BIOL214 Scientific Report: Spectroscopic Analysis of TCA Cycle Enzymes
VerifiedAdded on 2023/06/07
|11
|2156
|435
Report
AI Summary
This report details an experiment using spectroscopic methods to analyze the activity of malate dehydrogenase and succinate dehydrogenase, key enzymes in the Tricarboxylic Acid (TCA) cycle. The experiment measured the absorbance of these enzymes in mitochondrial and microsomal fractions of yeast cells, correlating the rate of NADH oxidation with reaction rates. Results, presented as absorbance versus time graphs, indicated enzyme activity in both fractions. The discussion interprets these findings in the context of existing literature, emphasizing the role of DCPIP and PMS in the succinate dehydrogenase assay and explaining the principles behind the spectrophotometric measurements. The report concludes by highlighting the importance of enzyme localization in cellular metabolism and the potential sources of error in the experimental procedure, emphasizing the need for precise dilutions and temperature control. Desklib provides access to similar reports and solved assignments for students.

Enzymes of the TCA cycle 1
ENZYMES OF THE TCA CYCLE
Name:
Department:
School:
Course:
Date:
ENZYMES OF THE TCA CYCLE
Name:
Department:
School:
Course:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Enzymes of the TCA cycle 2
Abstract
The Tricarboxylic acid (TCA) cycle is a major metabolic pathway accountable for
providing reduction potential for oxidative phosphorylation and anabolic substrates or cell repair,
growth and proliferation. The following experiment used a spectroscopic method of analysis to
measure the absorbance of malate and succinate dehydrogenase assay. The rate of decrease in
the absorbance was due to NADH oxidation which was the measure of the reaction rates.
Throughout the experiment, the fractions were stored on ice to avoid degradation. It is worth
noting, the rate of decrease or absence of substrates provided the black. Then, after the results of
the absorbance against the concentration over time were plotted, a linear graph with a negative
gradient was obtained.
Abstract
The Tricarboxylic acid (TCA) cycle is a major metabolic pathway accountable for
providing reduction potential for oxidative phosphorylation and anabolic substrates or cell repair,
growth and proliferation. The following experiment used a spectroscopic method of analysis to
measure the absorbance of malate and succinate dehydrogenase assay. The rate of decrease in
the absorbance was due to NADH oxidation which was the measure of the reaction rates.
Throughout the experiment, the fractions were stored on ice to avoid degradation. It is worth
noting, the rate of decrease or absence of substrates provided the black. Then, after the results of
the absorbance against the concentration over time were plotted, a linear graph with a negative
gradient was obtained.

Enzymes of the TCA cycle 3
Introduction
The Krebs cycles enzymes are membranes proteins found within the matrix of the
mitochondrial except for succinate dehydrogenase which is essential membrane protein locked to
the inner mitochondrial membrane (Chandel 2015, pp. 204). Acetyl-CoA joins with oxaloacetate
by citrate synthase, to create a 6-C molecule. Therefore, the compound releases citric acid from
the enzyme complex. The fragment of water moves from the third position on the citric acid
molecule and add to the fourth position by the enzyme aconitase resulting in isocitrate. Isocitrate
dehydrogenase compound catalysis the oxidation of the fourth position of OH group of isocitrate,
to produce alpha-ketoglutarate where one NAD molecule changes to NADH. Decarboxylation
happens to the alpha-ketoglutarate, changing another molecule of NAD to NADH, by alpha-
ketoglutarate dehydrogenase, producing succinyl CoA which is an unstable molecule (Intlekofer
et al. 2015, pp. 305). Succinyl-CoA synthesises the addition of a free phosphate group to
guanosine diphosphate, generating guanosine triphosphate. Thus, in the course, the CoA group
releases from succinyl-CoA, and the resulting molecule is succinate (Shi and Tu 2015, pp.127).
The release of two hydrogen atom from succinate occurs when the succinate dehydrogenase
reduces FAD to form FADH2, where the yield of the reaction builds fumarate (Ferro, Rodrigues
and De Souza 2015, pp. 258). The final result of the cycle comprises regeneration of
oxaloacetate by oxidation of L-malate by malate dehydrogenase where the conversion of one of
the molecules of NAD to NADH (West et al. 2015, pp. 553).
