Biodiesel Production: Reacting System Analysis - Student University
VerifiedAdded on 2023/06/13
|10
|1419
|81
Report
AI Summary
This report details an investigation into biodiesel production through transesterification, focusing on the analysis of reacting systems. The study utilizes karanja oil, methanol, and sodium hydroxide as a catalyst, examining the impact of various parameters such as mole ratio of methanol to oil, catalyst concentration, reaction temperature, and reaction time on biodiesel yield. Experiments were conducted using both batch and Continuous Stirred Tank Reactors (CSTR), with detailed methodologies outlined for each. The results indicate optimal conditions for maximizing biodiesel yield in both reactor types, with specific recommendations for catalyst concentration, temperature, and flow rates. The report concludes that continuous reactors like helical tube and RD reactors are preferable for biodiesel manufacturing due to their efficiency in alcohol usage. Desklib provides access to this and other solved assignments for students.
REACTING SYSTEM
[Student University]
[Student Name]
[Adm Number]
[Date]
[Student University]
[Student Name]
[Adm Number]
[Date]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
INTRODUCTION
According to (Li et al. 2005), the biodiesel is perceived as the best substitute of diesel oil because they have the same
properties, them being renewable energy. The biodiesel has many advantages in terms of usage since its environmental
friendly.
The most common way of producing the biodiesel is through the what we call transesterification which in this case refers
to the process of catalyzing chemical reaction which involves the oil from vegetables and also alcohol for the purpose of
vintage fatty acids methyl esters. According to (Freedman, Butterfield, and Pyrde 1986),It is worthy noting that the whole
process of transesterification reactions might be catalyzed with alkaline, acid or with enzymes. The process of
transesterification can be reversed and its completion process takes note of multiples parameters which in this case
includes the mole ratio of alcohol to the oil, catalysts, reaction temperature and time and the Free fatty acids .
According to (Li et al. 2005), the biodiesel is perceived as the best substitute of diesel oil because they have the same
properties, them being renewable energy. The biodiesel has many advantages in terms of usage since its environmental
friendly.
The most common way of producing the biodiesel is through the what we call transesterification which in this case refers
to the process of catalyzing chemical reaction which involves the oil from vegetables and also alcohol for the purpose of
vintage fatty acids methyl esters. According to (Freedman, Butterfield, and Pyrde 1986),It is worthy noting that the whole
process of transesterification reactions might be catalyzed with alkaline, acid or with enzymes. The process of
transesterification can be reversed and its completion process takes note of multiples parameters which in this case
includes the mole ratio of alcohol to the oil, catalysts, reaction temperature and time and the Free fatty acids .

MATERIALS AND METHODS
For this purpose, will use the following materials in the production of biodiesel, this includes
karanja oil from india , Methanol and alkaline of sodium hydroxide as a catalyst.
The karanja oil has all the traits which affects the transesterification process which includes
viscocity, density,acid value, saponification value and the FFA content.
The composition of the fatty acids and the diesel purity which was produced was then resoluted by
the use of the gas chromatograph of the DANI which was a make of DN biodiesel.
The output of the diesel was then calculated in relation to the quantity of the methyl esters which
was acquired over the unit quantity of the oil which was pretreated.
For this purpose, will use the following materials in the production of biodiesel, this includes
karanja oil from india , Methanol and alkaline of sodium hydroxide as a catalyst.
The karanja oil has all the traits which affects the transesterification process which includes
viscocity, density,acid value, saponification value and the FFA content.
The composition of the fatty acids and the diesel purity which was produced was then resoluted by
the use of the gas chromatograph of the DANI which was a make of DN biodiesel.
The output of the diesel was then calculated in relation to the quantity of the methyl esters which
was acquired over the unit quantity of the oil which was pretreated.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Cont’d
For Karanja oil, 750g of the oil was used and preheated at a temperature of 65 degrees Celsius. Methanol
with molar ratio of 5:1 was added alongside 2% concentrated Sulphuric acid when the temperature reached
65 degrees.
The temperature was the maintained at 65 degrees Celsius while stirring continues. In order to make
methanol vapor not to evaporate, reflux condenser was used in that case.
The mixture was then allowed for 2hours to completely react with the methanol with has alcohol content on
a molar ratio of 5:1. the acid value of 0.5-2mg NaOH/g was then calculated with an intention of checking if
at the solution is within the limit which is prescribed. The sample calculated is then used for reaction of
base catalyzed transesterification in both the batch and the CSTR reactors.
