove to be the bone extenders under the sustained influence of a
local bone graft (Nickoli & Hsu, 2014). Bioactive glass continues to extend the lumbar spine
at the fusion area under the influence of an osteoinductive stimulus while minimizing the
scope of post-operative spinal complications. The systematic utilization of bioactive glass in
the process of posterior spondylodesis leads to the generation of new bone across the
transverse processes of the fused location. The pattern of solid spinal fusion is further
5 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6 | P a g e
affirmed radiologically by the CT intervention in limited case scenarios, the remnants of the
bioactive glass remain embedded on the surface of the cancellous spinal bone after the
process of spinal surgical fusion intervention. However, no substantial evidence of spinal
fusion complications under the influence of bioactive glass remnants recorded in evidence
based clinical literature. Evidence-based research literature reports multiple benefits of
utilizing bioactive material as a stand-alone graft in spinal fusion interventions. The
utilization of bioactive material in the intertransverse vertebral space leads to 95% correction
of the vertebral spinal location (as emphasized by the findings in evidence-based clinical
literature) (Rantakokko, et al., 2012). The research literature has not revealed any evidence
regarding the predisposition of the candidates of spinal fusion in terms of acquiring the
debilitating disease outcomes attributing to feline leukemia, hepatitis virus, human
immunodeficiency virus and other virally transmitted diseases (Rantakokko, et al., 2012).
Evidence-based research literature does not recommend the sterilization, freeze drying or
freezing of the of the allograft bone (generated through bioactive material) with the objective
of retaining its osteoconductive and osteoinductive capacity (Rantakokko, et al., 2012).
Spinal fusion intervention evidentially increases the risk of the treated patients in terms of
acquiring adjacent segment disease and associated junctional spinal stenosis (Saavedra-Pozo,
Deusdara, & Benzel, 2014). The research professionals require undertaking prospective study
interventions with the objective of determining the possible change in bioactive glass
utilization methodology in spinal fusion interventions warranted for reducing the scope of
occurrence of post-operative spinal complications (including ASD and spinal stenosis). a
implantation of bioactive glass assisted hard tissue prosthesis in various spinal fusion
interventions brings excellent clinical outcomes. The porous biomaterial of the bioactive
glass assists in the fixation of the prosthetic device across the vertebral region (Krishnan &
Lakshmi, 2013). The extracellular elements of the bioactive glass lead facilitate an excellent
6 | P a g e
affirmed radiologically by the CT intervention in limited case scenarios, the remnants of the
bioactive glass remain embedded on the surface of the cancellous spinal bone after the
process of spinal surgical fusion intervention. However, no substantial evidence of spinal
fusion complications under the influence of bioactive glass remnants recorded in evidence
based clinical literature. Evidence-based research literature reports multiple benefits of
utilizing bioactive material as a stand-alone graft in spinal fusion interventions. The
utilization of bioactive material in the intertransverse vertebral space leads to 95% correction
of the vertebral spinal location (as emphasized by the findings in evidence-based clinical
literature) (Rantakokko, et al., 2012). The research literature has not revealed any evidence
regarding the predisposition of the candidates of spinal fusion in terms of acquiring the
debilitating disease outcomes attributing to feline leukemia, hepatitis virus, human
immunodeficiency virus and other virally transmitted diseases (Rantakokko, et al., 2012).
Evidence-based research literature does not recommend the sterilization, freeze drying or
freezing of the of the allograft bone (generated through bioactive material) with the objective
of retaining its osteoconductive and osteoinductive capacity (Rantakokko, et al., 2012).