For that reason, this report aims to determine dehydrogenase activity utilising artificial
oxidate such as dichlorophenolindophenol (DCPIP) for the assay. The paper assess the succinate
dehydrogenase distribution between the microsomal (microsomes and cytosol) and mitochondria
fractions. Finally, the report illustrate that two forms of malate dehydrogenase are present in
Introduction
The Krebs cycles enzymes are membranes proteins found within the matrix of the
mitochondrial except for succinate dehydrogenase which is essential membrane protein locked to
the inner mitochondrial membrane (Chandel 2015, pp. 204). Acetyl-CoA joins with oxaloacetate
by citrate synthase, to create a 6-C molecule. Therefore, the compound releases citric acid from
the enzyme complex. The fragment of water moves from the third position on the citric acid
molecule and add to the fourth position by the enzyme aconitase resulting in isocitrate. Isocitrate
dehydrogenase compound catalysis the oxidation of the fourth position of OH group of isocitrate,
to produce alpha-ketoglutarate where one NAD molecule changes to NADH. Decarboxylation
happens to the alpha-ketoglutarate, changing another molecule of NAD to NADH, by alpha-
ketoglutarate dehydrogenase, producing succinyl CoA which is an unstable molecule (Intlekofer
et al. 2015, pp. 305). Succinyl-CoA synthesises the addition of a free phosphate group to
guanosine diphosphate, generating guanosine triphosphate. Thus, in the course, the CoA group
releases from succinyl-CoA, and the resulting molecule is succinate (Shi and Tu 2015, pp.127).
The release of two hydrogen atom from succinate occurs when the succinate dehydrogenase
reduces FAD to form FADH2, where the yield of the reaction builds fumarate (Ferro, Rodrigues
and De Souza 2015, pp. 258). The final result of the cycle comprises regeneration of
oxaloacetate by oxidation of L-malate by malate dehydrogenase where the conversion of one of
the molecules of NAD to NADH (West et al. 2015, pp. 553).
For that reason, this report aims to determine dehydrogenase activity utilising artificial
oxidate such as dichlorophenolindophenol (DCPIP) for the assay. The paper assess the succinate
dehydrogenase distribution between the microsomal (microsomes and cytosol) and mitochondria
fractions. Finally, the report illustrate that two forms of malate dehydrogenase are present in
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Enzymes of the TCA cycle 4
yeast cells, one formation predominately in the mitochondria and other in the cytosol. In this
experiment, yeast (Saccharomyces cerevisiae) culture is grown, harvested, and disrupted in a
French press. Then, fractionation of homogenate into microsomal and mitochondrial will result,
whereby the fraction will be subdivided into small aliquots, snap frozen and kept in liquid
nitrogen, to avoid rapid degradation.
For both assays, we will utilise the spectroscopic method of analysis at the absorbance
wavelength of 340nm and 600 nm for the malate dehydrogenase and succinate dehydrogenase
respectively. For the malate dehydrogenase, one will use 4mg/ml of NADH, 50mM phosphate
buffer of pH 7.4 and 1.3mg/ml oxaloacetate. On the side of the succinate dehydrogenase, 50mM
phosphate buffer, 50 ml of 1.5mM DCPIP, 20ml of 12.5mM phenazine methosulphate, 30ml of
20mM KCN, and finally, subcellular fractions of mitochondrial and diluted fraction
mitochondrial fractions and microsomal is used.
yeast cells, one formation predominately in the mitochondria and other in the cytosol. In this
experiment, yeast (Saccharomyces cerevisiae) culture is grown, harvested, and disrupted in a
French press. Then, fractionation of homogenate into microsomal and mitochondrial will result,
whereby the fraction will be subdivided into small aliquots, snap frozen and kept in liquid
nitrogen, to avoid rapid degradation.
