Blood Grouping Practicals: ABO, Rhesus Typing, and Transfusion
VerifiedAdded on 2022/08/17
|14
|5018
|16
Practical Assignment
AI Summary
This document provides a comprehensive solution to a practical assignment focused on blood grouping techniques. The assignment covers the principles and procedures of ABO and Rhesus typing, including the use of anti-A, anti-B, and anti-D sera, as well as Diamed ID cards for column agglutination technology. Students are expected to perform forward and reverse blood grouping, interpret results, and identify blood groups. The assignment also delves into the causes of false-positive and false-negative reactions, and the strategies for red cell transfusion in patients with autoimmune hemolytic anemia. Furthermore, the document includes discussions on safe handling of blood and precautions against infection hazards. The solution provides detailed answers to the discussion points, including the interpretation of results, blood group compatibility, and the clinical considerations for transfusion reactions. This resource is designed to aid students in understanding the complexities of blood grouping and its clinical applications, offering a practical guide to the laboratory procedures and the underlying principles of blood transfusion medicine.

BMS3136 Transplantation, Transfusion and Specialist Biochemistry
Practicals for Blood grouping
Aim
To allow students to experience practical blood grouping
Learning objectives
After doing this practical class and researching the discussion points, students should be
able to:
Safely handle blood, and take suitable precautions against infection hazards from the
blood of others.
Describe the principles of ABO and Rhesus typing of blood.
Perform ABO and Rhesus typing of blood.
Explain the terms ‘forward blood grouping’ and ‘reverse blood grouping’
Describe and manually perform a common automated technique for blood grouping
used in UK hospital laboratories
Describe how antibody screening on blood samples is performed in the UK
Introduction
ABO blood grouping is the most important serological test performed in blood compatibility
testing – this is because the antibodies of the system (anti-A and anti-B) are preformed
and can cause a rapid transfusion reaction if the wrong group is given. The Rhesus system
is also important, but as the antibodies are not present unless there has been previous
exposure to the Rhesus antigen in a Rhesus negative individual this is less critical than
ABO grouping.
‘Forward’ ABO blood grouping is now done with monoclonal, separate anti-A and anti-B
reagents used against the patient’s red cells (historically, polyclonal anti-A, anti-B, and
anti-A, B together reagents were used). A and B red cells are also used for ‘reverse
grouping’, i.e. the patient’s serum is tested against known A and B group red blood cells
to check that it agglutinates them appropriately. Any discrepancies between the forward and
reverse group need to be investigated using the patient’s original blood sample rather than
any cell suspensions created from it to prevent accumulating errors.
Reminder about safe handling of human blood
For the purposes of this practical, we will supply you with screened donor blood. Although
1
Practicals for Blood grouping
Aim
To allow students to experience practical blood grouping
Learning objectives
After doing this practical class and researching the discussion points, students should be
able to:
Safely handle blood, and take suitable precautions against infection hazards from the
blood of others.
Describe the principles of ABO and Rhesus typing of blood.
Perform ABO and Rhesus typing of blood.
Explain the terms ‘forward blood grouping’ and ‘reverse blood grouping’
Describe and manually perform a common automated technique for blood grouping
used in UK hospital laboratories
Describe how antibody screening on blood samples is performed in the UK
Introduction
ABO blood grouping is the most important serological test performed in blood compatibility
testing – this is because the antibodies of the system (anti-A and anti-B) are preformed
and can cause a rapid transfusion reaction if the wrong group is given. The Rhesus system
is also important, but as the antibodies are not present unless there has been previous
exposure to the Rhesus antigen in a Rhesus negative individual this is less critical than
ABO grouping.
‘Forward’ ABO blood grouping is now done with monoclonal, separate anti-A and anti-B
reagents used against the patient’s red cells (historically, polyclonal anti-A, anti-B, and
anti-A, B together reagents were used). A and B red cells are also used for ‘reverse
grouping’, i.e. the patient’s serum is tested against known A and B group red blood cells
to check that it agglutinates them appropriately. Any discrepancies between the forward and
reverse group need to be investigated using the patient’s original blood sample rather than
any cell suspensions created from it to prevent accumulating errors.
Reminder about safe handling of human blood
For the purposes of this practical, we will supply you with screened donor blood. Although
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

the blood has been screened for HIV and hepatitis, you must still take all safety
precautions – there are undoubtedly new viruses as yet undiscovered! Please observe all
of the following safety points.
Ensure that any cuts or broken skin on your hands are covered with a plaster.
All the usual lab rules apply – no eating or drinking is particularly important.
Wear a lab coat, gloves, and safety glasses at all times.
Keep sources of blood sealed as much as you possibly can, i.e. replace the stoppers
or caps on containers of blood as soon as you can, and put lids on test tubes with
blood or solutions of blood in.
Never put an uncovered tube of blood into a centrifuge – it must have a lid.
Never mix a tube containing blood on a vortex mixer unless the tube has a lid on.
If you spill blood on the bench or floor, ask for help to clean it up safely.
If you break a glass slide that has blood on it, do not touch it – call for help to clear it up
with a dustpan and brush. Sharp fragments and foreign blood are potentially
dangerous.
Do not run the tap fast and pour blood (or solutions containing blood) into the water
stream – you risk creating aerosols of blood vapor! Pour solutions carefully down
the sink, then run the tap slowly afterward.
Ensure that all disposable materials that have touched blood during the practical are
disposed of in an appropriate hazardous waste bin (ask a demonstrator or technician if
you are uncertain of this). Non-disposable materials (e.g. hemocytometer) should be
soaked in Milton sterilizing fluid solution.
If you are unsure of how to proceed safely at any stage of these practicals, ask for
help.
If you injure yourself in any way with something potentially contaminated with blood, you
must immediately inform one of the staff.
1. ABO and Rhesus grouping
Mater i a l s
Anti-A, Anti-B and Anti-D sera
Red blood cell suspensions from 4 patients– CAUTION, INFECTION HAZARD
Tiles
Eppendorf tubes
2
precautions – there are undoubtedly new viruses as yet undiscovered! Please observe all
of the following safety points.
Ensure that any cuts or broken skin on your hands are covered with a plaster.
All the usual lab rules apply – no eating or drinking is particularly important.
Wear a lab coat, gloves, and safety glasses at all times.
Keep sources of blood sealed as much as you possibly can, i.e. replace the stoppers
or caps on containers of blood as soon as you can, and put lids on test tubes with
blood or solutions of blood in.
Never put an uncovered tube of blood into a centrifuge – it must have a lid.
Never mix a tube containing blood on a vortex mixer unless the tube has a lid on.
If you spill blood on the bench or floor, ask for help to clean it up safely.
If you break a glass slide that has blood on it, do not touch it – call for help to clear it up
with a dustpan and brush. Sharp fragments and foreign blood are potentially
dangerous.
Do not run the tap fast and pour blood (or solutions containing blood) into the water
stream – you risk creating aerosols of blood vapor! Pour solutions carefully down
the sink, then run the tap slowly afterward.
Ensure that all disposable materials that have touched blood during the practical are
disposed of in an appropriate hazardous waste bin (ask a demonstrator or technician if
you are uncertain of this). Non-disposable materials (e.g. hemocytometer) should be
soaked in Milton sterilizing fluid solution.
If you are unsure of how to proceed safely at any stage of these practicals, ask for
help.
If you injure yourself in any way with something potentially contaminated with blood, you
must immediately inform one of the staff.
