Durham College CHEM 2701/2703: Biochemistry Final Take-Home Assignment
VerifiedAdded on 2023/06/08
|5
|988
|220
Homework Assignment
AI Summary
This document provides a complete solution to a final take-home assignment for a Pre-Health Chemistry II course (CHEM 2701/2703) at Durham College. The assignment covers key biochemistry concepts, including categorizing biomolecules (sucrose, citric acid, taurine, aspartame, glutamine, potassium sorbate, fructose, caffeine, sucralose, and folic acid) based on their properties. It explores lactose intolerance, enzyme function, and the difference between lactose and lactase. The assignment also delves into the structure and impact of Olestra, a synthetic fat, including its effects on vitamin absorption. Furthermore, it examines DNA structure, including the sugars present in DNA and RNA, and the components of nucleotides. Finally, the assignment covers amino acids, protein structure levels (primary, secondary, tertiary, and quaternary), and factors affecting protein structure.
CHEM 2701/2703 – Final Take-Home Assignment (15% Final Evaluation)
Pre-Health Chemistry II – Durham College /45 marks
1) (10 marks) Using the information from the above reading, categorize the following as either:
(1) A source of energy
(2) An amino acid
(3) A stimulant
(4) A vitamin
(5) An artificial sweetener
(6) A preservative
(7) Related to metabolism
NB: Numbers can be used more than once or not at all
Biomolecules
of the products
sucrose citric acid taurine aspartame glutamine
Category #
from above
1. A source of
energy
7.Related
to
metabolism
3.A stimulant 5.An artificial
sweetener
2.An amino
acid
Biomolecules
of the products
potassium
sorbate
fructose caffeine sucralose folic acid
Category #
from above
6.A preservative 1.A source
of energy
3.A stimulant 5.An artificial
sweetener
7.Related to
metabolism
2: Lactose Intolerance (enzymes)
1. (5 marks) Number match the parts of an enzyme and substrate. More than one letter may
apply.
○ 1=enzyme A,C,E_____
○ 2=substrate _D, D____
○ 3=active site _A, E____
○ 4=enzyme-substrate complex __C,D___
1
Pre-Health Chemistry II – Durham College /45 marks
1) (10 marks) Using the information from the above reading, categorize the following as either:
(1) A source of energy
(2) An amino acid
(3) A stimulant
(4) A vitamin
(5) An artificial sweetener
(6) A preservative
(7) Related to metabolism
NB: Numbers can be used more than once or not at all
Biomolecules
of the products
sucrose citric acid taurine aspartame glutamine
Category #
from above
1. A source of
energy
7.Related
to
metabolism
3.A stimulant 5.An artificial
sweetener
2.An amino
acid
Biomolecules
of the products
potassium
sorbate
fructose caffeine sucralose folic acid
Category #
from above
6.A preservative 1.A source
of energy
3.A stimulant 5.An artificial
sweetener
7.Related to
metabolism
2: Lactose Intolerance (enzymes)
1. (5 marks) Number match the parts of an enzyme and substrate. More than one letter may
apply.
○ 1=enzyme A,C,E_____
○ 2=substrate _D, D____
○ 3=active site _A, E____
○ 4=enzyme-substrate complex __C,D___
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

○ 5=product _F, G____
2. (2 marks) Instead of drinking lactose free milk someone might take lactase tablets. Do you think
this would work? Explain why/how?
Yes, the lactase tablets help to break down the lactose in milk therefore the people can take the milk
with lactose and then take lactase pills instead of the lactose free milk.
3. (2 marks) What is the difference between lactose and lactase? 2 differences needed for full marks.
Lactose is a carbohydrate polymer which has two smaller sugars while lactase is an enzymatic
protein. Also, lactose is a disaccharide sugar, which is made up of one molecule of galactose and one
molecule of glucose while lactase is able to generate two monosaccharide molecule.
4. (2 marks) Identify 2 factors that can affect enzyme activity.
The concentration of the enzymes, temperature and substrate concentration
3: Olestra (lipids)
1. (2 marks) Describe the structure of a triglyceride.
Triglyceride is made of a glycerol and 3-fatty acid chains.
2. Olestra is a synthetic fat that was discovered in the 1990's. It was popular at the time because it
had the taste and texture of a real fat but did not cause weight gain. Olestra is also known as olean.
Research olestra/olean at the following links:
● https://onlinelibrary.wiley.com/doi/pdf/10.1002/clc.4960190508
● https://en.wikipedia.org/wiki/Olestra
a) (1 mark) Why was olestra/olean banned?