For both assays, we will utilise the spectroscopic method of analysis at the absorbance
wavelength of 340nm and 600 nm for the malate dehydrogenase and succinate dehydrogenase
respectively. For the malate dehydrogenase, one will use 4mg/ml of NADH, 50mM phosphate
buffer of pH 7.4 and 1.3mg/ml oxaloacetate. On the side of the succinate dehydrogenase, 50mM
phosphate buffer, 50 ml of 1.5mM DCPIP, 20ml of 12.5mM phenazine methosulphate, 30ml of
20mM KCN, and finally, subcellular fractions of mitochondrial and diluted fraction
mitochondrial fractions and microsomal is used.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Enzymes of the TCA cycle 5
Results
0 2 4 6 8 10 12
0
2
4
6
8
10
12
f(x) = NaN x + NaNf(x) = NaN x + NaN
concentration over time (min)
Absorbance at 340
mitochondrial fraction
microsomal fraction
Figure 1: Absorbance (340nm) vs. concentration over time (minutes), spectroscopic method of
analysis at the absorbance wavelength of 340nm for the malate dehydrogenase, one will use
4mg/ml of NADH, 50mM phosphate buffer of pH 7.4 and 1.3mg/ml oxaloacetate.
Results
0 2 4 6 8 10 12
0
2
4
6
8
10
12
f(x) = NaN x + NaNf(x) = NaN x + NaN
concentration over time (min)
Absorbance at 340
mitochondrial fraction
microsomal fraction
Figure 1: Absorbance (340nm) vs. concentration over time (minutes), spectroscopic method of
analysis at the absorbance wavelength of 340nm for the malate dehydrogenase, one will use
4mg/ml of NADH, 50mM phosphate buffer of pH 7.4 and 1.3mg/ml oxaloacetate.
Enzymes of the TCA cycle 6
0 5 10 15 20 25
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
f(x) = − 0.00645307692307692 x + 0.77889
f(x) = − 0.00608461538461539 x + 0.85058
concentration over time (min)
absorbance at 600
mitochondrial
fraction
microsomal fraction
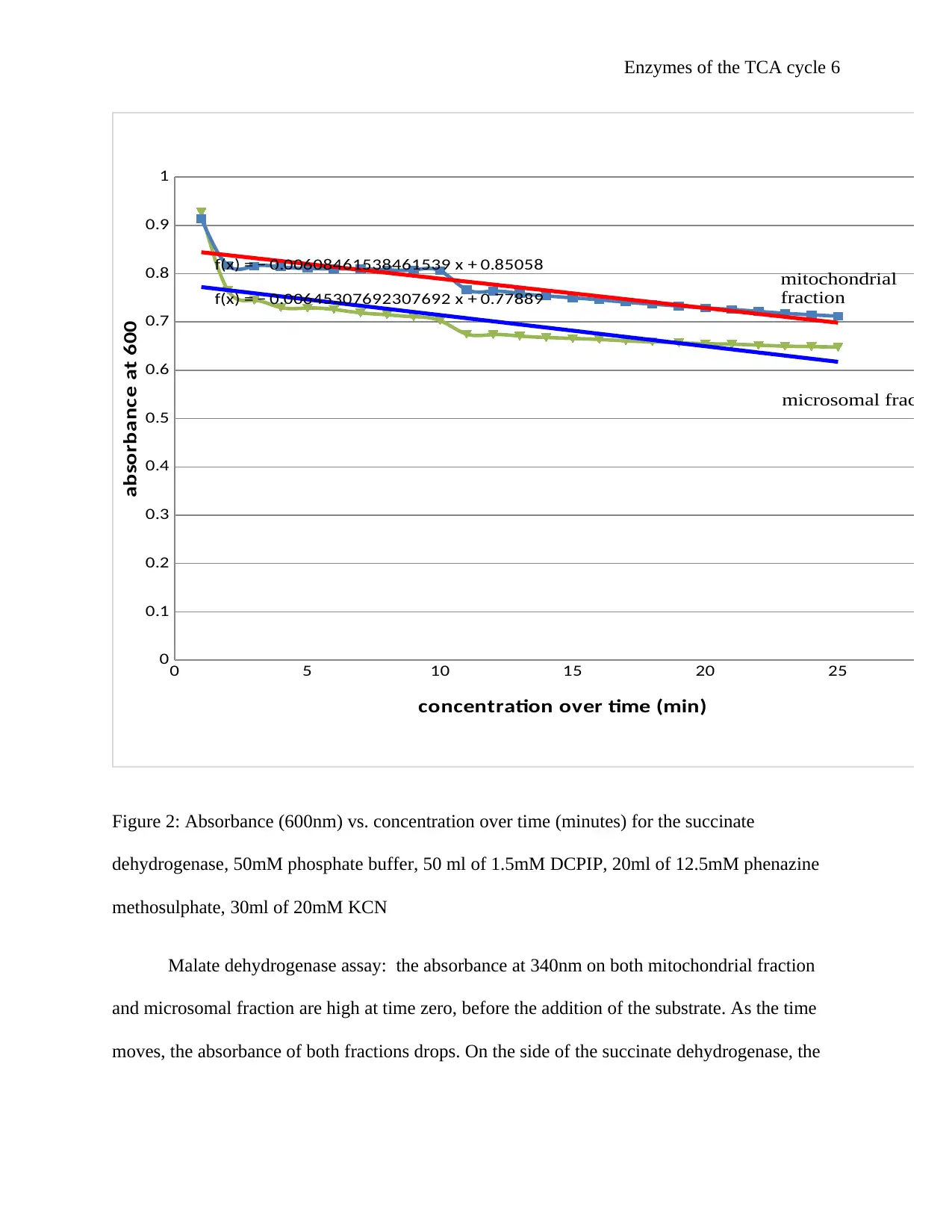
Figure 2: Absorbance (600nm) vs. concentration over time (minutes) for the succinate
dehydrogenase, 50mM phosphate buffer, 50 ml of 1.5mM DCPIP, 20ml of 12.5mM phenazine
methosulphate, 30ml of 20mM KCN
Malate dehydrogenase assay: the absorbance at 340nm on both mitochondrial fraction