1. ABO and Rhesus grouping
Mater i a l s
Anti-A, Anti-B and Anti-D sera
Red blood cell suspensions from 4 patients– CAUTION, INFECTION HAZARD
Tiles
Eppendorf tubes
2
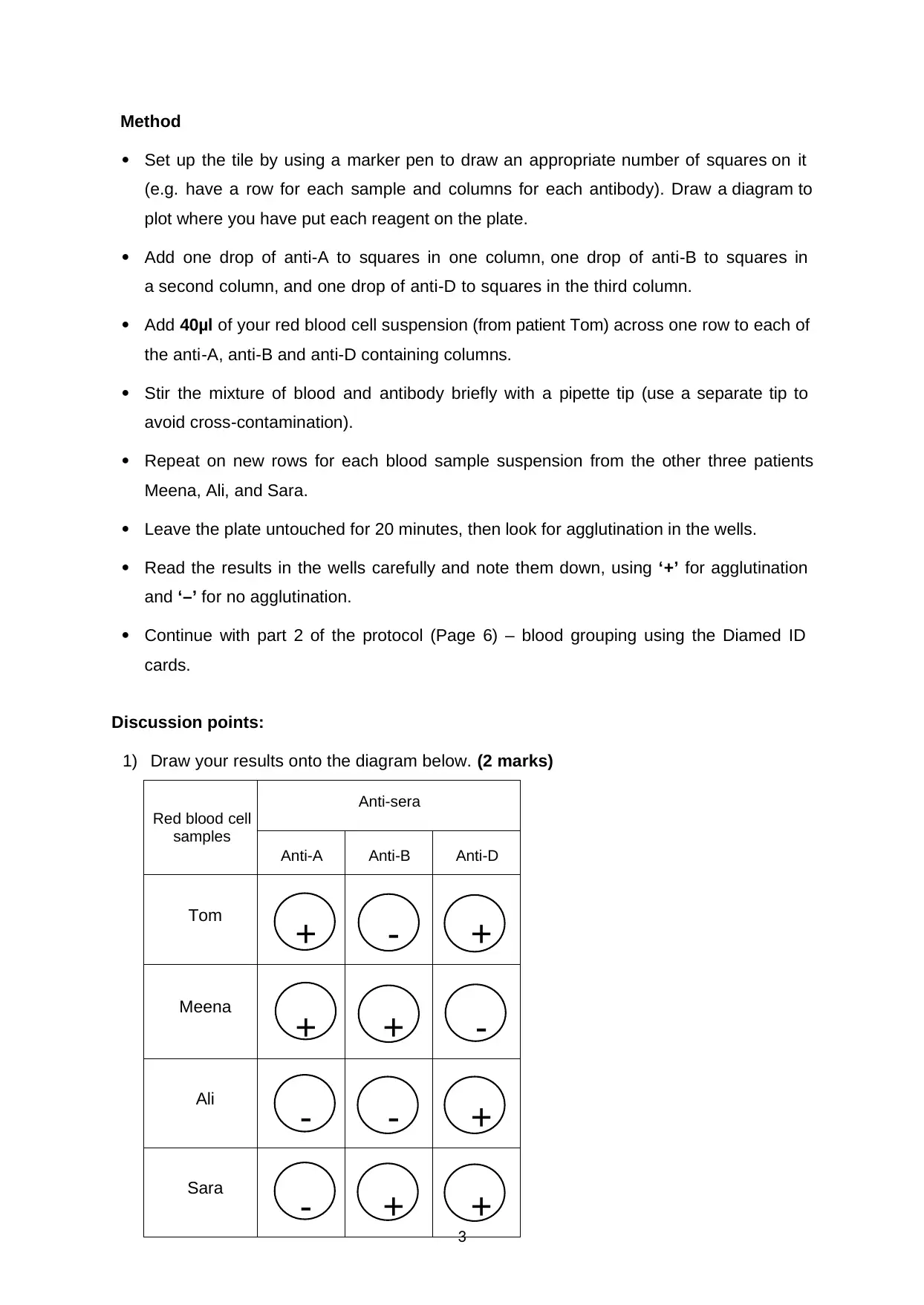
Method
Set up the tile by using a marker pen to draw an appropriate number of squares on it
(e.g. have a row for each sample and columns for each antibody). Draw a diagram to
plot where you have put each reagent on the plate.
Add one drop of anti-A to squares in one column, one drop of anti-B to squares in
a second column, and one drop of anti-D to squares in the third column.
Add 40μl of your red blood cell suspension (from patient Tom) across one row to each of
the anti-A, anti-B and anti-D containing columns.
Stir the mixture of blood and antibody briefly with a pipette tip (use a separate tip to
avoid cross-contamination).
Repeat on new rows for each blood sample suspension from the other three patients
Meena, Ali, and Sara.
Leave the plate untouched for 20 minutes, then look for agglutination in the wells.
Read the results in the wells carefully and note them down, using ‘+’ for agglutination
and ‘–’ for no agglutination.
Continue with part 2 of the protocol (Page 6) – blood grouping using the Diamed ID
cards.
Discussion points:
1) Draw your results onto the diagram below. (2 marks)
3
Red blood cell
samples
Anti-sera
Anti-A Anti-B Anti-D
Tom
+ - +
Meena
+ + -
Ali
- - +
Sara
- + +
Set up the tile by using a marker pen to draw an appropriate number of squares on it
(e.g. have a row for each sample and columns for each antibody). Draw a diagram to
plot where you have put each reagent on the plate.
Add one drop of anti-A to squares in one column, one drop of anti-B to squares in
a second column, and one drop of anti-D to squares in the third column.
Add 40μl of your red blood cell suspension (from patient Tom) across one row to each of
the anti-A, anti-B and anti-D containing columns.
Stir the mixture of blood and antibody briefly with a pipette tip (use a separate tip to
avoid cross-contamination).
Repeat on new rows for each blood sample suspension from the other three patients
Meena, Ali, and Sara.
Leave the plate untouched for 20 minutes, then look for agglutination in the wells.
Read the results in the wells carefully and note them down, using ‘+’ for agglutination
and ‘–’ for no agglutination.
Continue with part 2 of the protocol (Page 6) – blood grouping using the Diamed ID
cards.