Olestra/olean was banned due to its gaining up of weight due to the fake fat available, which includes
diarrhea, cramps, and leaky bowel syndrome
2
2. (2 marks) Instead of drinking lactose free milk someone might take lactase tablets. Do you think
this would work? Explain why/how?
Yes, the lactase tablets help to break down the lactose in milk therefore the people can take the milk
with lactose and then take lactase pills instead of the lactose free milk.
3. (2 marks) What is the difference between lactose and lactase? 2 differences needed for full marks.
Lactose is a carbohydrate polymer which has two smaller sugars while lactase is an enzymatic
protein. Also, lactose is a disaccharide sugar, which is made up of one molecule of galactose and one
molecule of glucose while lactase is able to generate two monosaccharide molecule.
4. (2 marks) Identify 2 factors that can affect enzyme activity.
The concentration of the enzymes, temperature and substrate concentration
3: Olestra (lipids)
1. (2 marks) Describe the structure of a triglyceride.
Triglyceride is made of a glycerol and 3-fatty acid chains.
2. Olestra is a synthetic fat that was discovered in the 1990's. It was popular at the time because it
had the taste and texture of a real fat but did not cause weight gain. Olestra is also known as olean.
Research olestra/olean at the following links:
● https://onlinelibrary.wiley.com/doi/pdf/10.1002/clc.4960190508
● https://en.wikipedia.org/wiki/Olestra
a) (1 mark) Why was olestra/olean banned?
Olestra/olean was banned due to its gaining up of weight due to the fake fat available, which includes
diarrhea, cramps, and leaky bowel syndrome
2

b) (1 mark) How is the structure of olean/olestra different from that of a normal fat/oil?
Olean has 6 fatty acids instead of the 3 for the normal fat has.
3. Read the information on olestra available here: https://recipes.howstuffworks.com/question526.htm
a) (1 mark) Which sugar/carbohydrate is present in olestra?
Sucrose
b) (1 mark) In a triglyceride, 3 fatty acid chains are attached to a glycerol backbone. In olestra,
how many chains are attached?
6 to 8 fatty acids
c) (2 marks) How does olestra/olean impact vitamin absorption?
The olestra is able to bid with the vitamins in the intestines and therefore inhibiting their absorption.
4: DNA Structure (nucleic acids)
1. A nucleotide contains a carbohydrate.
a. (2 marks) What is the name of the sugar/carbohydrate in DNA? How many carbon
atoms does it have?
Deoxyribose is the main sugar found in DNA. It has 5 carbon atoms.
b. (2 marks) What is the name of the sugar/carbohydrate in RNA? How many carbon
atoms does it have?
Ribose is the sugar found in RNA. Ribose has 5 major carbon atoms.
2. (1 mark) True/False: Uracil is a key component of DNA. Explain your answer.
False, Uracil is found in RNA and in DNA it is replaced by thymine and thus not a key component for
DNA.
3
Olean has 6 fatty acids instead of the 3 for the normal fat has.
3. Read the information on olestra available here: https://recipes.howstuffworks.com/question526.htm
a) (1 mark) Which sugar/carbohydrate is present in olestra?
Sucrose
b) (1 mark) In a triglyceride, 3 fatty acid chains are attached to a glycerol backbone. In olestra,
how many chains are attached?
6 to 8 fatty acids
c) (2 marks) How does olestra/olean impact vitamin absorption?
The olestra is able to bid with the vitamins in the intestines and therefore inhibiting their absorption.
4: DNA Structure (nucleic acids)
1. A nucleotide contains a carbohydrate.
a. (2 marks) What is the name of the sugar/carbohydrate in DNA? How many carbon
atoms does it have?
Deoxyribose is the main sugar found in DNA. It has 5 carbon atoms.
b. (2 marks) What is the name of the sugar/carbohydrate in RNA? How many carbon
atoms does it have?
Ribose is the sugar found in RNA. Ribose has 5 major carbon atoms.
2. (1 mark) True/False: Uracil is a key component of DNA. Explain your answer.