and microsomal fraction are high at time zero, before the addition of the substrate. As the time
moves, the absorbance of both fractions drops. On the side of the succinate dehydrogenase, the
0 5 10 15 20 25
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
f(x) = − 0.00645307692307692 x + 0.77889
f(x) = − 0.00608461538461539 x + 0.85058
concentration over time (min)
absorbance at 600
mitochondrial
fraction
microsomal fraction
Figure 2: Absorbance (600nm) vs. concentration over time (minutes) for the succinate
dehydrogenase, 50mM phosphate buffer, 50 ml of 1.5mM DCPIP, 20ml of 12.5mM phenazine
methosulphate, 30ml of 20mM KCN
Malate dehydrogenase assay: the absorbance at 340nm on both mitochondrial fraction
and microsomal fraction are high at time zero, before the addition of the substrate. As the time
moves, the absorbance of both fractions drops. On the side of the succinate dehydrogenase, the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Enzymes of the TCA cycle 7
scenario is the same as one for the malate dehydrogenase; the absorbance decreases with the
addition of the substrate.
Discussion
The reduction of oxaloacetate by NADH can be used as the measurement of malate
dehydrogenase (Martínez-Reyes et al. 2016, pp. 200). It is worth mentioning that the reaction of
oxalate and NADH in the presence of hydrogen ions lies far to the right. At a neutral pH and in a
slight excess of NADH, the reaction tends to be rapid, and oxaloacetate is quantitatively
converted to malate. Therefore, the rate of decrease in absorbance at the 340 can be accounted on
the NADH oxidation which entails the measurement of the speed of the reaction. It is also worth
noting that the mitochondrial fraction will comprise some NADH oxidase activity (West et al.
2015). A dilute mitochondrial fraction is used for this essay as the activity of malate
dehydrogenase is relatively high. Thus, the NADH oxidase activity under the above conditions is
low and does not typically interfere with the assay.
DCPIP and PMS can be reduced under an anaerobic condition to stable forms in the
presence of oxygen. PMS acts as intermediary electron carrier where succinate is the substrate.
