The Boiling Points of Organic Compounds: Factors and Analysis
VerifiedAdded on 2022/10/01
|8
|1402
|17
Report
AI Summary
This report examines the boiling points of four organic compounds: 3-Methylbutanal, Butanoic Acid, Hexanal, and Pentanal. It begins with an introduction to boiling points and their dependence on chemical composition. The report presents the boiling points of each compound and then delves into the factors that affect these points, including intermolecular forces (ionic, hydrogen bonding, dipole-dipole, and Van der Waals), polarity, molecular weight, and symmetry. The analysis reveals that Butanoic Acid has the highest boiling point due to strong hydrogen bonds, followed by Hexanal, Pentanal, and finally 3-Methylbutanal, with the lowest boiling point due to its lower molecular weight and branching. The report uses structural diagrams and references to support its conclusions, offering a detailed understanding of the relationship between molecular structure and boiling point in organic compounds. The report is a valuable resource for students studying chemistry, providing a clear explanation of the principles governing boiling points and the properties of the compounds.

Boiling Points of Organic Compounds 1
BOILING POINTS OF ORGANIC COMPOUNDS
by (Name)
The Name of the Class (Course)
Professor (Tutor)
The Name of the School (University)
The City and State where it is located
The Date
BOILING POINTS OF ORGANIC COMPOUNDS
by (Name)
The Name of the Class (Course)
Professor (Tutor)
The Name of the School (University)
The City and State where it is located
The Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Boiling Points of Organic Compounds 2
Boiling Points of 3-Methylbutanal, Butanoic Acid, Hexanal, And Pentanal
Introduction
Boiling point is a temperature at which the fluid’s (normally liquid) vapour pressure matches the
outside pressure surrounding it. Variation in chemicals boiling points is due to difference in their
chemical compositions (Timberlake, k. 2015). Determination of the boiling points can only be
attained experimentally, by taking the measurements. The compounds named above are all
organic compound.
Table 1. Compounds and their boiling points at a pressure of 760 mmHg
Compound Boiling points in degrees Celsius at
760 mmHg
Molecular weight (g/ mol)
Butanoic Acid 164.3 ± 3.0 88.11
Hexanal 127.9 ± 3.0 100.17
Pentanal 103.7 ± 3.0 86.13
3-Methylbutanal 92.5° 86.13
Order of the boiling points
Butanoic Acid
Hexanal
Pentanal
3-Methylbutanal.
Factors Affecting the Boiling Points of the Organic Compounds
Reducing
boiling points
Boiling Points of 3-Methylbutanal, Butanoic Acid, Hexanal, And Pentanal
Introduction
Boiling point is a temperature at which the fluid’s (normally liquid) vapour pressure matches the
outside pressure surrounding it. Variation in chemicals boiling points is due to difference in their
chemical compositions (Timberlake, k. 2015). Determination of the boiling points can only be
attained experimentally, by taking the measurements. The compounds named above are all
organic compound.
Table 1. Compounds and their boiling points at a pressure of 760 mmHg
Compound Boiling points in degrees Celsius at
760 mmHg
Molecular weight (g/ mol)
Butanoic Acid 164.3 ± 3.0 88.11
Hexanal 127.9 ± 3.0 100.17
Pentanal 103.7 ± 3.0 86.13
3-Methylbutanal 92.5° 86.13
Order of the boiling points
Butanoic Acid
Hexanal
Pentanal
3-Methylbutanal.
Factors Affecting the Boiling Points of the Organic Compounds
Reducing
boiling points

Boiling Points of Organic Compounds 3
The boiling points reveal the strength of forces among the molecules. The more they bond
together, the more energy it will take to discharge them into the air as gases. The following
factors determine the boiling points of the compounds subjected to the same external factors like
the atmospheric pressure (Sherwood & Dalby, 2018).
Nature of the intermolecular forces.