Discussion points:
1) Draw your results onto the diagram below. (2 marks)
3
Red blood cell
samples
Anti-sera
Anti-A Anti-B Anti-D
Tom
+ - +
Meena
+ + -
Ali
- - +
Sara
- + +
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(Butt et al, 2018)
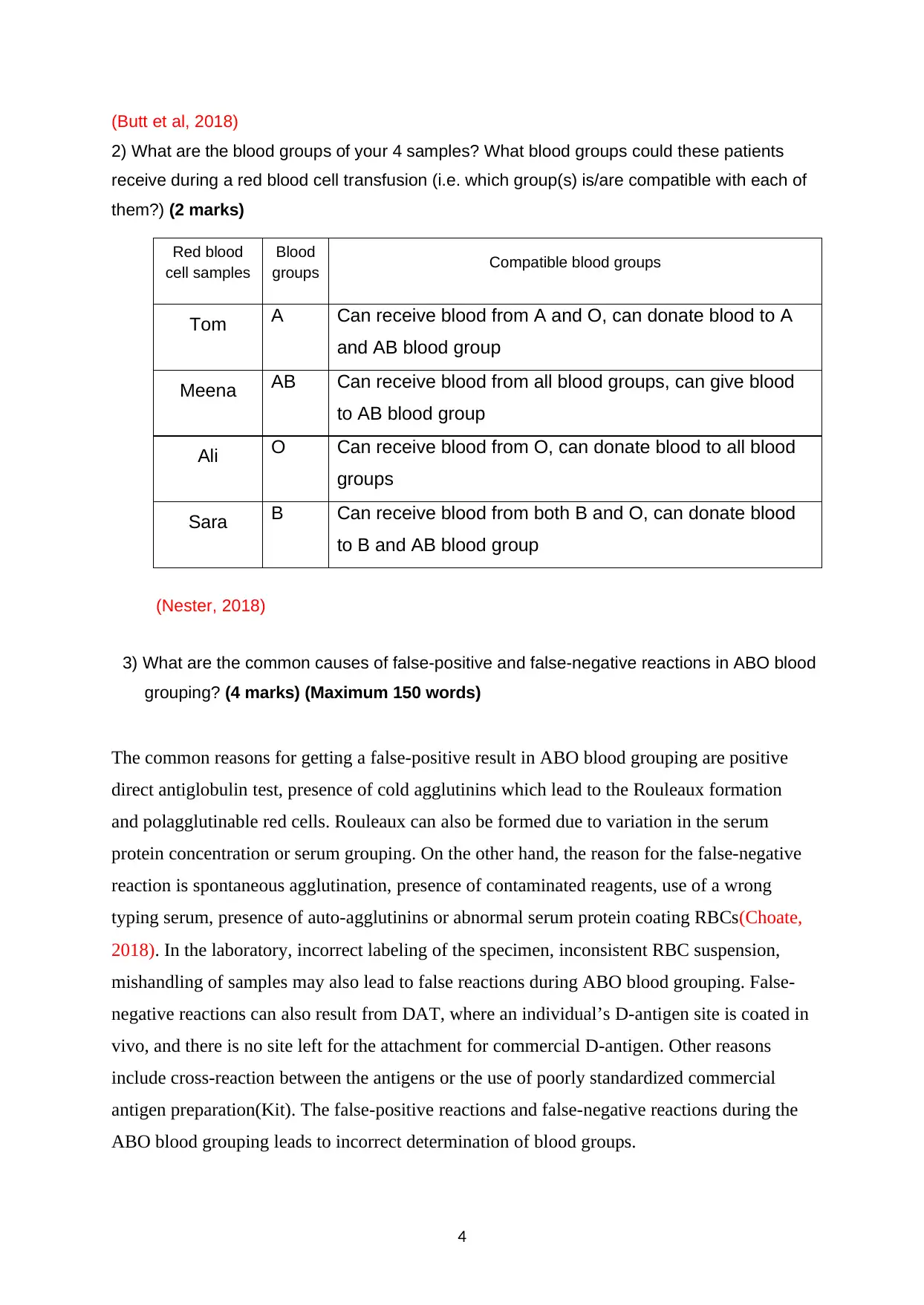
2) What are the blood groups of your 4 samples? What blood groups could these patients
receive during a red blood cell transfusion (i.e. which group(s) is/are compatible with each of
them?) (2 marks)
Red blood
cell samples
Blood
groups Compatible blood groups
Tom A Can receive blood from A and O, can donate blood to A
and AB blood group
Meena AB Can receive blood from all blood groups, can give blood
to AB blood group
Ali O Can receive blood from O, can donate blood to all blood
groups
Sara B Can receive blood from both B and O, can donate blood
to B and AB blood group
(Nester, 2018)
3) What are the common causes of false-positive and false-negative reactions in ABO blood
grouping? (4 marks) (Maximum 150 words)
The common reasons for getting a false-positive result in ABO blood grouping are positive
direct antiglobulin test, presence of cold agglutinins which lead to the Rouleaux formation
and polagglutinable red cells. Rouleaux can also be formed due to variation in the serum
protein concentration or serum grouping. On the other hand, the reason for the false-negative
reaction is spontaneous agglutination, presence of contaminated reagents, use of a wrong
typing serum, presence of auto-agglutinins or abnormal serum protein coating RBCs(Choate,
2018). In the laboratory, incorrect labeling of the specimen, inconsistent RBC suspension,
mishandling of samples may also lead to false reactions during ABO blood grouping. False-
negative reactions can also result from DAT, where an individual’s D-antigen site is coated in
vivo, and there is no site left for the attachment for commercial D-antigen. Other reasons
include cross-reaction between the antigens or the use of poorly standardized commercial
antigen preparation(Kit). The false-positive reactions and false-negative reactions during the
ABO blood grouping leads to incorrect determination of blood groups.
4
2) What are the blood groups of your 4 samples? What blood groups could these patients
receive during a red blood cell transfusion (i.e. which group(s) is/are compatible with each of
them?) (2 marks)
Red blood
cell samples
Blood
groups Compatible blood groups
Tom A Can receive blood from A and O, can donate blood to A
and AB blood group
Meena AB Can receive blood from all blood groups, can give blood
to AB blood group
Ali O Can receive blood from O, can donate blood to all blood
groups
Sara B Can receive blood from both B and O, can donate blood
to B and AB blood group
(Nester, 2018)
3) What are the common causes of false-positive and false-negative reactions in ABO blood
grouping? (4 marks) (Maximum 150 words)
The common reasons for getting a false-positive result in ABO blood grouping are positive
direct antiglobulin test, presence of cold agglutinins which lead to the Rouleaux formation
and polagglutinable red cells. Rouleaux can also be formed due to variation in the serum
protein concentration or serum grouping. On the other hand, the reason for the false-negative
reaction is spontaneous agglutination, presence of contaminated reagents, use of a wrong
typing serum, presence of auto-agglutinins or abnormal serum protein coating RBCs(Choate,
2018). In the laboratory, incorrect labeling of the specimen, inconsistent RBC suspension,
mishandling of samples may also lead to false reactions during ABO blood grouping. False-
negative reactions can also result from DAT, where an individual’s D-antigen site is coated in
vivo, and there is no site left for the attachment for commercial D-antigen. Other reasons
include cross-reaction between the antigens or the use of poorly standardized commercial
antigen preparation(Kit). The false-positive reactions and false-negative reactions during the
ABO blood grouping leads to incorrect determination of blood groups.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
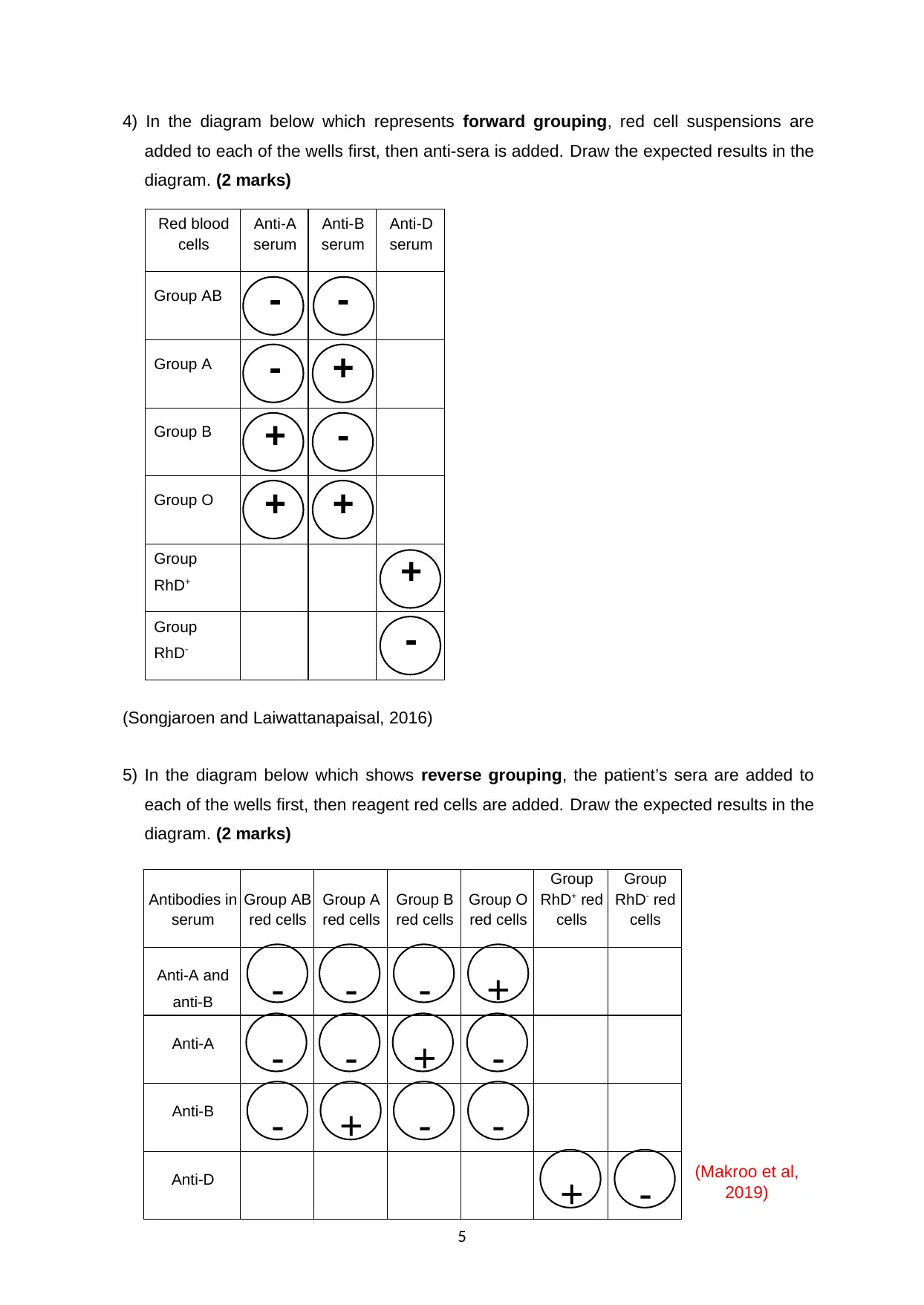
4) In the diagram below which represents forward grouping, red cell suspensions are
added to each of the wells first, then anti-sera is added. Draw the expected results in the
diagram. (2 marks)
Red blood
cells
Anti-A
serum
Anti-B
serum
Anti-D
serum
Group AB - -
Group A - +
Group B + -
Group O + +
Group
RhD+ +
Group
RhD- -
(Songjaroen and Laiwattanapaisal, 2016)
5) In the diagram below which shows reverse grouping, the patient’s sera are added to
each of the wells first, then reagent red cells are added. Draw the expected results in the
diagram. (2 marks)
(Makroo et al,
2019)
5
Antibodies in
serum
Group AB
red cells
Group A
red cells
Group B
red cells
Group O
red cells
Group
RhD+ red
cells
Group
RhD- red
cells
Anti-A and
anti-B - - - +
Anti-A
- - + -
Anti-B
- + - -
Anti-D
+ -
added to each of the wells first, then anti-sera is added. Draw the expected results in the
diagram. (2 marks)
Red blood
cells
Anti-A
serum
Anti-B
serum
Anti-D
serum