Succinate dehydrogenases catalysis the succinate oxidation to fumarate with the electrons passed
to the oxidised DCPIP (Birsoy et al. 2015, pp. 540). The disappearance of oxidised DCPIP was
followed spectrophotometrically at 600 nm, which represent the decrease in the absorbance in
the above graph. Therefore, the above statement fulfils the objective of an experiment which
assesses the succinate dehydrogenase dispersion between the microsomal and mitochondria.
Conclusion
scenario is the same as one for the malate dehydrogenase; the absorbance decreases with the
addition of the substrate.
Discussion
The reduction of oxaloacetate by NADH can be used as the measurement of malate
dehydrogenase (Martínez-Reyes et al. 2016, pp. 200). It is worth mentioning that the reaction of
oxalate and NADH in the presence of hydrogen ions lies far to the right. At a neutral pH and in a
slight excess of NADH, the reaction tends to be rapid, and oxaloacetate is quantitatively
converted to malate. Therefore, the rate of decrease in absorbance at the 340 can be accounted on
the NADH oxidation which entails the measurement of the speed of the reaction. It is also worth
noting that the mitochondrial fraction will comprise some NADH oxidase activity (West et al.
2015). A dilute mitochondrial fraction is used for this essay as the activity of malate
dehydrogenase is relatively high. Thus, the NADH oxidase activity under the above conditions is
low and does not typically interfere with the assay.
DCPIP and PMS can be reduced under an anaerobic condition to stable forms in the
presence of oxygen. PMS acts as intermediary electron carrier where succinate is the substrate.
Succinate dehydrogenases catalysis the succinate oxidation to fumarate with the electrons passed
to the oxidised DCPIP (Birsoy et al. 2015, pp. 540). The disappearance of oxidised DCPIP was
followed spectrophotometrically at 600 nm, which represent the decrease in the absorbance in
the above graph. Therefore, the above statement fulfils the objective of an experiment which
assesses the succinate dehydrogenase dispersion between the microsomal and mitochondria.
Conclusion
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Enzymes of the TCA cycle 8
Yeasts are ubiquitous unicellular fungi widespread in natural environment colonising
from terrestrial, aerial to aquatic surrounding, where the active colonisation is intimately
connected to their physiological adaptability to an extremely variable atmosphere. In yeast, just
like any other heterotrophic organism, the carbon and energy metabolism are inextricably
interlinked. ATP is offered by the organic molecules oxidation that also acts as carbon sources
for biosynthesis, and ultimately it is used as energy for all sorts of cellular work. All enzymes are
localised with one or more particular compartment of the cells. Two significant tactics can be
employed to assess the enzymes localization; cell disruption to release the intact organelles, and
preservation of the entire cell structure and detection of enzyme activity by histochemical
methods coupled with a microscopic assessment.
In the succinate dehydrogenase, the disappearance of oxidised DCPIP is well depicted
spectrophotometrically as the absorbance decreases as the substrate is added. The function of
PMS is to by-pass the cyanide-inhibited site of electron transport, cytochrome oxidase.
Therefore, electrons flow from PMS to DCPIP, whereas in vivo electrons flow from FADH to
coenzymes Q, cytochrome, cytochrome oxidase and finally to oxygen (Pietrocola, Galluzzi,
Bravo-San Pedro, Madeo and Kroemer 2015, pp. 806). The absorbance of both mitochondrial
and microsomal reduces due to a decrease in the rate as result of NADH, which is the measure of
the frequency of the reaction. The effect of the experiment demonstrates the dehydrogenase
activity using DCPIP for the essay. It also examines the distribution of succinate dehydrogenase
in microsomal and mitochondrial. The problem can arise as a result of inaccurate dilution
resulting in a concentration of extract being high. Therefore, this will cause an extremely high
malate dehydrogenase. Additionally, the fractions of the mitochondrial and microsomal are very
Yeasts are ubiquitous unicellular fungi widespread in natural environment colonising
from terrestrial, aerial to aquatic surrounding, where the active colonisation is intimately
connected to their physiological adaptability to an extremely variable atmosphere. In yeast, just
like any other heterotrophic organism, the carbon and energy metabolism are inextricably
interlinked. ATP is offered by the organic molecules oxidation that also acts as carbon sources
for biosynthesis, and ultimately it is used as energy for all sorts of cellular work. All enzymes are
localised with one or more particular compartment of the cells. Two significant tactics can be
employed to assess the enzymes localization; cell disruption to release the intact organelles, and
preservation of the entire cell structure and detection of enzyme activity by histochemical
methods coupled with a microscopic assessment.