The intermolecular forces between the molecules of the organic compounds are: ionic, hydrogen
bonding, dipole to dipole forces and the Van der Waals dispersion forces. Their strength reduces
in that order, ionic bonding being the strongest and the Van der Waals dispersion forces being
the weakest.
Polarity
This is the attraction between the molecules of a liquid as a result of a significant differences in
electronegativity between two or more elements making up the liquid molecules (Carey et al.,
2018). Some of the organic molecules are dipolar.
In polar compounds the elements with the unlike polarities attract each other, meaning the
positive end is attracted to the negative end of another molecule. The resultant charge effect
causes the molecules to attract each other. Functional group determines the polarity of a given
molecule. The more the functional groups the greater the polarity. And the greater the polarity
the higher the boiling points (Anon 2019).
The molecular weight
The boiling points reveal the strength of forces among the molecules. The more they bond
together, the more energy it will take to discharge them into the air as gases. The following
factors determine the boiling points of the compounds subjected to the same external factors like
the atmospheric pressure (Sherwood & Dalby, 2018).
Nature of the intermolecular forces.
The intermolecular forces between the molecules of the organic compounds are: ionic, hydrogen
bonding, dipole to dipole forces and the Van der Waals dispersion forces. Their strength reduces
in that order, ionic bonding being the strongest and the Van der Waals dispersion forces being
the weakest.
Polarity
This is the attraction between the molecules of a liquid as a result of a significant differences in
electronegativity between two or more elements making up the liquid molecules (Carey et al.,
2018). Some of the organic molecules are dipolar.
In polar compounds the elements with the unlike polarities attract each other, meaning the
positive end is attracted to the negative end of another molecule. The resultant charge effect
causes the molecules to attract each other. Functional group determines the polarity of a given
molecule. The more the functional groups the greater the polarity. And the greater the polarity
the higher the boiling points (Anon 2019).
The molecular weight
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Boiling Points of Organic Compounds 4
Increase in the number of the length of carbon-carbon chain in an organic compound increases
the boiling point. Increase in the number of the carbon atoms chain increases the surface area for
the organic compounds to interact more with each other by bonding (Carey et al., 2018). The
type of molecular attraction which occur at this level is the Van der Waals forces. Van der Waals
forces are weak electrostatic forces that attract neutral molecule to one another.
Symmetry of the organic compound
Branching affects the surface area of organic compound by reducing the surface area which plays
a major role in bonding. It reduces the surface area thereby limiting the attractive force between
the organic molecules (Timberlake, k. 2015). This results in boiling point reduction.
Analysis of the Compounds
Butanoic Acid
Butanoic acid has the highest boiling point compared to the other three organic substances. This
is because it has a more superior intermolecular forces known as the hydrogen bonds which is
more superior compared to the other types of forces binding the molecules together (Carey et al.,
2018). As illustrated and explained above the hydrogen bonds exist between the molecules of
butanoic acid due to difference in electronegativity between the functional group elements in the
butanoic acid molecules.
Illustration of hydrogen bonds
Increase in the number of the length of carbon-carbon chain in an organic compound increases
the boiling point. Increase in the number of the carbon atoms chain increases the surface area for
the organic compounds to interact more with each other by bonding (Carey et al., 2018). The
type of molecular attraction which occur at this level is the Van der Waals forces. Van der Waals
forces are weak electrostatic forces that attract neutral molecule to one another.
Symmetry of the organic compound
Branching affects the surface area of organic compound by reducing the surface area which plays
a major role in bonding. It reduces the surface area thereby limiting the attractive force between
the organic molecules (Timberlake, k. 2015). This results in boiling point reduction.
Analysis of the Compounds
Butanoic Acid
Butanoic acid has the highest boiling point compared to the other three organic substances. This
is because it has a more superior intermolecular forces known as the hydrogen bonds which is
more superior compared to the other types of forces binding the molecules together (Carey et al.,
2018). As illustrated and explained above the hydrogen bonds exist between the molecules of
butanoic acid due to difference in electronegativity between the functional group elements in the
butanoic acid molecules.
