Biomass and Biorefinery: Feedstock, Production, and Strategic Analysis
VerifiedAdded on 2022/10/19
|7
|1202
|320
Report
AI Summary
This report examines biomass and biorefinery processes, with a specific emphasis on feedstock selection and ethanol production. The analysis begins with a discussion on the optimal feedstock for ethanol conversion, highlighting bagasse from sugarcane due to its high cellulose and hemicellulose content. The report details the fermentation process, including the net reaction for ethanol formation and calculations for maximum ethanol yield. It then explores thermochemical biorefinery techniques such as pyrolysis and gasification, explaining how these methods convert biomass into valuable products like biocrude oil and syngas. The study also covers the production of gasoline from biomass, including hydrotreatment and catalytic processes. A strategic analysis is provided, emphasizing conversion and energy efficiency. The report includes a table correlating key biorefinery processes, and provides a list of relevant references.

BIOMASS AND BIOREFINERY.
Among all the research done on feedstock have always put their focus accurately on maximizing
yields, minimizing losses and all the costs therein involved, harvesting and collecting efficiently,
among others. The choice of the best feedstock for the conversion of ethanol lies between
different considerations, i.e., lignocellulosic feedstock has a composition of three parts namely
lignin, cellulose, and hemicelluloses. These components are required in various concentrations
for their efficiency performance(Bruins ME and Sanders JPM,2012).
Preferably, the feedstock with a relatively higher percentage of both cellulose and hemicelluloses
are mostly preferred in the biochemical conversion process.
In our case, the best feedstock that will be preferred will be Bagasse from sugarcane; this will be
preferred just because it has the highest quantity of cellulose and at the same time a higher
amount of hemicelluloses and more specifically as Cellulose 35%, Hemicellulose 40% and lignin
25%( Bussemaker MJ and Zhang D,2013).
C6H10O5 + H2O ------- C6H12O6 (12.1)
Ethanol is formed by the process of fermentation, summarized by the net reaction:
C6H12O6 (1 molecule of glucose) - 2C2H5OH (2 molecules of ethanol) + 2CO2(Carvalheiro
F, Duarte LC, and Gírio FM,2008).
Among all the research done on feedstock have always put their focus accurately on maximizing
yields, minimizing losses and all the costs therein involved, harvesting and collecting efficiently,
among others. The choice of the best feedstock for the conversion of ethanol lies between
different considerations, i.e., lignocellulosic feedstock has a composition of three parts namely
lignin, cellulose, and hemicelluloses. These components are required in various concentrations
for their efficiency performance(Bruins ME and Sanders JPM,2012).
Preferably, the feedstock with a relatively higher percentage of both cellulose and hemicelluloses
are mostly preferred in the biochemical conversion process.
In our case, the best feedstock that will be preferred will be Bagasse from sugarcane; this will be
preferred just because it has the highest quantity of cellulose and at the same time a higher
amount of hemicelluloses and more specifically as Cellulose 35%, Hemicellulose 40% and lignin
25%( Bussemaker MJ and Zhang D,2013).