In the succinate dehydrogenase, the disappearance of oxidised DCPIP is well depicted
spectrophotometrically as the absorbance decreases as the substrate is added. The function of
PMS is to by-pass the cyanide-inhibited site of electron transport, cytochrome oxidase.
Therefore, electrons flow from PMS to DCPIP, whereas in vivo electrons flow from FADH to
coenzymes Q, cytochrome, cytochrome oxidase and finally to oxygen (Pietrocola, Galluzzi,
Bravo-San Pedro, Madeo and Kroemer 2015, pp. 806). The absorbance of both mitochondrial
and microsomal reduces due to a decrease in the rate as result of NADH, which is the measure of
the frequency of the reaction. The effect of the experiment demonstrates the dehydrogenase
activity using DCPIP for the essay. It also examines the distribution of succinate dehydrogenase
in microsomal and mitochondrial. The problem can arise as a result of inaccurate dilution
resulting in a concentration of extract being high. Therefore, this will cause an extremely high
malate dehydrogenase. Additionally, the fractions of the mitochondrial and microsomal are very

Enzymes of the TCA cycle 9
sensitive to degradation and thus exposing them to high temperature will alter the samples. Thus,
the samples should always be stored or kept on ice at all times.
sensitive to degradation and thus exposing them to high temperature will alter the samples. Thus,
the samples should always be stored or kept on ice at all times.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Enzymes of the TCA cycle 10
References
Birsoy, K., Wang, T., Chen, W.W., Freinkman, E., Abu-Remaileh, M. and Sabatini, D.M., 2015.
An essential role of the mitochondrial electron transport chain in cell proliferation is to enable
aspartate synthesis. Cell, 162(3), Elsevier, pp.540-551. [Online]. Available from:
https://doi.org/10.1016/j.cell.2015.07.016, [Accessed on 17 September 2018].
Chandel, N.S., 2015. Evolution of mitochondria as signaling organelles. Cell
metabolism, Elsevier 22(2), pp.204-206. [Online]. Available from:
https://doi.org/10.1016/j.cmet.2015.05.013, [Accessed on 17 September 2018].
Ferro, M.S., Rodrigues, G.M. and De Souza, R.R., 2015. The role of mitochondria in physical
activity and its adaptation on aging. Journal of Morphological Sciences, 32(4), pp.257-263.
[Online]. Available from: http://jms.org.br/PDF/v32n4a07.pdf, [Accessed on 17 September
2018].
Intlekofer, A.M., Dematteo, R.G., Venneti, S., Finley, L.W., Lu, C., Judkins, A.R., Rustenburg,
A.S., Grinaway, P.B., Chodera, J.D., Cross, J.R. and Thompson, C.B., 2015. Hypoxia induces
production of L-2-hydroxyglutarate. Cell metabolism, 22(2), Elsevier, pp.304-311. [Online].
Available from: https://doi.org/10.1016/j.cmet.2015.06.023, [Accessed on 17 September 2018].
Martínez-Reyes, I., Diebold, L.P., Kong, H., Schieber, M., Huang, H., Hensley, C.T., Mehta,
M.M., Wang, T., Santos, J.H., Woychik, R. and Dufour, E., 2016. TCA cycle and mitochondrial
membrane potential are necessary for diverse biological functions. Molecular cell, 61(2),
Elsevier, pp.199-209. [Online]. Available from: https://doi.org/10.1016/j.molcel.2015.12.002,
[Accessed on 17 September 2018].
References
Birsoy, K., Wang, T., Chen, W.W., Freinkman, E., Abu-Remaileh, M. and Sabatini, D.M., 2015.