Illustration of hydrogen bonds
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Boiling Points of Organic Compounds 5
The boiling point of butanoic acid is higher too than that of hexanal, pentanal and 3-
methylbutanal even though all have hydrogen bonds due to the difference in the number of the
oxygen atoms making up the functional groups. Butanoic acid has two oxygen atoms within the
functional group while hexanal has one oxygen atom within the functional group. Butanoic acid
therefore has two highly electronegative oxygen within the functional group making its structure,
while hexanal, butanal and 3-methylbutanal have only one highly electronegative oxygen atom
within their functional group making up their structure (Carey et al., 2018). Therefore, butanoic
acid forms two stronger hydrogen bonds between the neighbouring molecules unlike the rest of
the three compounds which their molecules make only one stronger hydrogen bonds with the
surrounding molecules.
Butanoic acid has a higher boiling point than hexanal though it has a lower molecular weight
because the extra hydrogen bond it got between its molecules is more superior than the Van der
Waals forces gained by hexanal through the additional carbon-carbon chain in its structure.
Hexanal
Hexanal has a higher boiling points than pentanal and 3-methylbutanal organic compounds due
to one reason: it has a longer carbon–carbon atoms chain. Hexanal has the highest molecular
weight than both compounds. Increase in molecular weight or carbon–carbon atoms chain
Structure of Butanoic acid
The boiling point of butanoic acid is higher too than that of hexanal, pentanal and 3-
methylbutanal even though all have hydrogen bonds due to the difference in the number of the
oxygen atoms making up the functional groups. Butanoic acid has two oxygen atoms within the
functional group while hexanal has one oxygen atom within the functional group. Butanoic acid
therefore has two highly electronegative oxygen within the functional group making its structure,
while hexanal, butanal and 3-methylbutanal have only one highly electronegative oxygen atom
within their functional group making up their structure (Carey et al., 2018). Therefore, butanoic
acid forms two stronger hydrogen bonds between the neighbouring molecules unlike the rest of
the three compounds which their molecules make only one stronger hydrogen bonds with the
surrounding molecules.