For Karanja oil, 750g of the oil was used and preheated at a temperature of 65 degrees Celsius. Methanol
with molar ratio of 5:1 was added alongside 2% concentrated Sulphuric acid when the temperature reached
65 degrees.
The temperature was the maintained at 65 degrees Celsius while stirring continues. In order to make
methanol vapor not to evaporate, reflux condenser was used in that case.
The mixture was then allowed for 2hours to completely react with the methanol with has alcohol content on
a molar ratio of 5:1. the acid value of 0.5-2mg NaOH/g was then calculated with an intention of checking if
at the solution is within the limit which is prescribed. The sample calculated is then used for reaction of
base catalyzed transesterification in both the batch and the CSTR reactors.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
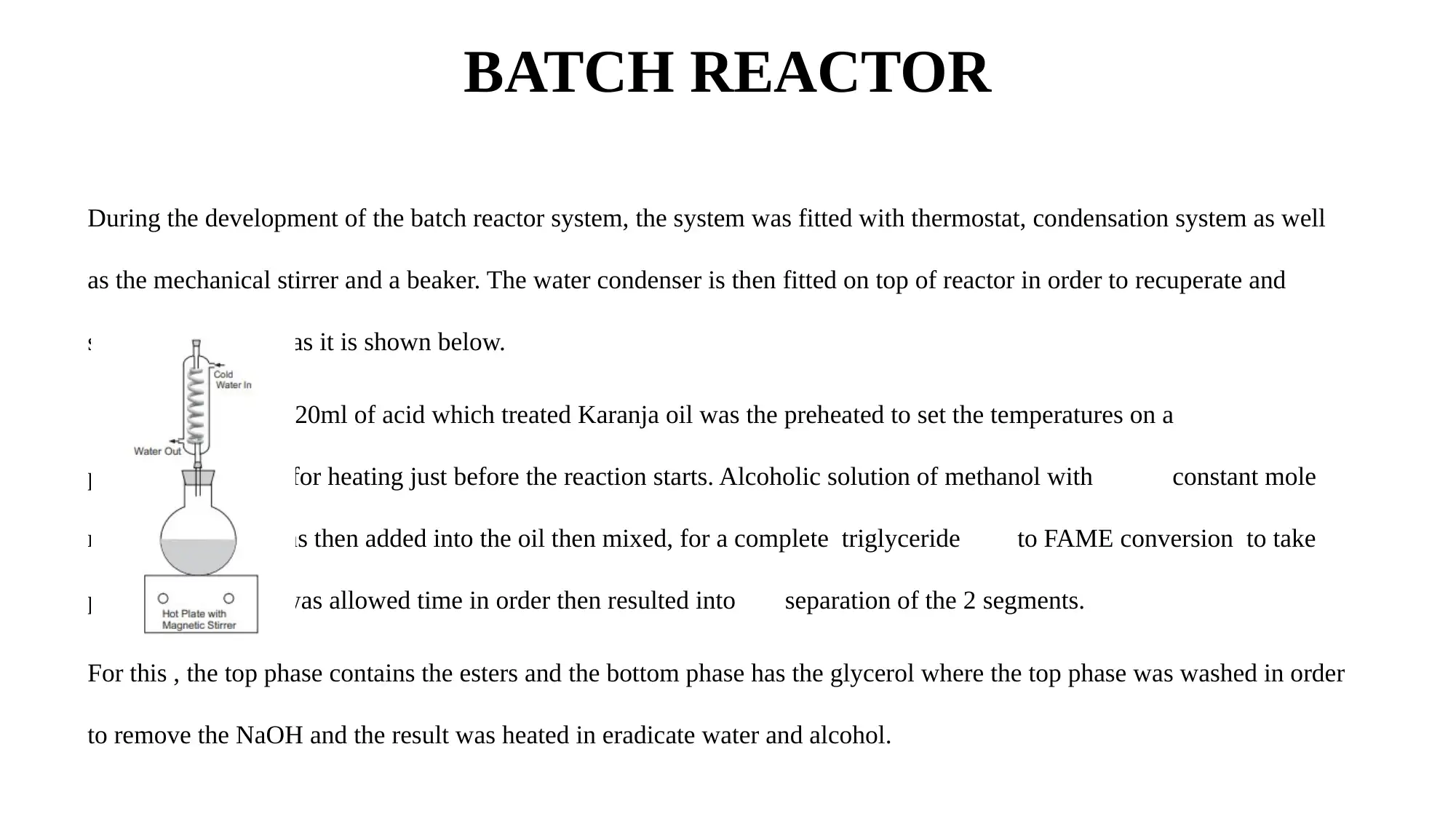
BATCH REACTOR
During the development of the batch reactor system, the system was fitted with thermostat, condensation system as well
as the mechanical stirrer and a beaker. The water condenser is then fitted on top of reactor in order to recuperate and
salvage the alcohol as it is shown below.