An essential role of the mitochondrial electron transport chain in cell proliferation is to enable
aspartate synthesis. Cell, 162(3), Elsevier, pp.540-551. [Online]. Available from:
https://doi.org/10.1016/j.cell.2015.07.016, [Accessed on 17 September 2018].
Chandel, N.S., 2015. Evolution of mitochondria as signaling organelles. Cell
metabolism, Elsevier 22(2), pp.204-206. [Online]. Available from:
https://doi.org/10.1016/j.cmet.2015.05.013, [Accessed on 17 September 2018].
Ferro, M.S., Rodrigues, G.M. and De Souza, R.R., 2015. The role of mitochondria in physical
activity and its adaptation on aging. Journal of Morphological Sciences, 32(4), pp.257-263.
[Online]. Available from: http://jms.org.br/PDF/v32n4a07.pdf, [Accessed on 17 September
2018].
Intlekofer, A.M., Dematteo, R.G., Venneti, S., Finley, L.W., Lu, C., Judkins, A.R., Rustenburg,
A.S., Grinaway, P.B., Chodera, J.D., Cross, J.R. and Thompson, C.B., 2015. Hypoxia induces
production of L-2-hydroxyglutarate. Cell metabolism, 22(2), Elsevier, pp.304-311. [Online].
Available from: https://doi.org/10.1016/j.cmet.2015.06.023, [Accessed on 17 September 2018].
Martínez-Reyes, I., Diebold, L.P., Kong, H., Schieber, M., Huang, H., Hensley, C.T., Mehta,
M.M., Wang, T., Santos, J.H., Woychik, R. and Dufour, E., 2016. TCA cycle and mitochondrial
membrane potential are necessary for diverse biological functions. Molecular cell, 61(2),
Elsevier, pp.199-209. [Online]. Available from: https://doi.org/10.1016/j.molcel.2015.12.002,
[Accessed on 17 September 2018].
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Enzymes of the TCA cycle 11
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J.M., Madeo, F. and Kroemer, G., 2015. Acetyl
coenzyme A: a central metabolite and second messenger. Cell metabolism, 21(6), Elsevier,
pp.805-821. [Online]. Available from: https://doi.org/10.1016/j.cmet.2015.05.014, [Accessed on
17 September 2018].f
Shi, L. and Tu, B.P., 2015. Acetyl-CoA and the regulation of metabolism: mechanisms and
consequences. Current opinion in cell biology, 33, Elsevier, pp.125-131, Elsevier, [Online].
Available from: https://doi.org/10.1016/j.ceb.2015.02.003, [Accessed on 17 September 2018].
West, A.P., Khoury-Hanold, W., Staron, M., Tal, M.C., Pineda, C.M., Lang, S.M., Bestwick, M.,
Duguay, B.A., Raimundo, N., MacDuff, D.A. and Kaech, S.M., 2015. Mitochondrial DNA stress
primes the antiviral innate immune response. Nature, 520(7548), p.553. [Online]. Available
from: https://doi.org/10.1038/nature14156, [Accessed on 17 September 2018].
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J.M., Madeo, F. and Kroemer, G., 2015. Acetyl
coenzyme A: a central metabolite and second messenger. Cell metabolism, 21(6), Elsevier,
pp.805-821. [Online]. Available from: https://doi.org/10.1016/j.cmet.2015.05.014, [Accessed on
17 September 2018].f
Shi, L. and Tu, B.P., 2015. Acetyl-CoA and the regulation of metabolism: mechanisms and
consequences. Current opinion in cell biology, 33, Elsevier, pp.125-131, Elsevier, [Online].
Available from: https://doi.org/10.1016/j.ceb.2015.02.003, [Accessed on 17 September 2018].
West, A.P., Khoury-Hanold, W., Staron, M., Tal, M.C., Pineda, C.M., Lang, S.M., Bestwick, M.,
Duguay, B.A., Raimundo, N., MacDuff, D.A. and Kaech, S.M., 2015. Mitochondrial DNA stress
primes the antiviral innate immune response. Nature, 520(7548), p.553. [Online]. Available
from: https://doi.org/10.1038/nature14156, [Accessed on 17 September 2018].
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





