BIOCHEMISTRY Homework: Analysis and Preparation of PBS Solutions
VerifiedAdded on 2023/06/06
|6
|1097
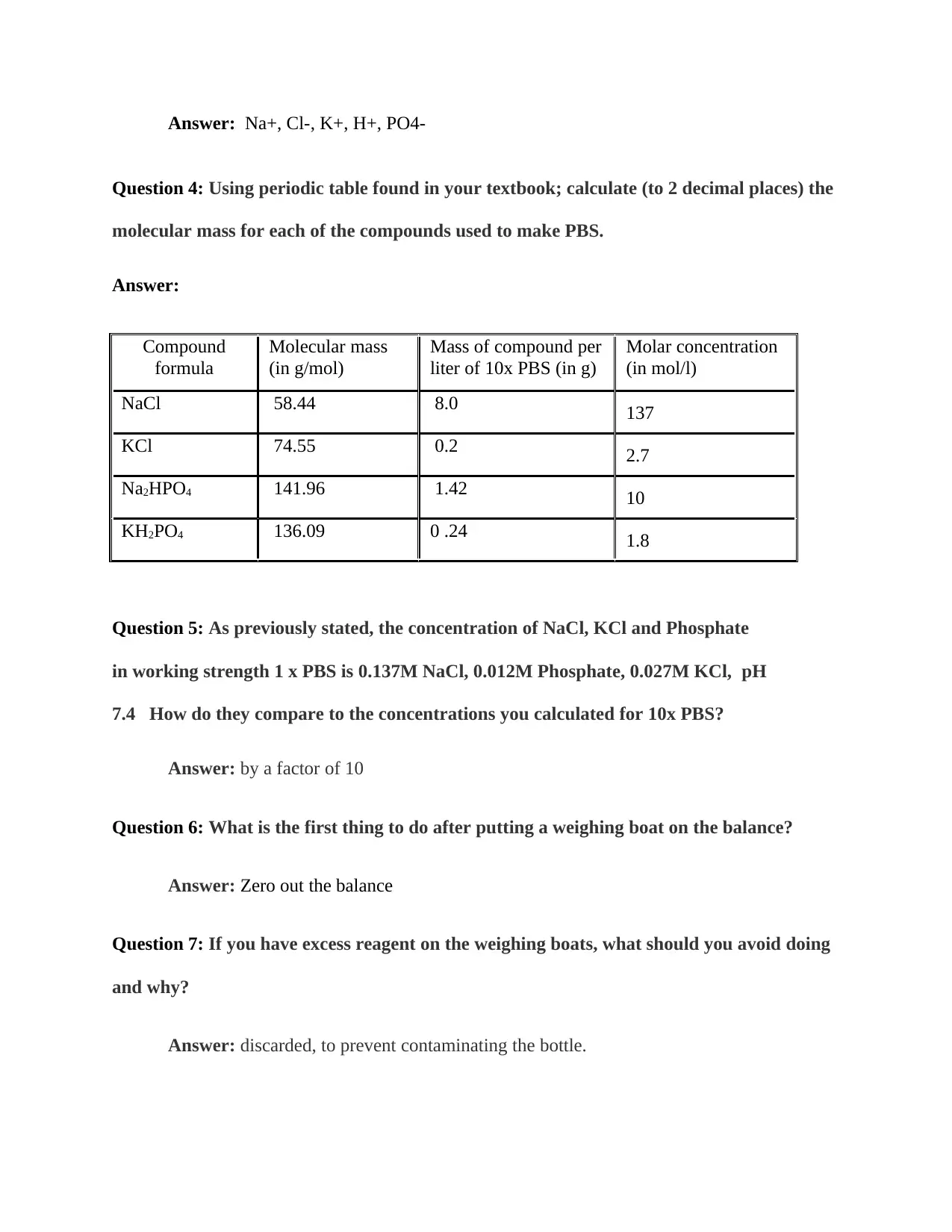
|182
Homework Assignment
AI Summary
This homework assignment delves into the analysis and preparation of a Phosphate Buffered Saline (PBS) solution, a common buffer used in biochemical experiments. The assignment begins by exploring the relationship between pH and H3O+ concentration, explaining how dilution affects the pH of acids and bases. It then identifies the acid and base in PBS, determines the ions produced, and calculates the molecular mass and molar concentrations of the compounds used. The solution compares the calculated concentrations for 10x PBS with those of a 1x working solution. The assignment also covers practical aspects of solution preparation, such as the correct use of lab equipment (weighing boats, graduated cylinders), calibration of pH meters, and the rationale behind specific protocol steps, including autoclaving. The solution provides a detailed step-by-step guide on how to prepare a 1x working solution of PBS, including glassware, reagents, and pH adjustments.
1 out of 6








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)