C6H10O5 + H2O ------- C6H12O6 (12.1)
Ethanol is formed by the process of fermentation, summarized by the net reaction:
C6H12O6 (1 molecule of glucose) - 2C2H5OH (2 molecules of ethanol) + 2CO2(Carvalheiro
F, Duarte LC, and Gírio FM,2008).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
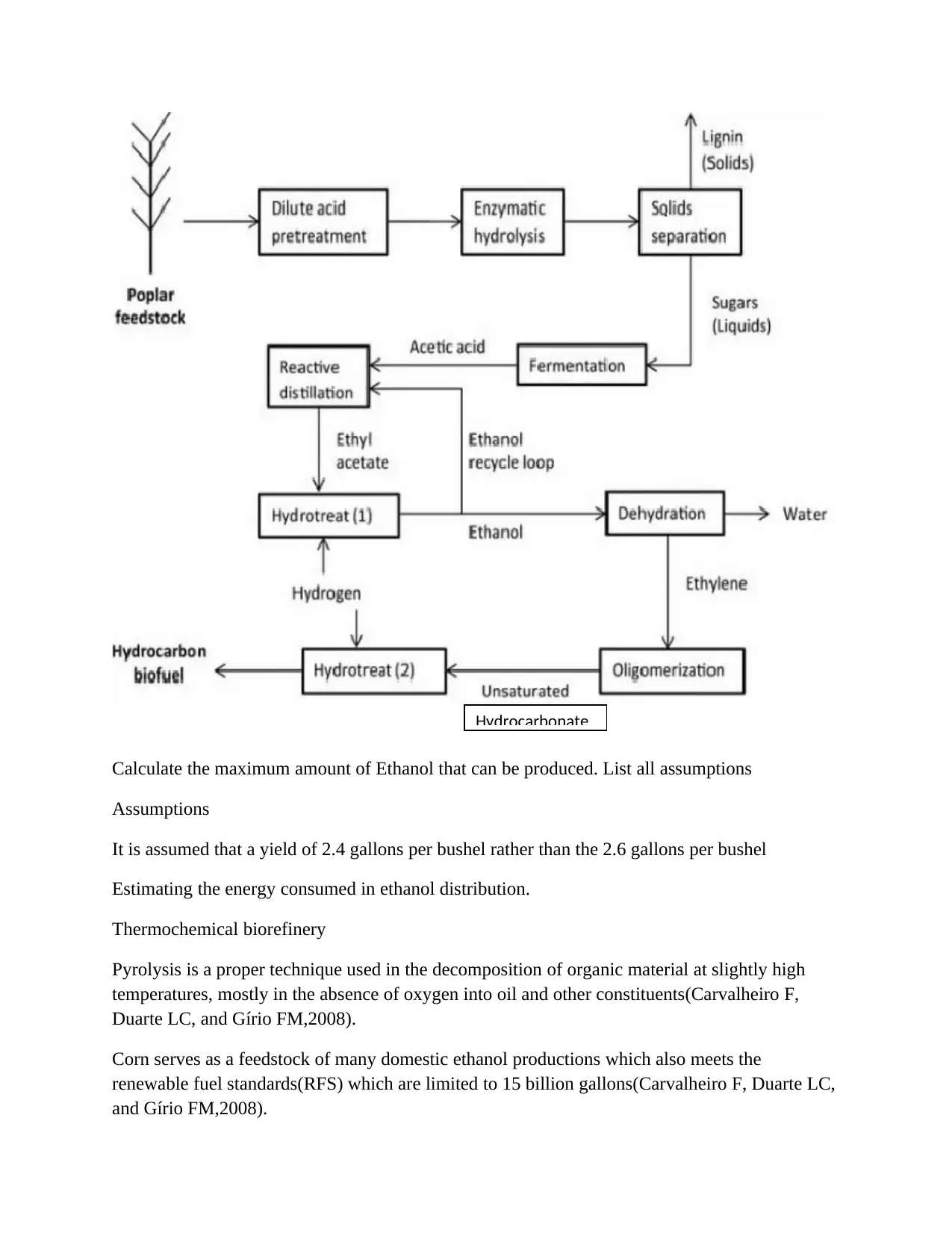
Calculate the maximum amount of Ethanol that can be produced. List all assumptions
Assumptions
It is assumed that a yield of 2.4 gallons per bushel rather than the 2.6 gallons per bushel
Estimating the energy consumed in ethanol distribution.
Thermochemical biorefinery
Pyrolysis is a proper technique used in the decomposition of organic material at slightly high
temperatures, mostly in the absence of oxygen into oil and other constituents(Carvalheiro F,
Duarte LC, and Gírio FM,2008).
Corn serves as a feedstock of many domestic ethanol productions which also meets the
renewable fuel standards(RFS) which are limited to 15 billion gallons(Carvalheiro F, Duarte LC,
and Gírio FM,2008).
Hydrocarbonate
Assumptions
It is assumed that a yield of 2.4 gallons per bushel rather than the 2.6 gallons per bushel
Estimating the energy consumed in ethanol distribution.
Thermochemical biorefinery
Pyrolysis is a proper technique used in the decomposition of organic material at slightly high
temperatures, mostly in the absence of oxygen into oil and other constituents(Carvalheiro F,
Duarte LC, and Gírio FM,2008).