Butanoic acid has a higher boiling point than hexanal though it has a lower molecular weight
because the extra hydrogen bond it got between its molecules is more superior than the Van der
Waals forces gained by hexanal through the additional carbon-carbon chain in its structure.
Hexanal
Hexanal has a higher boiling points than pentanal and 3-methylbutanal organic compounds due
to one reason: it has a longer carbon–carbon atoms chain. Hexanal has the highest molecular
weight than both compounds. Increase in molecular weight or carbon–carbon atoms chain
Structure of Butanoic acid
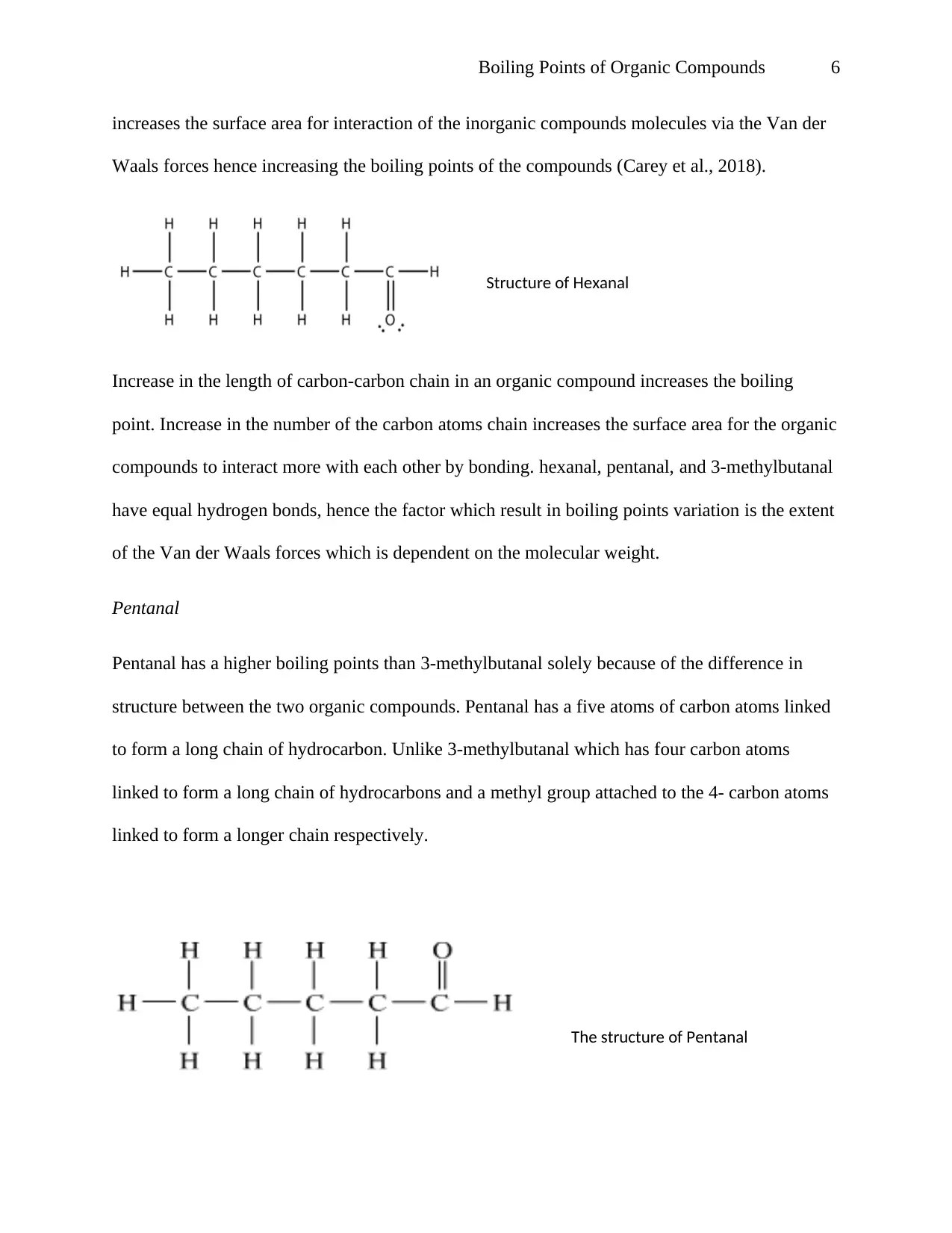
Boiling Points of Organic Compounds 6
increases the surface area for interaction of the inorganic compounds molecules via the Van der
Waals forces hence increasing the boiling points of the compounds (Carey et al., 2018).
Increase in the length of carbon-carbon chain in an organic compound increases the boiling
point. Increase in the number of the carbon atoms chain increases the surface area for the organic
compounds to interact more with each other by bonding. hexanal, pentanal, and 3-methylbutanal
have equal hydrogen bonds, hence the factor which result in boiling points variation is the extent
of the Van der Waals forces which is dependent on the molecular weight.
Pentanal
Pentanal has a higher boiling points than 3-methylbutanal solely because of the difference in
structure between the two organic compounds. Pentanal has a five atoms of carbon atoms linked
to form a long chain of hydrocarbon. Unlike 3-methylbutanal which has four carbon atoms
linked to form a long chain of hydrocarbons and a methyl group attached to the 4- carbon atoms
linked to form a longer chain respectively.
Structure of Hexanal
The structure of Pentanal
increases the surface area for interaction of the inorganic compounds molecules via the Van der
Waals forces hence increasing the boiling points of the compounds (Carey et al., 2018).
Increase in the length of carbon-carbon chain in an organic compound increases the boiling
point. Increase in the number of the carbon atoms chain increases the surface area for the organic
compounds to interact more with each other by bonding. hexanal, pentanal, and 3-methylbutanal
have equal hydrogen bonds, hence the factor which result in boiling points variation is the extent
of the Van der Waals forces which is dependent on the molecular weight.