Spinal fusion intervention evidentially increases the risk of the treated patients in terms of
acquiring adjacent segment disease and associated junctional spinal stenosis (Saavedra-Pozo,
Deusdara, & Benzel, 2014). The research professionals require undertaking prospective study
interventions with the objective of determining the possible change in bioactive glass
utilization methodology in spinal fusion interventions warranted for reducing the scope of
occurrence of post-operative spinal complications (including ASD and spinal stenosis). a
implantation of bioactive glass assisted hard tissue prosthesis in various spinal fusion
interventions brings excellent clinical outcomes. The porous biomaterial of the bioactive
glass assists in the fixation of the prosthetic device across the vertebral region (Krishnan &
Lakshmi, 2013). The extracellular elements of the bioactive glass lead facilitate an excellent
6 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7 | P a g e
movement of nutrients, vascularity as well as cell occupancy. However, the utilization of
bioactive glass matrix in spinal fusion interventions does not reduce the risk of the corrected
spine in terms of acquiring spinal fractures under stressful conditions (Krishnan & Lakshmi,
2013). Therefore, the clinicians and research professionals require undertaking prospective
research interventions with the objective of developing innovative bone matrix preparation
strategies for reducing the risk of occurrence of spinal fractures following their corrective
fusion interventions.
Data collection methods
The systematic study data regarding bioactive glass material was sequentially
retrieved through PubMed, Cochrane and Research Gate for its evidence-based analysis. The
following search term pattern was utilized for selecting the articles of interest that included
the pattern of systematic, observatory as well as quantitative research interventions. Indeed,
out of 456 selected research interventions, 6 studies that appropriately matched with the
subject of interest were selected and analysed for retrieving the evidence-based findings.
1. “Bioactive glass” and spinal fusion”
2. “Bioactive glass” or “ceramic products”
3. “β TCP” and “Bioactive glass” and “spinal fusion”
4. “Hydroxypartite” and “Bioactive glass” and “spinal fusion”
5. Healos” and Bioactive glass” and spinal fusion”
6. Bioactive glass” and orthopaedic surgery”
7. Bioactive glass” and vertebral fusion management”
8. Bioactive glass” and vertebral fusion” and bone substitute”
9. Bioactive glass” and intervertebral regeneration”
10. Bioactive glass” and vertebral fractures”
11. Bioactive glass management”
7 | P a g e
movement of nutrients, vascularity as well as cell occupancy. However, the utilization of
bioactive glass matrix in spinal fusion interventions does not reduce the risk of the corrected
spine in terms of acquiring spinal fractures under stressful conditions (Krishnan & Lakshmi,
2013). Therefore, the clinicians and research professionals require undertaking prospective
research interventions with the objective of developing innovative bone matrix preparation
strategies for reducing the risk of occurrence of spinal fractures following their corrective
fusion interventions.
Data collection methods
The systematic study data regarding bioactive glass material was sequentially
retrieved through PubMed, Cochrane and Research Gate for its evidence-based analysis. The
following search term pattern was utilized for selecting the articles of interest that included
the pattern of systematic, observatory as well as quantitative research interventions. Indeed,
out of 456 selected research interventions, 6 studies that appropriately matched with the
subject of interest were selected and analysed for retrieving the evidence-based findings.
1. “Bioactive glass” and spinal fusion”
2. “Bioactive glass” or “ceramic products”
3. “β TCP” and “Bioactive glass” and “spinal fusion”
4. “Hydroxypartite” and “Bioactive glass” and “spinal fusion”
5. Healos” and Bioactive glass” and spinal fusion”
6. Bioactive glass” and orthopaedic surgery”
7. Bioactive glass” and vertebral fusion management”
8. Bioactive glass” and vertebral fusion” and bone substitute”
9. Bioactive glass” and intervertebral regeneration”
10. Bioactive glass” and vertebral fractures”
11. Bioactive glass management”
7 | P a g e

8 | P a g e
12. Bioactive glass” and intervertebral complications”
Results
The findings of the evaluated research interventions confirm the elevated capacity of
bioactive glass in terms of facilitating the process of spinal surgical fusion. The minimum
post-operative complications that the surgery candidates experience after spinal fusion
(because of the effective utilization of bioactive glass) makes it as a material of choice for
undertaking corrective interventions of the human spine. The findings of the presented
systematic analysis were rationally authenticated with the systematic utilization of the CASP
tool. Critical Appraisal Skills Programme (CASP) is indeed regarded as an evidence-based
tool warranting utilization for the systematic assessment for the methodological quality and
results of numerous research interventions (Zeng, et al., 2015). The study review addressed a
focussed research question (related to bioactive material utilization and its comparative
analysis with other ceramic products) and research papers of the selected subject were
evidentially explored for generating the desirable study outcomes. The irrelevant studies were
summarily excluded from the research interventions. The precision of the study results
appeared precise, rational unless and otherwise negated by the findings of the prospective
research interventions.