Averagely, 120ml of acid which treated Karanja oil was the preheated to set the temperatures on a
plate which is used for heating just before the reaction starts. Alcoholic solution of methanol with constant mole
ratio with NaOH was then added into the oil then mixed, for a complete triglyceride to FAME conversion to take
place, the solution was allowed time in order then resulted into separation of the 2 segments.
For this , the top phase contains the esters and the bottom phase has the glycerol where the top phase was washed in order
to remove the NaOH and the result was heated in eradicate water and alcohol.
During the development of the batch reactor system, the system was fitted with thermostat, condensation system as well
as the mechanical stirrer and a beaker. The water condenser is then fitted on top of reactor in order to recuperate and
salvage the alcohol as it is shown below.
Averagely, 120ml of acid which treated Karanja oil was the preheated to set the temperatures on a
plate which is used for heating just before the reaction starts. Alcoholic solution of methanol with constant mole
ratio with NaOH was then added into the oil then mixed, for a complete triglyceride to FAME conversion to take
place, the solution was allowed time in order then resulted into separation of the 2 segments.
For this , the top phase contains the esters and the bottom phase has the glycerol where the top phase was washed in order
to remove the NaOH and the result was heated in eradicate water and alcohol.
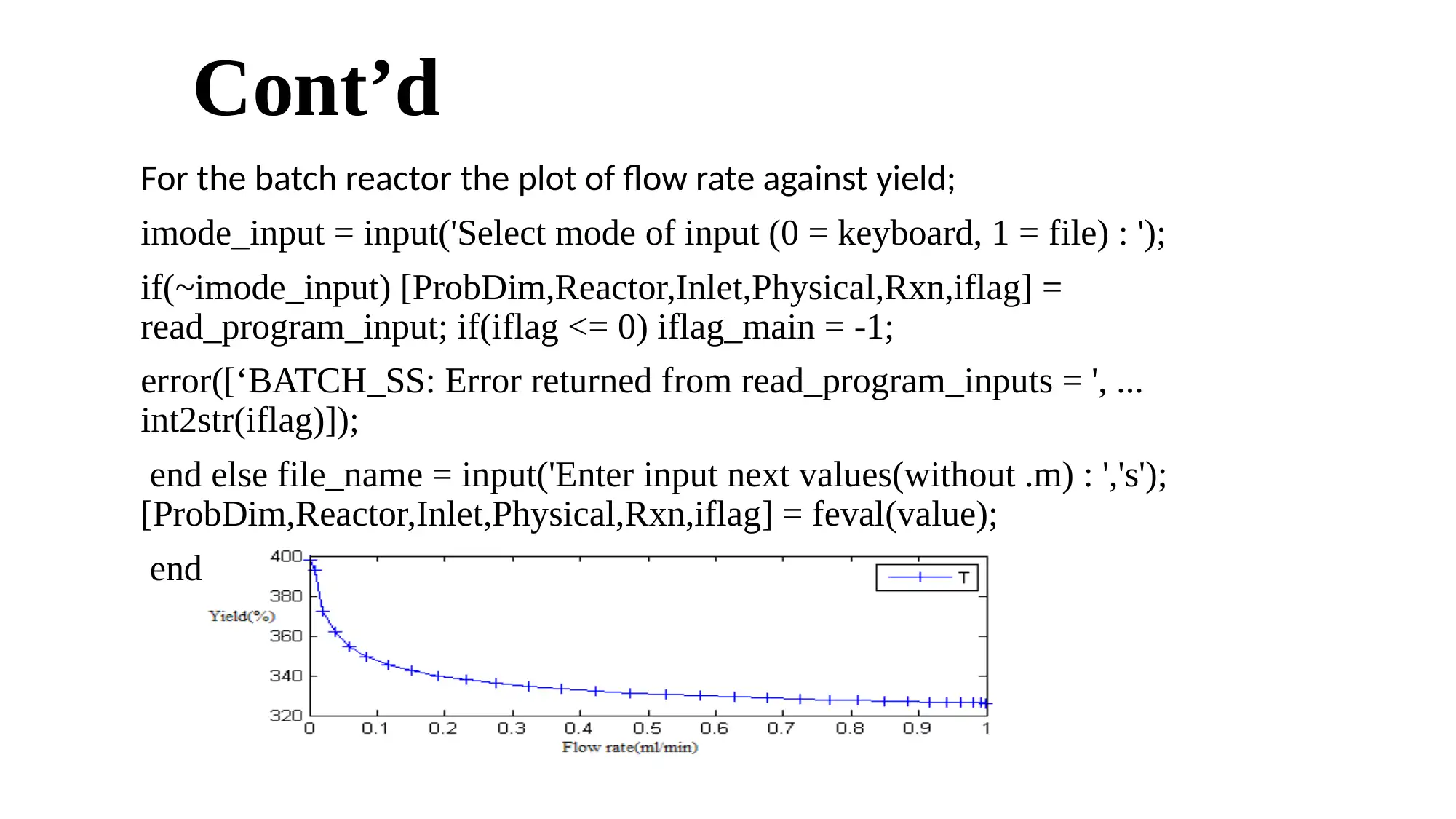
Cont’d
For the batch reactor the plot of flow rate against yield;
imode_input = input('Select mode of input (0 = keyboard, 1 = file) : ');