Group AB - -
Group A - +
Group B + -
Group O + +
Group
RhD+ +
Group
RhD- -
(Songjaroen and Laiwattanapaisal, 2016)
5) In the diagram below which shows reverse grouping, the patient’s sera are added to
each of the wells first, then reagent red cells are added. Draw the expected results in the
diagram. (2 marks)
(Makroo et al,
2019)
5
Antibodies in
serum
Group AB
red cells
Group A
red cells
Group B
red cells
Group O
red cells
Group
RhD+ red
cells
Group
RhD- red
cells
Anti-A and
anti-B - - - +
Anti-A
- - + -
Anti-B
- + - -
Anti-D
+ -

2. Column agglutination technology
Most hospital laboratories in the UK used automated systems to group patients’ blood
samples, and column agglutination techniques are in common use. These are based on
the principles of size exclusion chromatography. They use the same system for ABO and
Rhesus grouping that you have used (i.e. anti-A, anti-B and anti-D [the latter in duplicate]
for forwarding grouping, and known group A and B red cells for reverse grouping), but
reagents are in small tubes containing a gel, within a larger cassette. The patient’s red
cells are added to the small tubes containing anti-A, anti-B or anti-D, and their plasma to
tubes containing group A and group B cells, and once an agglutination reaction has had
time to occur, the cassette is spun in a centrifuge. Where agglutination has occurred, the
large clumps of cells are unable to pass through the small spaces in the gel, and the clump
remains at the top of the column. Where agglutination has not occurred, the small, free
cells are able to pass through the column and can be seen at the base of the tube in the
cassette. The principle of size exclusion chromatography will be demonstrated, and you
will use two samples of patient red blood cells and serum on Diamed blood grouping
cassettes to establish (and cross-check) the ABO and Rhesus groups of these patients.
Materials
Diamed ABO/D+ reverse grouping ID cards (two cards per group)
Known blood samples (red cell suspension and sera) (See ABO and Rh grouping above).
Unknown blood samples (red cell suspension and sera).
Methods
1. Label two Diamed ID cards with your initials and an appropriate label of your known
samples (the first card) and unknown samples (the second card).
2. Hold the Diamed ID card upright and peel back the foil from the wells.
3. To both ID cards: Pipette 10 μl of known blood group A cells into lane 5. Pipette 10 μl of
known blood group B cells into lane 6.
4. To card 1 only: Pipette 10 μl of Tom’s known red cells into lanes 1- 4, and 10 μl of
the correspondent serum of Tom’s (ser-Tom) into lanes 5 and 6.
5. To card 2 only: Pipette 10 μl of the unknown red cell suspension (Un.) into lanes 1- 4,
and 10 μl of the corresponding unknown serum (ser-Un.) into lanes 5 and 6.
6. Centrifuge the cards for 10 minutes in the Diamed centrifuge.
7. Read and record the reactions below.
6
Most hospital laboratories in the UK used automated systems to group patients’ blood
samples, and column agglutination techniques are in common use. These are based on
the principles of size exclusion chromatography. They use the same system for ABO and
Rhesus grouping that you have used (i.e. anti-A, anti-B and anti-D [the latter in duplicate]
for forwarding grouping, and known group A and B red cells for reverse grouping), but
reagents are in small tubes containing a gel, within a larger cassette. The patient’s red
cells are added to the small tubes containing anti-A, anti-B or anti-D, and their plasma to
tubes containing group A and group B cells, and once an agglutination reaction has had
time to occur, the cassette is spun in a centrifuge. Where agglutination has occurred, the
large clumps of cells are unable to pass through the small spaces in the gel, and the clump
remains at the top of the column. Where agglutination has not occurred, the small, free
cells are able to pass through the column and can be seen at the base of the tube in the
cassette. The principle of size exclusion chromatography will be demonstrated, and you
will use two samples of patient red blood cells and serum on Diamed blood grouping
cassettes to establish (and cross-check) the ABO and Rhesus groups of these patients.
Materials
Diamed ABO/D+ reverse grouping ID cards (two cards per group)
Known blood samples (red cell suspension and sera) (See ABO and Rh grouping above).
Unknown blood samples (red cell suspension and sera).
Methods
1. Label two Diamed ID cards with your initials and an appropriate label of your known
samples (the first card) and unknown samples (the second card).
2. Hold the Diamed ID card upright and peel back the foil from the wells.
3. To both ID cards: Pipette 10 μl of known blood group A cells into lane 5. Pipette 10 μl of
known blood group B cells into lane 6.
4. To card 1 only: Pipette 10 μl of Tom’s known red cells into lanes 1- 4, and 10 μl of
the correspondent serum of Tom’s (ser-Tom) into lanes 5 and 6.
5. To card 2 only: Pipette 10 μl of the unknown red cell suspension (Un.) into lanes 1- 4,
and 10 μl of the corresponding unknown serum (ser-Un.) into lanes 5 and 6.