Corn serves as a feedstock of many domestic ethanol productions which also meets the
renewable fuel standards(RFS) which are limited to 15 billion gallons(Carvalheiro F, Duarte LC,
and Gírio FM,2008).
Hydrocarbonate
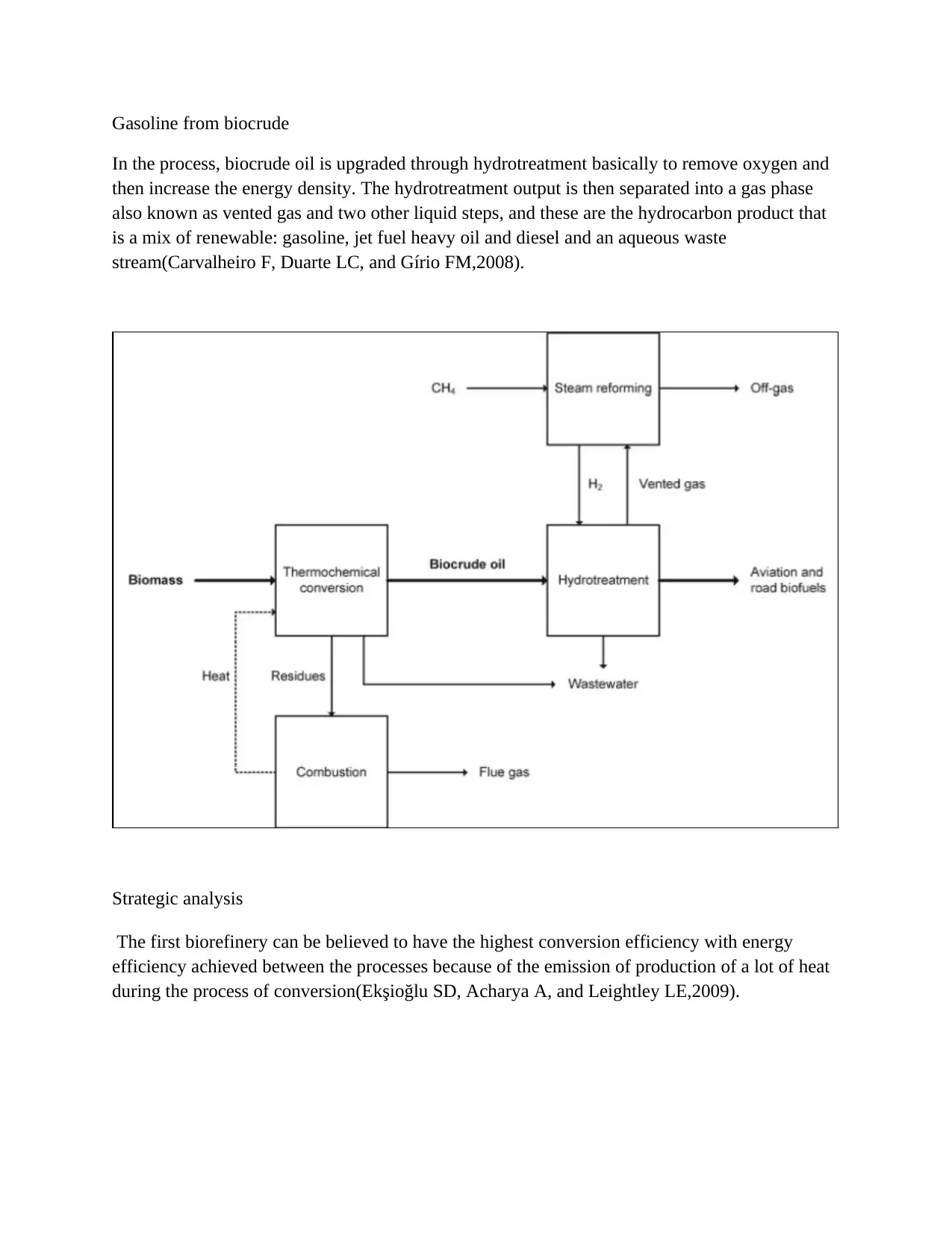
Gasoline from biocrude
In the process, biocrude oil is upgraded through hydrotreatment basically to remove oxygen and
then increase the energy density. The hydrotreatment output is then separated into a gas phase
also known as vented gas and two other liquid steps, and these are the hydrocarbon product that
is a mix of renewable: gasoline, jet fuel heavy oil and diesel and an aqueous waste
stream(Carvalheiro F, Duarte LC, and Gírio FM,2008).