if(~imode_input) [ProbDim,Reactor,Inlet,Physical,Rxn,iflag] =
read_program_input; if(iflag <= 0) iflag_main = -1;
error([‘BATCH_SS: Error returned from read_program_inputs = ', ...
int2str(iflag)]);
end else file_name = input('Enter input next values(without .m) : ','s');
[ProbDim,Reactor,Inlet,Physical,Rxn,iflag] = feval(value);
end
For the batch reactor the plot of flow rate against yield;
imode_input = input('Select mode of input (0 = keyboard, 1 = file) : ');
if(~imode_input) [ProbDim,Reactor,Inlet,Physical,Rxn,iflag] =
read_program_input; if(iflag <= 0) iflag_main = -1;
error([‘BATCH_SS: Error returned from read_program_inputs = ', ...
int2str(iflag)]);
end else file_name = input('Enter input next values(without .m) : ','s');
[ProbDim,Reactor,Inlet,Physical,Rxn,iflag] = feval(value);
end
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
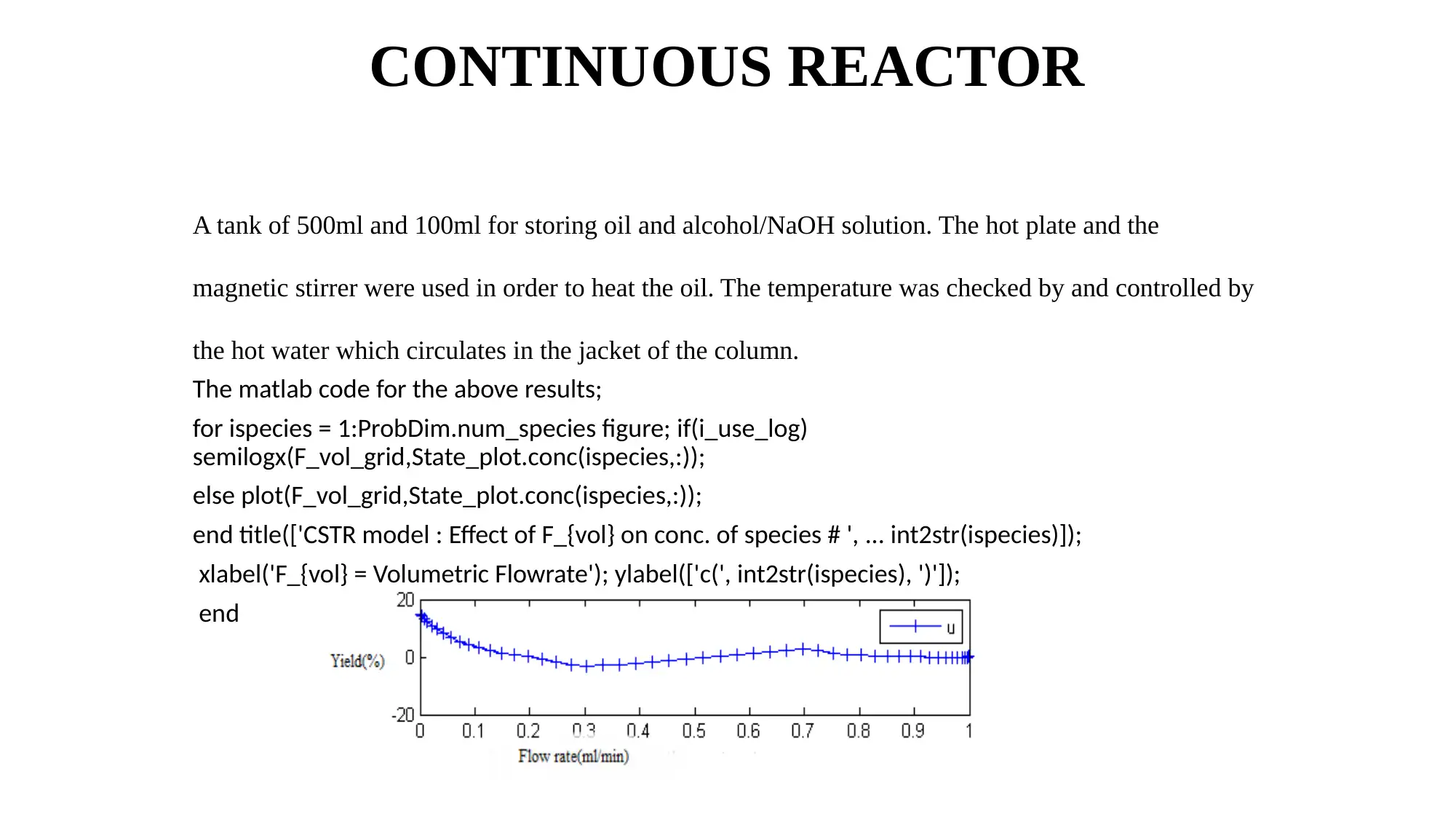
CONTINUOUS REACTOR
A tank of 500ml and 100ml for storing oil and alcohol/NaOH solution. The hot plate and the
magnetic stirrer were used in order to heat the oil. The temperature was checked by and controlled by
the hot water which circulates in the jacket of the column.
The matlab code for the above results;
for ispecies = 1:ProbDim.num_species figure; if(i_use_log)
semilogx(F_vol_grid,State_plot.conc(ispecies,:));
else plot(F_vol_grid,State_plot.conc(ispecies,:));
end title(['CSTR model : Effect of F_{vol} on conc. of species # ', ... int2str(ispecies)]);
xlabel('F_{vol} = Volumetric Flowrate'); ylabel(['c(', int2str(ispecies), ')']);
end
A tank of 500ml and 100ml for storing oil and alcohol/NaOH solution. The hot plate and the
magnetic stirrer were used in order to heat the oil. The temperature was checked by and controlled by