6. Centrifuge the cards for 10 minutes in the Diamed centrifuge.
7. Read and record the reactions below.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
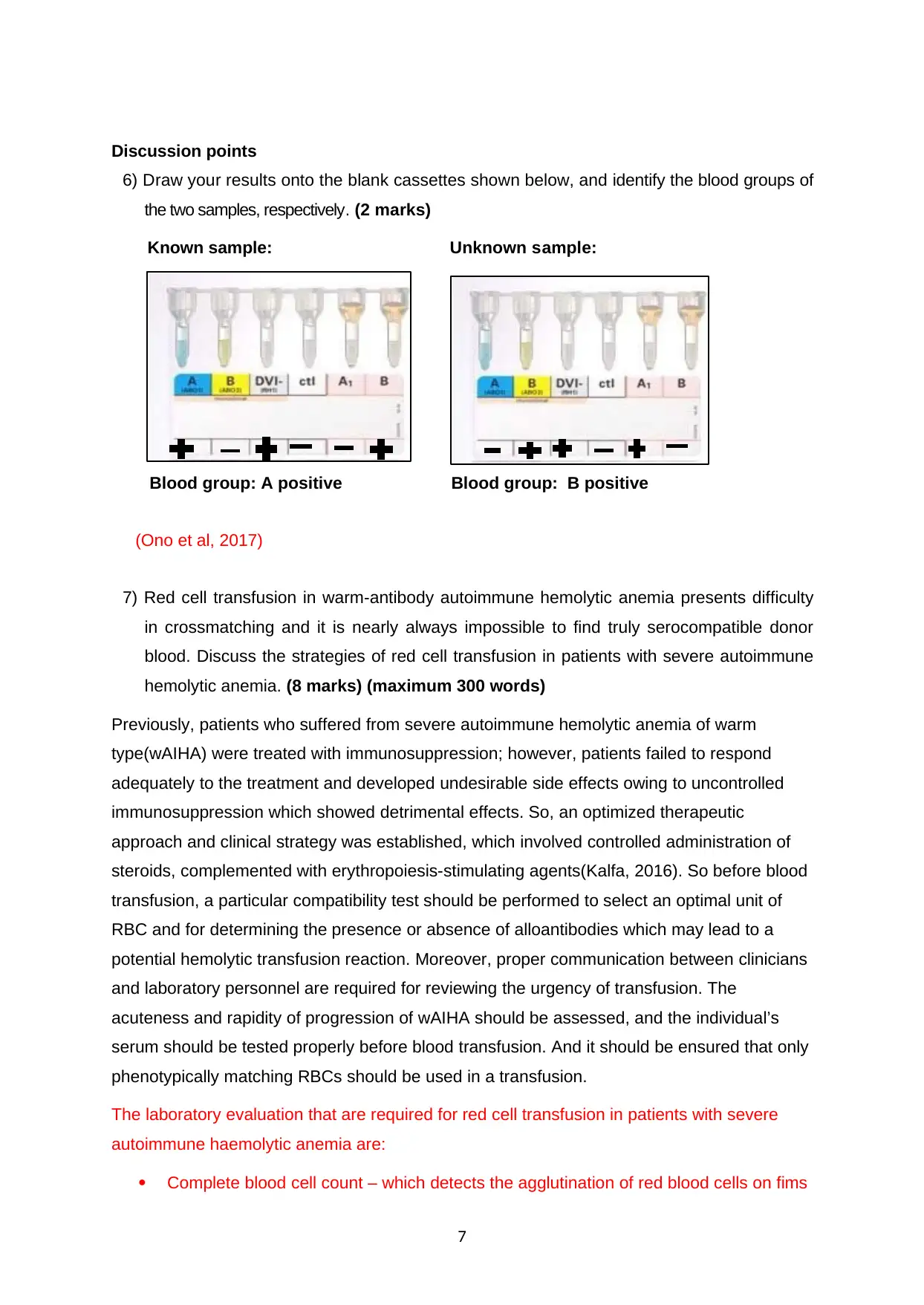
Discussion points
6) Draw your results onto the blank cassettes shown below, and identify the blood groups of
the two samples, respectively. (2 marks)
Known sample: Unknown sample:
Blood group: A positive Blood group: B positive
(Ono et al, 2017)
7) Red cell transfusion in warm-antibody autoimmune hemolytic anemia presents difficulty
in crossmatching and it is nearly always impossible to find truly serocompatible donor
blood. Discuss the strategies of red cell transfusion in patients with severe autoimmune
hemolytic anemia. (8 marks) (maximum 300 words)
Previously, patients who suffered from severe autoimmune hemolytic anemia of warm
type(wAIHA) were treated with immunosuppression; however, patients failed to respond
adequately to the treatment and developed undesirable side effects owing to uncontrolled
immunosuppression which showed detrimental effects. So, an optimized therapeutic
approach and clinical strategy was established, which involved controlled administration of
steroids, complemented with erythropoiesis-stimulating agents(Kalfa, 2016). So before blood
transfusion, a particular compatibility test should be performed to select an optimal unit of
RBC and for determining the presence or absence of alloantibodies which may lead to a
potential hemolytic transfusion reaction. Moreover, proper communication between clinicians
and laboratory personnel are required for reviewing the urgency of transfusion. The
acuteness and rapidity of progression of wAIHA should be assessed, and the individual’s
serum should be tested properly before blood transfusion. And it should be ensured that only
phenotypically matching RBCs should be used in a transfusion.
The laboratory evaluation that are required for red cell transfusion in patients with severe
autoimmune haemolytic anemia are:
Complete blood cell count – which detects the agglutination of red blood cells on fims
7
6) Draw your results onto the blank cassettes shown below, and identify the blood groups of
the two samples, respectively. (2 marks)
Known sample: Unknown sample:
Blood group: A positive Blood group: B positive
(Ono et al, 2017)
7) Red cell transfusion in warm-antibody autoimmune hemolytic anemia presents difficulty
in crossmatching and it is nearly always impossible to find truly serocompatible donor
blood. Discuss the strategies of red cell transfusion in patients with severe autoimmune
hemolytic anemia. (8 marks) (maximum 300 words)
Previously, patients who suffered from severe autoimmune hemolytic anemia of warm
type(wAIHA) were treated with immunosuppression; however, patients failed to respond
adequately to the treatment and developed undesirable side effects owing to uncontrolled
immunosuppression which showed detrimental effects. So, an optimized therapeutic
approach and clinical strategy was established, which involved controlled administration of
steroids, complemented with erythropoiesis-stimulating agents(Kalfa, 2016). So before blood
transfusion, a particular compatibility test should be performed to select an optimal unit of
RBC and for determining the presence or absence of alloantibodies which may lead to a
potential hemolytic transfusion reaction. Moreover, proper communication between clinicians
and laboratory personnel are required for reviewing the urgency of transfusion. The
acuteness and rapidity of progression of wAIHA should be assessed, and the individual’s
serum should be tested properly before blood transfusion. And it should be ensured that only
phenotypically matching RBCs should be used in a transfusion.
The laboratory evaluation that are required for red cell transfusion in patients with severe
autoimmune haemolytic anemia are:
Complete blood cell count – which detects the agglutination of red blood cells on fims
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Peripheral blood smear test – used for identifying spherocytes, fragmented RBCs,
nucleated RBCs and polychromasia
DAT test or Coombs Test- It is also known as an antiglobulin test. It is of two types-
the direct Coombs test and the indirect Coombs test. Direct Coombs test helps in the
detection of antibodies which coats the donor RBC, whereas, indirect Coombs test
helps in detecting the presence of antibodies in the recipient blood before transfusion.
ABO related acute hemolytic transfusion may lead to a positive anti-globulin reaction,
which signifies the presence of complement (C3d) on the circulating RBC along with
the recipient’s anti-A and anti-B or anti-A, B. Majority are positive, may give negative
result if all incompatible cells are destroyed(Hill et al, 2017).