Discussion
The evidence-based findings regarding the utilization of bioactive glass in spinal
fusion interventions indicate its potential in terms of intervertebral bone generation and
facilitation of the healing process during the post-operative period. However, the utilization
of bioactive glass does not effectively subside the entire risks associated with spinal
interventions related outcomes. For example, no concrete methodology exists for effectively
reducing the risk of occurrence of spinal fractures (during post-operative period) under the
influence of weak tensile strength of the bioactive glass material. Therefore, the concept of
8 | P a g e
12. Bioactive glass” and intervertebral complications”
Results
The findings of the evaluated research interventions confirm the elevated capacity of
bioactive glass in terms of facilitating the process of spinal surgical fusion. The minimum
post-operative complications that the surgery candidates experience after spinal fusion
(because of the effective utilization of bioactive glass) makes it as a material of choice for
undertaking corrective interventions of the human spine. The findings of the presented
systematic analysis were rationally authenticated with the systematic utilization of the CASP
tool. Critical Appraisal Skills Programme (CASP) is indeed regarded as an evidence-based
tool warranting utilization for the systematic assessment for the methodological quality and
results of numerous research interventions (Zeng, et al., 2015). The study review addressed a
focussed research question (related to bioactive material utilization and its comparative
analysis with other ceramic products) and research papers of the selected subject were
evidentially explored for generating the desirable study outcomes. The irrelevant studies were
summarily excluded from the research interventions. The precision of the study results
appeared precise, rational unless and otherwise negated by the findings of the prospective
research interventions.
Discussion
The evidence-based findings regarding the utilization of bioactive glass in spinal
fusion interventions indicate its potential in terms of intervertebral bone generation and
facilitation of the healing process during the post-operative period. However, the utilization
of bioactive glass does not effectively subside the entire risks associated with spinal
interventions related outcomes. For example, no concrete methodology exists for effectively
reducing the risk of occurrence of spinal fractures (during post-operative period) under the
influence of weak tensile strength of the bioactive glass material. Therefore, the concept of
8 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9 | P a g e
system engineering management requires effective incorporation for minimizing the risk of
post-surgical complications associated with the utilization of bioactive glass material in
spinal fusion interventions (Erasmus & Doeben-Henisch, 2011). Accordingly, the
developmental phasing of the bioactive glass material would require integrated teaming
approach with the objective of systematically testing the process of integration, assembly and
fabrication of the bioactive glass material. The developmental, integration, implementation,
deployment, operational and phaseout requirements of the bioactive glass preparation would
require step-by-step acquisition in the context of configuring an elevated quality material
with minimal disease predisposition implications (Erasmus & Doeben-Henisch, 2011). The
systematic technical management of the bioactive glass manufacturing process with the
utilization of quality driven approaches will rationally improve the design solution and the
eventual surgical outcomes. The systematic deployment and integration of the human factors
since the initiation of bioactive glass material extraction intervention and its eventual
utilization in spinal fusion surgery would substantially increase the quality of the desirable
health outcomes in the post-operative tenure (Erasmus & Doeben-Henisch, 2011). This
rationally indicates that the safe, sustainable and reliable administration of bioactive glass
assisted spinal fusion interventions warrants the systematic deployment of system
engineering management approaches by the healthcare manufacturers, quality enhancement
teams, clinicians and surgeons (Erasmus & Doeben-Henisch, 2011). Prospective research
studies require administration with the objective of exploring the scope of improvement in
the management concepts for effectively reducing the problems and hindrances encountered
while promoting the vertebral fusion interventions and bone substitute assessment in the
subjects of interest.