False, Uracil is found in RNA and in DNA it is replaced by thymine and thus not a key component for
DNA.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
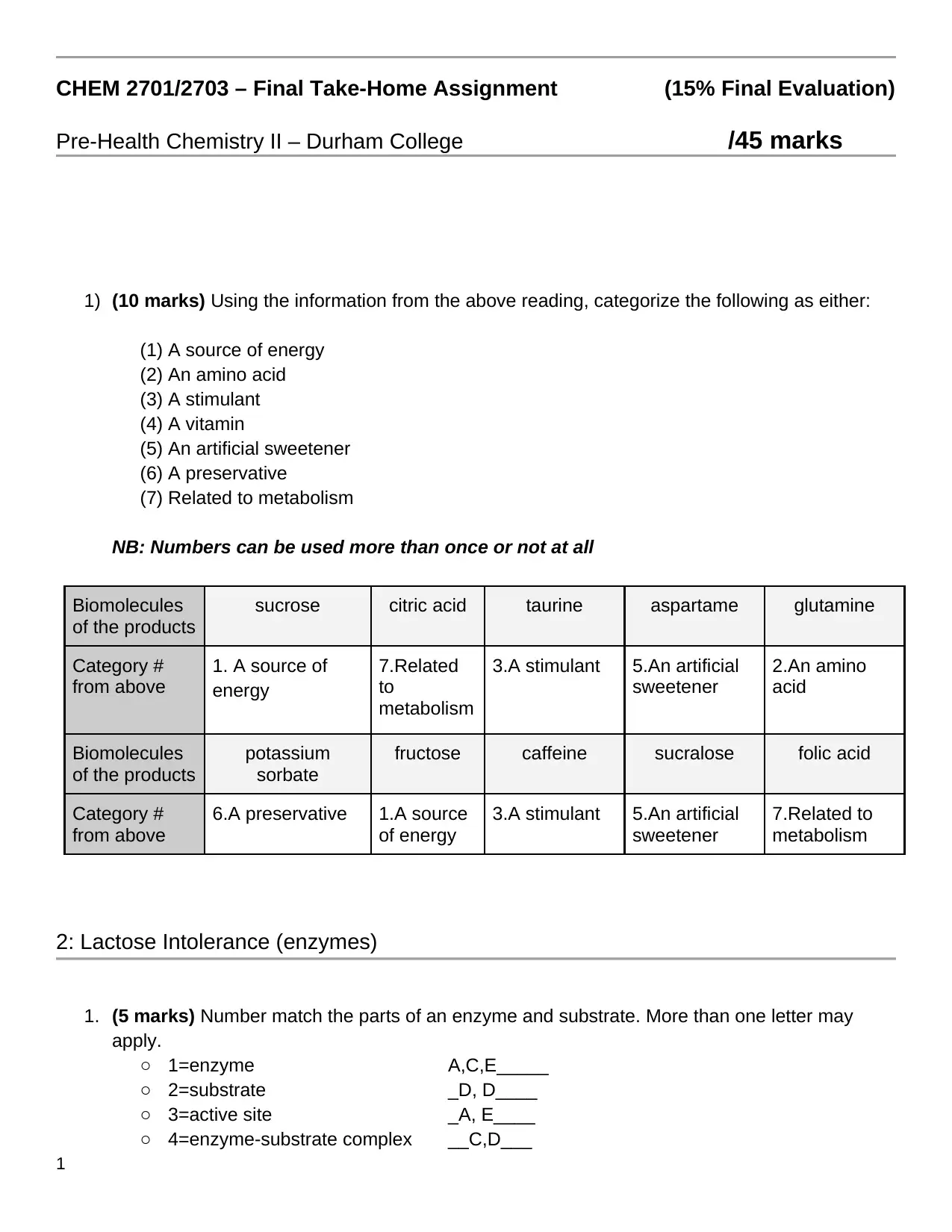
3. (3 marks) Multiselect: Choose the following that are part of the DNA structure.
❏ Single stranded
❏ Double stranded
❏ Contains a nucleotide
❏ Sugar-phosphate backbone
❏ Contains ribose
❏ Contains deoxyribose
5: Amino Acids and Protein Structure
(proteins)
1. (3 marks) Multiselect: Choose all parts that make up an
amino acid:
❏ alpha amino group
❏ nitrogenous base
❏ r group
❏ fatty acid
❏ 4 fused cyclical rings
❏ disaccharide
❏ phosphate
❏ active site
❏ acid group
❏ glycoside linkage
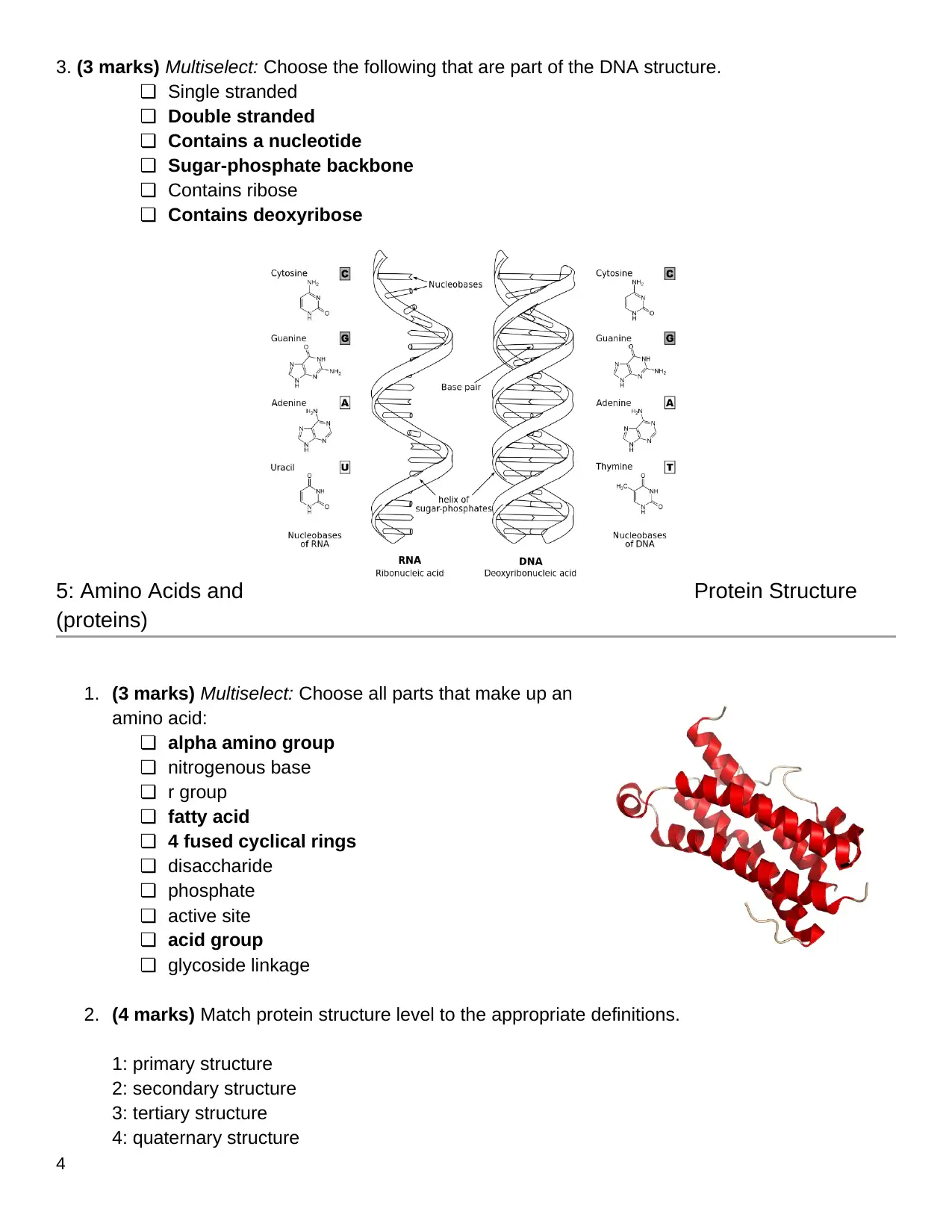
2. (4 marks) Match protein structure level to the appropriate definitions.
1: primary structure
2: secondary structure
3: tertiary structure
4: quaternary structure
4
❏ Single stranded
❏ Double stranded
❏ Contains a nucleotide
❏ Sugar-phosphate backbone
❏ Contains ribose
❏ Contains deoxyribose
5: Amino Acids and Protein Structure
(proteins)
1. (3 marks) Multiselect: Choose all parts that make up an
amino acid:
❏ alpha amino group
❏ nitrogenous base
❏ r group
❏ fatty acid
❏ 4 fused cyclical rings
❏ disaccharide
❏ phosphate
❏ active site
❏ acid group
❏ glycoside linkage
2. (4 marks) Match protein structure level to the appropriate definitions.
1: primary structure
2: secondary structure
3: tertiary structure
4: quaternary structure
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2 Alpha helix and beta sheets
3 3-dimensional folding of a protein
1 Linear sequence of amino acids
4 Protein consisting of more than 1 amino acid chain
3. (1 mark) Explain what happens to the structure of a protein if there is heat applied to it or a pH
change has occurred.
The heat and pH increase is able to disrupt the hydrogen and and the non-polar hydrophobic
interactions. This increases the kinetic energy and therefore causing the molecule to vibrate the bond.
5
3 3-dimensional folding of a protein
1 Linear sequence of amino acids
4 Protein consisting of more than 1 amino acid chain
3. (1 mark) Explain what happens to the structure of a protein if there is heat applied to it or a pH
change has occurred.
The heat and pH increase is able to disrupt the hydrogen and and the non-polar hydrophobic
interactions. This increases the kinetic energy and therefore causing the molecule to vibrate the bond.
5
1 out of 5
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