Strategic analysis
The first biorefinery can be believed to have the highest conversion efficiency with energy
efficiency achieved between the processes because of the emission of production of a lot of heat
during the process of conversion(Ekşioğlu SD, Acharya A, and Leightley LE,2009).
In the process, biocrude oil is upgraded through hydrotreatment basically to remove oxygen and
then increase the energy density. The hydrotreatment output is then separated into a gas phase
also known as vented gas and two other liquid steps, and these are the hydrocarbon product that
is a mix of renewable: gasoline, jet fuel heavy oil and diesel and an aqueous waste
stream(Carvalheiro F, Duarte LC, and Gírio FM,2008).
Strategic analysis
The first biorefinery can be believed to have the highest conversion efficiency with energy
efficiency achieved between the processes because of the emission of production of a lot of heat
during the process of conversion(Ekşioğlu SD, Acharya A, and Leightley LE,2009).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Gasification is a process which converts organic or fuel-based carbonaceous materials directly
into carbon monoxide, hydrogen, and carbon dioxide. In the process the gas mixture is referred
to as syngas or producer gas and is itself a fuel(Nobre BP, Villalobos F, Barragan BE, and
Oliveira AC)
For this case,
Cellulose is the best among the feedstocks
How to produce gasoline from biomass
Solid biomass, such as wood and garbage, can be burned directly to produce heat. Biomass can
also be converted into a gas called biogas or into liquid biofuels such as ethanol and biodiesel.
These fuels can then be burned for energy(Nobre BP, Villalobos F, Barragan BE, and Oliveira
AC).
Ethanol is produced from biomass mostly through a fermentation process using glucose derived
from sugars (sugar cane, sugar beet, and molasses), starch (grains, corns, grains) or cellulose
(forest products) as raw materials. Synthetic ethanol can also be produced from non-renewable
sources like coal and gas(Nobre BP, Villalobos F, Barragan BE, and Oliveira AC).
One involves heating biomass to a high temperature and under pressure in a controlled amount of
oxygen which leads to the production of synthesis gas (a mixture of carbon monoxide and
hydrogen. This process is known as gasification. Synthesis gas can then be converted into many
critical industrial compounds. Gasification leading to kerosine and diesel fuels is discussed in the
unit on Biofuels(Ekşioğlu SD, Acharya A and Leightley LE(2009).
The second method again involves heating the biomass to a high temperature, but in this case in
the absence of air. This process is known as pyrolysis. To form useful products, the reaction time
must be short, otherwise the original product will be carbon(Nobre BP, Villalobos F, Barragan
BE and Oliveira AC(2013). This process is thus called fast pyrolysis, and the primary product is
an oil known as bio-oil.The production of bio-oil is discussed in more detail in the unit on
Biofuels(Labbé N, Kline LM, Moens L, Kim K and Kim PC(2012).
into carbon monoxide, hydrogen, and carbon dioxide. In the process the gas mixture is referred
to as syngas or producer gas and is itself a fuel(Nobre BP, Villalobos F, Barragan BE, and
Oliveira AC)
For this case,
Cellulose is the best among the feedstocks
How to produce gasoline from biomass
Solid biomass, such as wood and garbage, can be burned directly to produce heat. Biomass can
also be converted into a gas called biogas or into liquid biofuels such as ethanol and biodiesel.
These fuels can then be burned for energy(Nobre BP, Villalobos F, Barragan BE, and Oliveira
AC).
Ethanol is produced from biomass mostly through a fermentation process using glucose derived
from sugars (sugar cane, sugar beet, and molasses), starch (grains, corns, grains) or cellulose
(forest products) as raw materials. Synthetic ethanol can also be produced from non-renewable
sources like coal and gas(Nobre BP, Villalobos F, Barragan BE, and Oliveira AC).