9 | P a g e
system engineering management requires effective incorporation for minimizing the risk of
post-surgical complications associated with the utilization of bioactive glass material in
spinal fusion interventions (Erasmus & Doeben-Henisch, 2011). Accordingly, the
developmental phasing of the bioactive glass material would require integrated teaming
approach with the objective of systematically testing the process of integration, assembly and
fabrication of the bioactive glass material. The developmental, integration, implementation,
deployment, operational and phaseout requirements of the bioactive glass preparation would
require step-by-step acquisition in the context of configuring an elevated quality material
with minimal disease predisposition implications (Erasmus & Doeben-Henisch, 2011). The
systematic technical management of the bioactive glass manufacturing process with the
utilization of quality driven approaches will rationally improve the design solution and the
eventual surgical outcomes. The systematic deployment and integration of the human factors
since the initiation of bioactive glass material extraction intervention and its eventual
utilization in spinal fusion surgery would substantially increase the quality of the desirable
health outcomes in the post-operative tenure (Erasmus & Doeben-Henisch, 2011). This
rationally indicates that the safe, sustainable and reliable administration of bioactive glass
assisted spinal fusion interventions warrants the systematic deployment of system
engineering management approaches by the healthcare manufacturers, quality enhancement
teams, clinicians and surgeons (Erasmus & Doeben-Henisch, 2011). Prospective research
studies require administration with the objective of exploring the scope of improvement in
the management concepts for effectively reducing the problems and hindrances encountered
while promoting the vertebral fusion interventions and bone substitute assessment in the
subjects of interest.
9 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
10 | P a g e
10 | P a g e
10 | P a g e

11 | P a g e
Conclusion
Bioactive glass material highly recommended by the clinicians and researchers on a
wider scale in terms of its utilization in spinal fusion interventions for the systematic
acquisition of the healthcare outcomes. The porous surface of the bioactive glass material
facilitates its adjustment with the prosthetic implant and reduces the scope of development of
its mechanical complications. The risks of utilizing bioactive glass material in spinal fusion
interventions require effective minimization with the utilization of system engineering
management approaches. The incorporation of the integrated teaming approaches in the
process of bioactive glass material preparation and its eventual application in spinal fusion
surgeries will lead to the substantial reduction in post-surgical complications and associated
adverse health outcomes in the population of interest. The regular monitoring of each step of
the bioactive material preparation and its utilization in spinal fusion will assist the clinicians
and healthcare manufacturers in terms of modifying the bioactive glass preparation
approaches and associated surgical intervention procedures for reducing the scope of
occurrence of spinal trauma or associated clinical complications in the predisposed surgery
candidates.
11 | P a g e
Conclusion
Bioactive glass material highly recommended by the clinicians and researchers on a
wider scale in terms of its utilization in spinal fusion interventions for the systematic
acquisition of the healthcare outcomes. The porous surface of the bioactive glass material
facilitates its adjustment with the prosthetic implant and reduces the scope of development of
its mechanical complications. The risks of utilizing bioactive glass material in spinal fusion
interventions require effective minimization with the utilization of system engineering
management approaches. The incorporation of the integrated teaming approaches in the
process of bioactive glass material preparation and its eventual application in spinal fusion
surgeries will lead to the substantial reduction in post-surgical complications and associated
adverse health outcomes in the population of interest. The regular monitoring of each step of
the bioactive material preparation and its utilization in spinal fusion will assist the clinicians
and healthcare manufacturers in terms of modifying the bioactive glass preparation
approaches and associated surgical intervention procedures for reducing the scope of
occurrence of spinal trauma or associated clinical complications in the predisposed surgery
candidates.
11 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