Coagulation screening – used for the detection of disseminated intravascular
coagulation
Compatibility testing – it is used for detecting the presence of alloantibodies
Haptoglobin and plasma/ unirany haemoglobin test – used for the identifying both
intravascular and extravascular haemolysis
8) Describe the consequences of receiving ABO-mismatched red blood transfusions.
Explain the mechanisms by which the consequences may arise and summarise the
clinical features associated with the consequences. (10 marks) (maximum 400 words)
ABO-mismatched blood transfusion results in both Immune and non-immune mediated
acute haemolytic transfusion. Immune mediated acute haemolytic transfusion is caused by
the infusion of RBCs which are not compatible with the patient’s anti-A, anti-B or other RBC
antibodies. It can also result from failed identification of patient’s blood group during
specimen identification or transfusion. Non-immune acute haemolytic transfusion results
from haemolysis of the red blood cells by factors such as coadministration of RBC with an
incompatible crystalloid solution of 5% dextrose solution, improper storage of blood or by
non-validated blood administration methods. Acute hemolytic reactions result from ABO
incompatibility. The recipient’s blood contains antibodies against A and B blood groups,
which destroys the donor RBCs also activating the blood clotting factor XII which leads to
intravascular coagulation and damage of kidney. They also induce complement activation,
resulting in the release of cytokinins like IL-1, IL—6, which cause a lowering of blood
pressure.
The pathogenesis of acute haemolytic anaemia involves MAC (Membrane Attack Complex)
formation which leads to osmotic lysis of the erythrocytes and activates the complement
system, releasing C3a or C5a. This leads to activation of the White blood cells and release
of cytokines. Moreover, the autoantibodies are also able to destroy RBC with the help of
8
nucleated RBCs and polychromasia
DAT test or Coombs Test- It is also known as an antiglobulin test. It is of two types-
the direct Coombs test and the indirect Coombs test. Direct Coombs test helps in the
detection of antibodies which coats the donor RBC, whereas, indirect Coombs test
helps in detecting the presence of antibodies in the recipient blood before transfusion.
ABO related acute hemolytic transfusion may lead to a positive anti-globulin reaction,
which signifies the presence of complement (C3d) on the circulating RBC along with
the recipient’s anti-A and anti-B or anti-A, B. Majority are positive, may give negative
result if all incompatible cells are destroyed(Hill et al, 2017).
Coagulation screening – used for the detection of disseminated intravascular
coagulation
Compatibility testing – it is used for detecting the presence of alloantibodies
Haptoglobin and plasma/ unirany haemoglobin test – used for the identifying both
intravascular and extravascular haemolysis
8) Describe the consequences of receiving ABO-mismatched red blood transfusions.
Explain the mechanisms by which the consequences may arise and summarise the
clinical features associated with the consequences. (10 marks) (maximum 400 words)
ABO-mismatched blood transfusion results in both Immune and non-immune mediated
acute haemolytic transfusion. Immune mediated acute haemolytic transfusion is caused by
the infusion of RBCs which are not compatible with the patient’s anti-A, anti-B or other RBC
antibodies. It can also result from failed identification of patient’s blood group during
specimen identification or transfusion. Non-immune acute haemolytic transfusion results
from haemolysis of the red blood cells by factors such as coadministration of RBC with an
incompatible crystalloid solution of 5% dextrose solution, improper storage of blood or by
non-validated blood administration methods. Acute hemolytic reactions result from ABO
incompatibility. The recipient’s blood contains antibodies against A and B blood groups,
which destroys the donor RBCs also activating the blood clotting factor XII which leads to
intravascular coagulation and damage of kidney. They also induce complement activation,
resulting in the release of cytokinins like IL-1, IL—6, which cause a lowering of blood
pressure.
The pathogenesis of acute haemolytic anaemia involves MAC (Membrane Attack Complex)
formation which leads to osmotic lysis of the erythrocytes and activates the complement
system, releasing C3a or C5a. This leads to activation of the White blood cells and release
of cytokines. Moreover, the autoantibodies are also able to destroy RBC with the help of
8

antibody dependent cell mediated cytotoxity (ADCC), which is itself mediated by CD8+ T
cells and natural killer cells. These cells helps to transport membrane receptors to the Fc
portion of immunoglobulin G (IgG). At last, the activated macrophages carrying those Fc
receptors recognize and phagocytose the erythrocytes which are already opsonized by
autoantibodies and complement. While direct complement-mediated lysis occurs mostly in
the blood circulation and liver, antibody dependent cell mediated cytotoxity takes place
preferably in the spleen and lymphoid organs(Worel, 2016).
The mismatched antigen-antibody reaction may lead to intravascular or extravascular
haemolysis, along with fever and chills, dyspnoea and hypotension among individuals. The
other clinical presentation of acute haemolytic transfusion reaction are tachychardia, pain in
abdomen and chest area accompanied with nausea and vomiting. It can also result in
haemoglobinuria( a condition where haemoglobin protein is found in exceptionally high
amount in the urine of an individual), renal failure and disseminated intravascular
coagulation(DIC), a condition affecting the blood’s ability to clot leading to major blood loss
from the body. DIC results due to formation of abnormal clumps of blood or clots within the
blood vessels which utilizes the factors responsible for blood clotting.
9) Discuss the laboratory investigations for patients with suspected acute hemolytic
transfusion reactions. (8.5 marks) (maximum 350 words)
The laboratory investigations performed for patients who are suspected with acute hemolytic
transfusion reactions are visual inspection of the recipient’s plasma followed by the urine
test, retyping of the recipient and donor RBC and direct antiglobulin test or Coombs test.
Overview of the laboratory test:
1. Antibody screening test – It determines the presence of an unexpected antibody,
other anti-A, and anti-B by testing patient serum against the screening cells.
2. Coombs test – It is also known as an antiglobulin test. It is of two types- the direct
Coombs test and the indirect Coombs test. Direct Coombs test helps in the detection
of antibodies which coats the donor RBC, whereas, indirect Coombs test helps in
detecting the presence of antibodies in the recipient blood before transfusion. ABO
related acute hemolytic transfusion may lead to a positive anti-globulin reaction,
which signifies the presence of complement (C3d) on the circulating RBC along with
the recipient’s anti-A and anti-B or anti-A, B.
3. Urinalysis – An urinalysis is a test to detect urinary tract infection, diabetes, and
kidney problems by checking the appearance, composition, and concentration of the
9
cells and natural killer cells. These cells helps to transport membrane receptors to the Fc
portion of immunoglobulin G (IgG). At last, the activated macrophages carrying those Fc
receptors recognize and phagocytose the erythrocytes which are already opsonized by
autoantibodies and complement. While direct complement-mediated lysis occurs mostly in
the blood circulation and liver, antibody dependent cell mediated cytotoxity takes place
preferably in the spleen and lymphoid organs(Worel, 2016).
The mismatched antigen-antibody reaction may lead to intravascular or extravascular
haemolysis, along with fever and chills, dyspnoea and hypotension among individuals. The
other clinical presentation of acute haemolytic transfusion reaction are tachychardia, pain in
abdomen and chest area accompanied with nausea and vomiting. It can also result in
haemoglobinuria( a condition where haemoglobin protein is found in exceptionally high
amount in the urine of an individual), renal failure and disseminated intravascular
coagulation(DIC), a condition affecting the blood’s ability to clot leading to major blood loss
from the body. DIC results due to formation of abnormal clumps of blood or clots within the
blood vessels which utilizes the factors responsible for blood clotting.
9) Discuss the laboratory investigations for patients with suspected acute hemolytic
transfusion reactions. (8.5 marks) (maximum 350 words)
The laboratory investigations performed for patients who are suspected with acute hemolytic
transfusion reactions are visual inspection of the recipient’s plasma followed by the urine
test, retyping of the recipient and donor RBC and direct antiglobulin test or Coombs test.