One involves heating biomass to a high temperature and under pressure in a controlled amount of
oxygen which leads to the production of synthesis gas (a mixture of carbon monoxide and
hydrogen. This process is known as gasification. Synthesis gas can then be converted into many
critical industrial compounds. Gasification leading to kerosine and diesel fuels is discussed in the
unit on Biofuels(Ekşioğlu SD, Acharya A and Leightley LE(2009).
The second method again involves heating the biomass to a high temperature, but in this case in
the absence of air. This process is known as pyrolysis. To form useful products, the reaction time
must be short, otherwise the original product will be carbon(Nobre BP, Villalobos F, Barragan
BE and Oliveira AC(2013). This process is thus called fast pyrolysis, and the primary product is
an oil known as bio-oil.The production of bio-oil is discussed in more detail in the unit on
Biofuels(Labbé N, Kline LM, Moens L, Kim K and Kim PC(2012).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
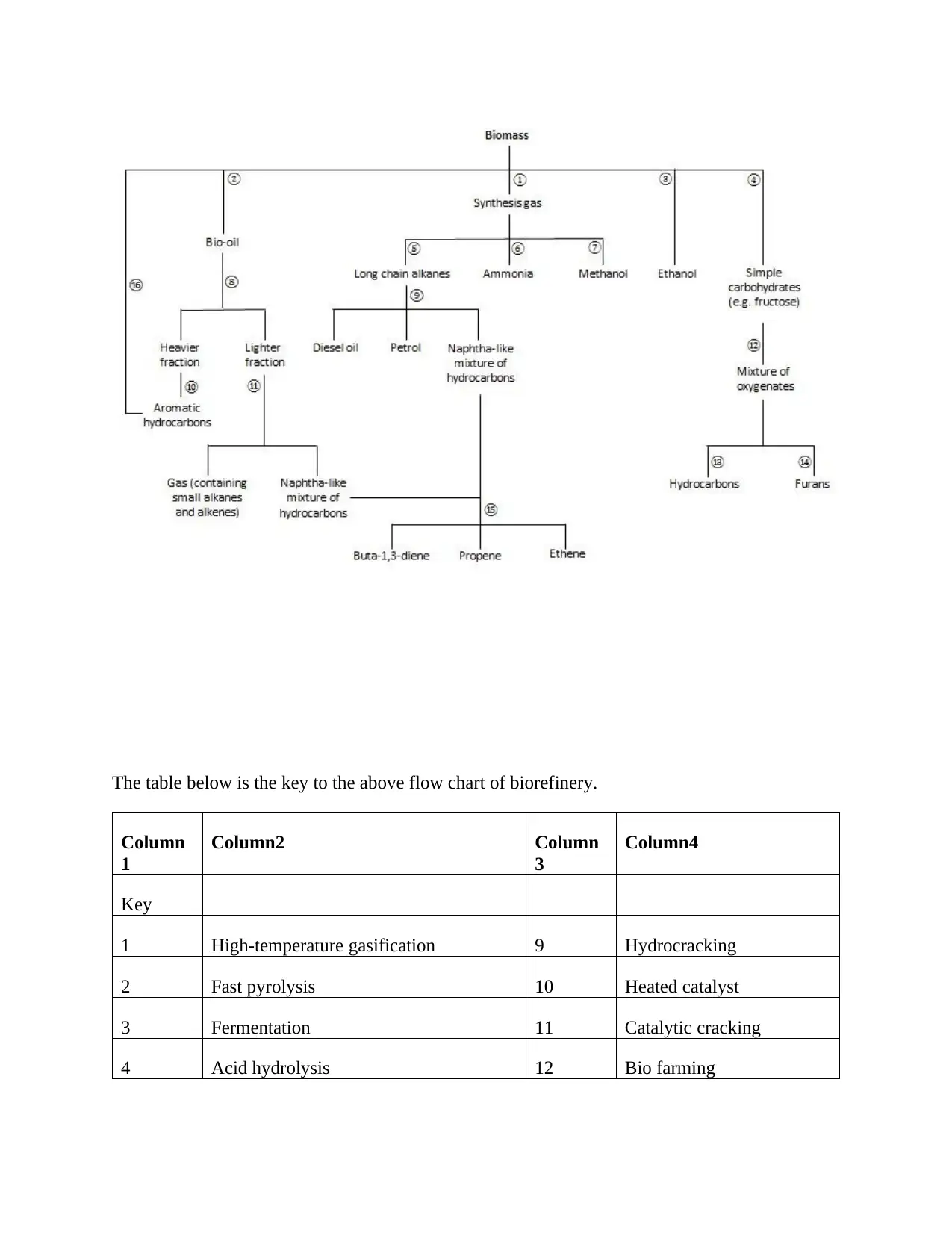
The table below is the key to the above flow chart of biorefinery.