Pentanal
Pentanal has a higher boiling points than 3-methylbutanal solely because of the difference in
structure between the two organic compounds. Pentanal has a five atoms of carbon atoms linked
to form a long chain of hydrocarbon. Unlike 3-methylbutanal which has four carbon atoms
linked to form a long chain of hydrocarbons and a methyl group attached to the 4- carbon atoms
linked to form a longer chain respectively.
Structure of Hexanal
The structure of Pentanal
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Boiling Points of Organic Compounds 7
Therefore, pentanal has geometry which increases the surface area favouring intraction of the
compounds molecules through Van der Waals forces, thereby increasing its boiling points.
Branching caused by the methyl group lowers the surface area in 3-methybutanal, hence,
reducing its surface area
3-Methylbutanal
3-methylbutanal is a volatile oily liquid. It is a butanal made of four carbon atom long chain with
methyl group substituting hydrogen atom at position 3. It naturally found in olives.
3-Methylbutanal has the lowest boiling point because it has the least molecular weight and also
has branching. Lower molecular weight and branching reduces the surface area for the
interaction of the molecules, thereby reducing the strength of Van de Waals forces (Timberlake,
k. 2015). Reduction in Van der Waals results in lowering of boiling point.
References
Carey, F. A., Giuliano, R. M., Allison, N. T., and Tuttle, S. L. B., 2018. Organic chemistry.
New York, NY: McGraw-Hill Education.
The structure of 3-Methylbutanal
Therefore, pentanal has geometry which increases the surface area favouring intraction of the
compounds molecules through Van der Waals forces, thereby increasing its boiling points.
Branching caused by the methyl group lowers the surface area in 3-methybutanal, hence,
reducing its surface area
3-Methylbutanal
3-methylbutanal is a volatile oily liquid. It is a butanal made of four carbon atom long chain with
methyl group substituting hydrogen atom at position 3. It naturally found in olives.
3-Methylbutanal has the lowest boiling point because it has the least molecular weight and also
has branching. Lower molecular weight and branching reduces the surface area for the
interaction of the molecules, thereby reducing the strength of Van de Waals forces (Timberlake,
k. 2015). Reduction in Van der Waals results in lowering of boiling point.
References
Carey, F. A., Giuliano, R. M., Allison, N. T., and Tuttle, S. L. B., 2018. Organic chemistry.
New York, NY: McGraw-Hill Education.
The structure of 3-Methylbutanal
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Boiling Points of Organic Compounds 8
Libretexts, 2019. Boiling Points [online]. Chemistry LibreTexts. Available from:
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Or
ganic_Chemistry)/Fundamentals/Intermolecular_Forces/Boiling_Points [Accessed 3 Oct
2019].
Anon., 2019. Polarity of Organic Compounds. Available from:
http://chemistry.elmhurst.edu/vchembook/213organicfcgp.html [Accessed 3 Oct 2019].
Sherwood, D. and Dalby, P., 2018. Boiling points and melting points. Oxford Scholarship
Online.
Timberlake, K. C., 2015. Chemistry: an introduction to general, organic, and biological
chemistry. Boston: Pearson.
Libretexts, 2019. Boiling Points [online]. Chemistry LibreTexts. Available from:
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Or
ganic_Chemistry)/Fundamentals/Intermolecular_Forces/Boiling_Points [Accessed 3 Oct
2019].
Anon., 2019. Polarity of Organic Compounds. Available from:
http://chemistry.elmhurst.edu/vchembook/213organicfcgp.html [Accessed 3 Oct 2019].
Sherwood, D. and Dalby, P., 2018. Boiling points and melting points. Oxford Scholarship
Online.
Timberlake, K. C., 2015. Chemistry: an introduction to general, organic, and biological
chemistry. Boston: Pearson.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