Overview of the laboratory test:
1. Antibody screening test – It determines the presence of an unexpected antibody,
other anti-A, and anti-B by testing patient serum against the screening cells.
2. Coombs test – It is also known as an antiglobulin test. It is of two types- the direct
Coombs test and the indirect Coombs test. Direct Coombs test helps in the detection
of antibodies which coats the donor RBC, whereas, indirect Coombs test helps in
detecting the presence of antibodies in the recipient blood before transfusion. ABO
related acute hemolytic transfusion may lead to a positive anti-globulin reaction,
which signifies the presence of complement (C3d) on the circulating RBC along with
the recipient’s anti-A and anti-B or anti-A, B.
3. Urinalysis – An urinalysis is a test to detect urinary tract infection, diabetes, and
kidney problems by checking the appearance, composition, and concentration of the
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

urine. Urinalysis may also detect hemoglobinuria. When the level of hemoglobin rises
in the urine, the urine color changes to reddish-brown(Green and Slayten, 2018).
4. Repeat ABO typing donor should be performed by collecting the blood sample post-
transfusion reactions and should be checked for any discrepancies between the
results of the original ABO type and repeat ABO type(Siddon and Tormey, 2019).
5. Compatibility testing – it is an Indirect Antiglobulin Test (IAT) where antibody is
screened and IAT crossmatched using pre and post transfusion sample. It provides
evidence for the presence of alloantibodies. Elution of antibodies from post-
transfused RBC may help in identification of antibody or in confirming specificities that
are identified in serum, in case of non-ABO incompatibility(Hill et al, 2017).
6. Urea/Creatitine and electrolytes blood test- Used to test the proper functioning of
the kidney
7. Screening of blood coagulation- This test is performed for detecting the presence
of Disseminated intravascular coagulation
8. Full blood count- used to check the agglutianation of red blood cells on blood fims.
9. Plasma/ urinary haemoglobin test- detects for the presence of intravascular
haemolysis
3. Antibody screening
Antibody screening is usually undertaken at the same time as blood grouping, and is
important in antenatal patients (who may have or develop antibodies that will affect the
fetus/baby), and prior to transfusion. It works by taking some of the patient’s serum and
mixing it with at least two different preparations of group O red blood cells (i.e. eliminating
the potential effects of anti-A and anti-B antibodies that the patient has anyway – these
cannot react against group O cells). These group O cells will have been tested and are
known to express a minimum set of antigens. In the UK, the minimum set of antigens is:
C, c, D, E, K, k, Fya, Fyb, Jka, Jkb, S, s, M, N, and Lea
Ideally, red cells should be homozygous for antigens (e.g. one set of Group O cells should
be SS and the other ss) to get the maximum agglutination reaction from any antibodies
present in the patient’s serum, so multiple sets of Group O cells are needed.
Like ABO and Rhesus grouping, most hospital labs now use automated systems for
antibody screening, and column agglutination is a common method that is demonstrated
here. Small volumes of the patient’s serum are added to each tube within a cassette –
each tube contains Group O red blood cells which express known antigens, and an anti-
human immunoglobulin. If the patient’s serum contains antibodies against any of the
antigens on the red blood cells, binding of the antibody to the antigen occurs. The anti-
human globulin present in the tube then causes the antibody-coated red blood cells to
10
in the urine, the urine color changes to reddish-brown(Green and Slayten, 2018).
4. Repeat ABO typing donor should be performed by collecting the blood sample post-
transfusion reactions and should be checked for any discrepancies between the
results of the original ABO type and repeat ABO type(Siddon and Tormey, 2019).
5. Compatibility testing – it is an Indirect Antiglobulin Test (IAT) where antibody is
screened and IAT crossmatched using pre and post transfusion sample. It provides
evidence for the presence of alloantibodies. Elution of antibodies from post-
transfused RBC may help in identification of antibody or in confirming specificities that
are identified in serum, in case of non-ABO incompatibility(Hill et al, 2017).
6. Urea/Creatitine and electrolytes blood test- Used to test the proper functioning of
the kidney
7. Screening of blood coagulation- This test is performed for detecting the presence
of Disseminated intravascular coagulation
8. Full blood count- used to check the agglutianation of red blood cells on blood fims.
9. Plasma/ urinary haemoglobin test- detects for the presence of intravascular
haemolysis
3. Antibody screening
Antibody screening is usually undertaken at the same time as blood grouping, and is
important in antenatal patients (who may have or develop antibodies that will affect the
fetus/baby), and prior to transfusion. It works by taking some of the patient’s serum and
mixing it with at least two different preparations of group O red blood cells (i.e. eliminating
the potential effects of anti-A and anti-B antibodies that the patient has anyway – these
cannot react against group O cells). These group O cells will have been tested and are
known to express a minimum set of antigens. In the UK, the minimum set of antigens is:
C, c, D, E, K, k, Fya, Fyb, Jka, Jkb, S, s, M, N, and Lea
Ideally, red cells should be homozygous for antigens (e.g. one set of Group O cells should
be SS and the other ss) to get the maximum agglutination reaction from any antibodies
present in the patient’s serum, so multiple sets of Group O cells are needed.
Like ABO and Rhesus grouping, most hospital labs now use automated systems for
antibody screening, and column agglutination is a common method that is demonstrated
here. Small volumes of the patient’s serum are added to each tube within a cassette –
each tube contains Group O red blood cells which express known antigens, and an anti-
human immunoglobulin. If the patient’s serum contains antibodies against any of the
antigens on the red blood cells, binding of the antibody to the antigen occurs. The anti-
human globulin present in the tube then causes the antibody-coated red blood cells to
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
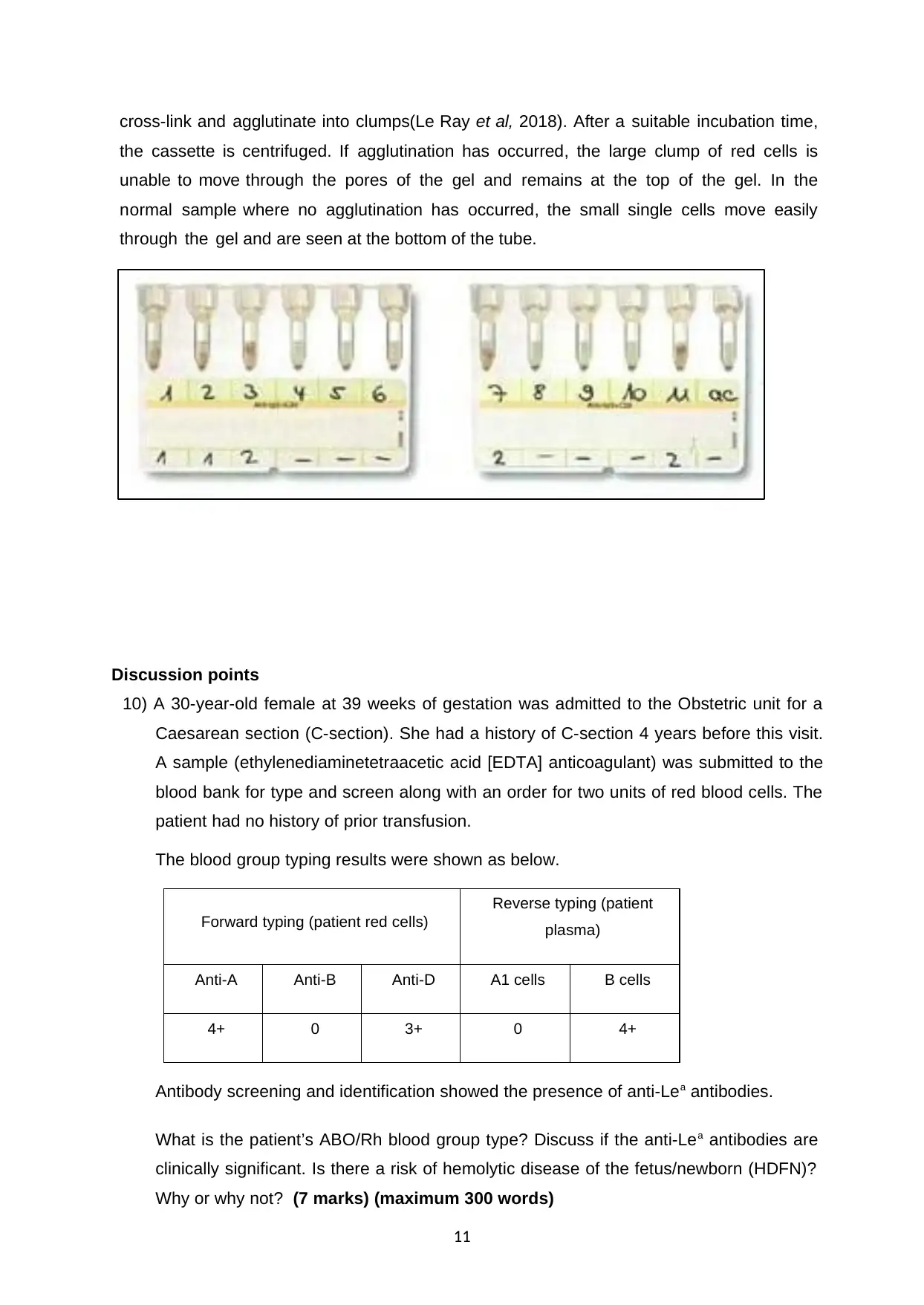
cross-link and agglutinate into clumps(Le Ray et al, 2018). After a suitable incubation time,
the cassette is centrifuged. If agglutination has occurred, the large clump of red cells is
unable to move through the pores of the gel and remains at the top of the gel. In the
normal sample where no agglutination has occurred, the small single cells move easily
through the gel and are seen at the bottom of the tube.