the hot water which circulates in the jacket of the column.
The matlab code for the above results;
for ispecies = 1:ProbDim.num_species figure; if(i_use_log)
semilogx(F_vol_grid,State_plot.conc(ispecies,:));
else plot(F_vol_grid,State_plot.conc(ispecies,:));
end title(['CSTR model : Effect of F_{vol} on conc. of species # ', ... int2str(ispecies)]);
xlabel('F_{vol} = Volumetric Flowrate'); ylabel(['c(', int2str(ispecies), ')']);
end
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

RESULTS AND DISCUSSION
From the properties of Karanja oil, the acid content is enough to be trans esterified using the base catalyst.
Some of the parameters which are affecting the biodiesel yield includes the mole ratio of methanol to oil,
concentration of the catalyst, the temperature reaction and the time of the reaction
When the catalyst concentration increases to 0.7%, the yield in biodiesel also increases to 94% though
further increase in concentration decrease the biodiesel yield.
High yield is witnessed when the viscosity of the is low with the mole ratio of 7:1
The yield increased when the temperature increased though the yield remained constant when the reaction
time was from 60mins.
From the properties of Karanja oil, the acid content is enough to be trans esterified using the base catalyst.
Some of the parameters which are affecting the biodiesel yield includes the mole ratio of methanol to oil,
concentration of the catalyst, the temperature reaction and the time of the reaction
When the catalyst concentration increases to 0.7%, the yield in biodiesel also increases to 94% though
further increase in concentration decrease the biodiesel yield.