Column
1
Column2 Column
3
Column4
Key
1 High-temperature gasification 9 Hydrocracking
2 Fast pyrolysis 10 Heated catalyst
3 Fermentation 11 Catalytic cracking
4 Acid hydrolysis 12 Bio farming
Column
1
Column2 Column
3
Column4
Key
1 High-temperature gasification 9 Hydrocracking
2 Fast pyrolysis 10 Heated catalyst
3 Fermentation 11 Catalytic cracking
4 Acid hydrolysis 12 Bio farming

5 Fisher-Tropsch (Shell Middle Distillate)
process
13 Catalytic reduction
6 Haber process 14 Dehydration
7 High temperature and pressure with a
catalyst
15 Steam cracking
8 Distillation 16 Heating sawdust over a
catalyst
References.
1)Bruins ME and Sanders JPM(2012) - Biofuels, bioproducts and biorefining, 2012 - Wiley
Online Library
2)Bussemaker MJ and Zhang D(2013) - Industrial & Engineering Chemistry …, 2013 - ACS
Publications.
3)Carvalheiro F, Duarte LC and Gírio FM(2008) - Journal of Scientific & …, 2008 -
repositorio.lneg.pt.
4)Ekşioğlu SD, Acharya A and Leightley LE(2009)… - Computers & Industrial …, 2009 –
Elsevier.
5)Ekşioğlu SD, Acharya A and Leightley LE(2009)… - Computers & Industrial …, 2009 –
Elsevier.
6)Labbé N, Kline LM, Moens L, Kim K, and Kim PC(2012)… - Bioresource …, 2012 –
Elsevier.
7)Parsell T, Yohe S, Degenstein J, Jarrell T, Klein L(2015)… - Green …, 2015 - pubs.rsc.org.
process
13 Catalytic reduction
6 Haber process 14 Dehydration
7 High temperature and pressure with a
catalyst
15 Steam cracking
8 Distillation 16 Heating sawdust over a
catalyst
References.
1)Bruins ME and Sanders JPM(2012) - Biofuels, bioproducts and biorefining, 2012 - Wiley
Online Library
2)Bussemaker MJ and Zhang D(2013) - Industrial & Engineering Chemistry …, 2013 - ACS
Publications.
3)Carvalheiro F, Duarte LC and Gírio FM(2008) - Journal of Scientific & …, 2008 -
repositorio.lneg.pt.
4)Ekşioğlu SD, Acharya A and Leightley LE(2009)… - Computers & Industrial …, 2009 –
Elsevier.
5)Ekşioğlu SD, Acharya A and Leightley LE(2009)… - Computers & Industrial …, 2009 –
Elsevier.
6)Labbé N, Kline LM, Moens L, Kim K, and Kim PC(2012)… - Bioresource …, 2012 –
Elsevier.
7)Parsell T, Yohe S, Degenstein J, Jarrell T, Klein L(2015)… - Green …, 2015 - pubs.rsc.org.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

8)Knutsen JS and Liberatore, MW(2009) - Journal of Rheology, 2009 - sor.scitation.org.
9)Nobre BP, Villalobos F, Barragan BE and Oliveira AC(2013)… - Bioresource …, 2013 –
Elsevier.
10)Stöcker M(2008) - Angewandte Chemie International Edition, 2008 - Wiley Online Library.
9)Nobre BP, Villalobos F, Barragan BE and Oliveira AC(2013)… - Bioresource …, 2013 –
Elsevier.
10)Stöcker M(2008) - Angewandte Chemie International Edition, 2008 - Wiley Online Library.
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.