Discussion points
10) A 30-year-old female at 39 weeks of gestation was admitted to the Obstetric unit for a
Caesarean section (C-section). She had a history of C-section 4 years before this visit.
A sample (ethylenediaminetetraacetic acid [EDTA] anticoagulant) was submitted to the
blood bank for type and screen along with an order for two units of red blood cells. The
patient had no history of prior transfusion.
The blood group typing results were shown as below.
Forward typing (patient red cells)
Reverse typing (patient
plasma)
Anti-A Anti-B Anti-D A1 cells B cells
4+ 0 3+ 0 4+
Antibody screening and identification showed the presence of anti-Lea antibodies.
What is the patient’s ABO/Rh blood group type? Discuss if the anti-Lea antibodies are
clinically significant. Is there a risk of hemolytic disease of the fetus/newborn (HDFN)?
Why or why not? (7 marks) (maximum 300 words)
11
the cassette is centrifuged. If agglutination has occurred, the large clump of red cells is
unable to move through the pores of the gel and remains at the top of the gel. In the
normal sample where no agglutination has occurred, the small single cells move easily
through the gel and are seen at the bottom of the tube.
Discussion points
10) A 30-year-old female at 39 weeks of gestation was admitted to the Obstetric unit for a
Caesarean section (C-section). She had a history of C-section 4 years before this visit.
A sample (ethylenediaminetetraacetic acid [EDTA] anticoagulant) was submitted to the
blood bank for type and screen along with an order for two units of red blood cells. The
patient had no history of prior transfusion.
The blood group typing results were shown as below.
Forward typing (patient red cells)
Reverse typing (patient
plasma)
Anti-A Anti-B Anti-D A1 cells B cells
4+ 0 3+ 0 4+
Antibody screening and identification showed the presence of anti-Lea antibodies.
What is the patient’s ABO/Rh blood group type? Discuss if the anti-Lea antibodies are
clinically significant. Is there a risk of hemolytic disease of the fetus/newborn (HDFN)?
Why or why not? (7 marks) (maximum 300 words)
11

Here the blood group of the patient is A since the value of the forward typing with Anti-A
is 4+ which in reverse typing gets reversed so that the value of B cells in plasma is 4+.
The anti-Lea antibodies do not usually have any clinical significance due to the
presence of abundant Lewis substances in the serum, which leads to neutralization of
the antibodies during the crossmatch in vitro or during transfusion in vivo. However, it
can be useful immunohematology since they are the only antigen that is not produced
by RBC itself. They are present in the body secretion. The lewis positive donor cells
can become lewis negative after transfusion due to their easy dissociation from the
cells. No, the patient has a low chance of facing the hemolytic disease of the fetus.
Since the anti-Lea antibodies are not clinically significant so they will not be able to
reduce the survival of the red cells, hence the chance of hemolytic disease of the fetus
is less(Srijinda et al, 2017).
Hemolytic disease of the newborn, also known as erythroblastosis fetalis is a blood-
related disorder in newborn babies. It happens as a result of incompatibility between
mother and fetus blood. It usually happens when the Rh-negative mother has a baby
with an Rh-positive father. If the baby is also Rh positive, then it can cross the placenta
and get into the bloodstream of the mother. The immune system of the mother
recognizes the Rh+ of the baby as foreign and destroy it. An antibody can only cause
fetal hemolytic disease if it crosses the placenta and reacts with the antigens present
on the surface of the RBC, but lewis antibodies cannot cross the placenta, they cannot
cause HDFN(Azuonwu et al, 2016). So the baby is not at the risk of HDFN and the
mother can be safely transfused. Since the mother’s blood contains Anti-D, so it will
destroy any RhD + blood cells that may cross the placenta and gets into the
bloodstream during the delivery. As a result, the blood will not be able to produce
antibodies which will reduce the risk of the baby to suffer from the hemolytic disease
REFERENCE:
12
is 4+ which in reverse typing gets reversed so that the value of B cells in plasma is 4+.
The anti-Lea antibodies do not usually have any clinical significance due to the
presence of abundant Lewis substances in the serum, which leads to neutralization of
the antibodies during the crossmatch in vitro or during transfusion in vivo. However, it
can be useful immunohematology since they are the only antigen that is not produced
by RBC itself. They are present in the body secretion. The lewis positive donor cells
can become lewis negative after transfusion due to their easy dissociation from the
cells. No, the patient has a low chance of facing the hemolytic disease of the fetus.
Since the anti-Lea antibodies are not clinically significant so they will not be able to
reduce the survival of the red cells, hence the chance of hemolytic disease of the fetus
is less(Srijinda et al, 2017).
Hemolytic disease of the newborn, also known as erythroblastosis fetalis is a blood-
related disorder in newborn babies. It happens as a result of incompatibility between
mother and fetus blood. It usually happens when the Rh-negative mother has a baby
with an Rh-positive father. If the baby is also Rh positive, then it can cross the placenta
and get into the bloodstream of the mother. The immune system of the mother
recognizes the Rh+ of the baby as foreign and destroy it. An antibody can only cause
fetal hemolytic disease if it crosses the placenta and reacts with the antigens present
on the surface of the RBC, but lewis antibodies cannot cross the placenta, they cannot
cause HDFN(Azuonwu et al, 2016). So the baby is not at the risk of HDFN and the
mother can be safely transfused. Since the mother’s blood contains Anti-D, so it will
destroy any RhD + blood cells that may cross the placenta and gets into the
bloodstream during the delivery. As a result, the blood will not be able to produce
antibodies which will reduce the risk of the baby to suffer from the hemolytic disease
REFERENCE:
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