High yield is witnessed when the viscosity of the is low with the mole ratio of 7:1
The yield increased when the temperature increased though the yield remained constant when the reaction
time was from 60mins.

CONCLUSION
The whole process of transesterification took place at very much separate investigation parameters in both the batch and
the CSTR. As far as the experiments are concerned, the following crucial conclusions are therefore recommend;
The batch reactor can operate suitably under a state of 7:1 which is mole ratio of methanol to oil, the concentration od
the catalyst to be 0.7 and a creation temperature of 65 degrees Celsius, and finally the reaction time is one hour and the
highest yield which is found is 94%.
For the CSTR, the highest yield obtained is 91.8% at a methanol to oil mole ratio of 7:1, same 0.7% concentration of
catalyst , same 65 degrees of temperature with 6.0ml/min oil flow rate.
Therefore the continuous reactors like the helical tube and the RD are the ones which are therefore recommended for the
manufacture of the biodiesel though in main cases the RD is mostly chosen is less alcohol is needed in order to
distinguish the excess alcohol. This is done in the same unit then reusing back to the column.
The whole process of transesterification took place at very much separate investigation parameters in both the batch and
the CSTR. As far as the experiments are concerned, the following crucial conclusions are therefore recommend;
The batch reactor can operate suitably under a state of 7:1 which is mole ratio of methanol to oil, the concentration od
the catalyst to be 0.7 and a creation temperature of 65 degrees Celsius, and finally the reaction time is one hour and the
highest yield which is found is 94%.
For the CSTR, the highest yield obtained is 91.8% at a methanol to oil mole ratio of 7:1, same 0.7% concentration of
catalyst , same 65 degrees of temperature with 6.0ml/min oil flow rate.
Therefore the continuous reactors like the helical tube and the RD are the ones which are therefore recommended for the
manufacture of the biodiesel though in main cases the RD is mostly chosen is less alcohol is needed in order to
distinguish the excess alcohol. This is done in the same unit then reusing back to the column.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

REFERENCES
Gillespie, D. T. (2001). Approximate accelerated stochastic simulation of
chemically reacting systems. The Journal of Chemical Physics, 115(4), 1716-1733.
Rathinam, M., Petzold, L. R., Cao, Y., & Gillespie, D. T. (2003). Stiffness in
stochastic chemically reacting systems: The implicit tau-leaping method. The
Journal of Chemical Physics, 119(24), 12784-12794.
Aksoy, L. 2011. Opium poppy (papaver somniferum L.) oil for preparation of
biodiesel: Optimization of conditions. Applied Energy 88:4713–4718.
Al-Widyan, M. I., and A. O. Al-Shyoukh. 2002. Experimental evaluation of the
transesterification of waste palm oil into biodiesel. Bioresource Technology
85:253–56.
Al-Zuhair, S. 2007. Production of biodiesel: Possibilities and challenges. Biofuels,
Bioproducts and Biorefining 1:57–66.
Burton, R., F. A. N. Xiaohu, and G. Austic. 2010. Evaluation of two-step reaction
and enzyme catalysis approaches for biodiesel production from spent coffee
grounds. International Journal of Green Energy 7:530–36
Gillespie, D. T. (2001). Approximate accelerated stochastic simulation of
chemically reacting systems. The Journal of Chemical Physics, 115(4), 1716-1733.
Rathinam, M., Petzold, L. R., Cao, Y., & Gillespie, D. T. (2003). Stiffness in
stochastic chemically reacting systems: The implicit tau-leaping method. The
Journal of Chemical Physics, 119(24), 12784-12794.
Aksoy, L. 2011. Opium poppy (papaver somniferum L.) oil for preparation of
biodiesel: Optimization of conditions. Applied Energy 88:4713–4718.
Al-Widyan, M. I., and A. O. Al-Shyoukh. 2002. Experimental evaluation of the
transesterification of waste palm oil into biodiesel. Bioresource Technology
85:253–56.
Al-Zuhair, S. 2007. Production of biodiesel: Possibilities and challenges. Biofuels,
Bioproducts and Biorefining 1:57–66.
Burton, R., F. A. N. Xiaohu, and G. Austic. 2010. Evaluation of two-step reaction
and enzyme catalysis approaches for biodiesel production from spent coffee
grounds. International Journal of Green Energy 7:530–36
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

