Secondary Breast Cancer Brain Metastasis: A Literature Review
VerifiedAdded on 2022/12/27
|17
|5695
|72
Literature Review
AI Summary
This literature review examines secondary breast cancer brain metastasis, a stage IV condition where cancer cells spread to the brain. It explores risk factors like obesity and hormone therapy, and the lack of a cure. The review delves into the role of the BIG3 gene (ARFGEF3) and its interaction with PHB2 in estrogen signaling, highlighting its potential as a therapeutic target. The document discusses prognostic factors, treatment modalities including surgery, radiation, chemotherapy, and targeted therapies. It emphasizes the 'seed and soil' hypothesis and the impact of cell-surface sialylation. The review also touches upon the genetic characterization of brain metastases, the role of EGFR mutations, and the potential of ERAP as a novel anti-tumor drug. The review presents various studies and figures on the impact of the BIG3 gene and its interaction with other proteins, which highlights its role in tumor suppression and potential therapeutic strategies. This assignment is a detailed overview of the current research and understanding of the topic.

Running head: DISSERTATION
Literature review
Name of the Student
Name of the University
Author Note
Literature review
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1DISSERTATION
Chapter 2: Literature review
Breast cancer refers to the cancer that generally develops in the breast tissue and the
common signs and symptoms of this condition comprise of lump development in the breast,
change in shape of the breast, fluid ejection from the nipple, or scaly red patch on the skin
(Miller et al. 2016). Major risk factors that are responsible for the development of breast
cancer comprise of reduced physical activity, alcohol consumption, obesity, hormone
replacement therapy, and/or early menstruation age (Neuhouser et al. 2015). Secondary
breast cancer, also referred to as metastatic breast cancer refers to stage IV of the disease
where the cancerous cells have spread to sites that are distant from the breast, farther than the
axillary lymph nodes (DeSantis, Ma, Bryan and Jemal 2014). Currently, no cure has been
discovered for secondary breast cancer. It typically occurs more than a few years after
diagnosis of the primary breast cancer, though it is occasionally diagnosed at the similar time
or, seldom, prior to diagnosis of primary breast cancer (Abu-Helalah et al. 2015).
Research evidences elaborate on the fact that breast cancer predominantly metastasizes to the
lungs, bone, liver, regional lymph nodes, and the brain, with bone being one of the most
common region (Aceto et al. 2014). Treatment of secondary breast cancer is dependent on the
site of the metastatic tumours and encompasses several means such as radiation, surgery,
biological therapy, chemotherapy, and hormonal therapy. The brain has been identified as a
unique organ where breast cancer metastasis occurs owing to the fact that the tumour cells
have to cross the blood-brain barrier for development of micro metastasis. According to Chen
and Bourguignon (2014) CD44 is a cell-surface transmembrane glycoprotein that acts as a
hyaluronic acid specific receptor that is responsible for cell adhesion, which in turn is brought
about by binding to particular components of the extracellular matrix. In addition, CD44 also
controls adhesion of the breast cancer cells that are circulating in the brain, at the secondary
site to the endothelium, via the hyaluronate matrix ligand and/or cytoplasmic attachments.
Chapter 2: Literature review
Breast cancer refers to the cancer that generally develops in the breast tissue and the
common signs and symptoms of this condition comprise of lump development in the breast,
change in shape of the breast, fluid ejection from the nipple, or scaly red patch on the skin
(Miller et al. 2016). Major risk factors that are responsible for the development of breast
cancer comprise of reduced physical activity, alcohol consumption, obesity, hormone
replacement therapy, and/or early menstruation age (Neuhouser et al. 2015). Secondary
breast cancer, also referred to as metastatic breast cancer refers to stage IV of the disease
where the cancerous cells have spread to sites that are distant from the breast, farther than the
axillary lymph nodes (DeSantis, Ma, Bryan and Jemal 2014). Currently, no cure has been
discovered for secondary breast cancer. It typically occurs more than a few years after
diagnosis of the primary breast cancer, though it is occasionally diagnosed at the similar time
or, seldom, prior to diagnosis of primary breast cancer (Abu-Helalah et al. 2015).
Research evidences elaborate on the fact that breast cancer predominantly metastasizes to the
lungs, bone, liver, regional lymph nodes, and the brain, with bone being one of the most
common region (Aceto et al. 2014). Treatment of secondary breast cancer is dependent on the
site of the metastatic tumours and encompasses several means such as radiation, surgery,
biological therapy, chemotherapy, and hormonal therapy. The brain has been identified as a
unique organ where breast cancer metastasis occurs owing to the fact that the tumour cells
have to cross the blood-brain barrier for development of micro metastasis. According to Chen
and Bourguignon (2014) CD44 is a cell-surface transmembrane glycoprotein that acts as a
hyaluronic acid specific receptor that is responsible for cell adhesion, which in turn is brought
about by binding to particular components of the extracellular matrix. In addition, CD44 also
controls adhesion of the breast cancer cells that are circulating in the brain, at the secondary
site to the endothelium, via the hyaluronate matrix ligand and/or cytoplasmic attachments.

2DISSERTATION
(Freag 2017) The primary aim of treatment is to regulate and reduce down the spread of
cancerous cells to the brain.
Leone (2015) highlights breast cancer as the common cause of brain metastases and it
is a catastrophic event for patients as it is associated with poor prognosis and survival of
patient. Investigations regarding prognostic factors indicate that tumour subtypes is a
prognostic factor that influences overall survival rate. Another prognostic factor is the
performance status of patients at the time of diagnosis of brain metastases. Some of the local
therapy modalities available for the treatment of brain metastasis include surgical resection,
stereotatic radiosurgery and the whole brain radiation therapy. The advantage of surgical
therapy is that it is associated with improvements in focal deficits and decrease in intracranial
hypertension. In contrast, whole brain radiation therapy plays a role in controlling
macroscopic metastases. However, as brain metastases represent at unmet need in breast
cancer patient, a better understanding of the molecular underpinnings of CNS progression is
needed in future.
According to the "seed and soil" hypothesis particular organs trigger metastases from
one kind of cancer by promoting their proliferation and growth, when compared to other
cancer types. This interaction is generally found to be reciprocal and dynamic, owing to the
capability of the cancer cells to transform the environment where they are present (Liu et al.
2017). Hence, this hypothesis considers tumour embolus as the seed and suggests that the
target organs that are affected by cancer act as the substratum or soil. Secondary breast cancer
in the brain has also been associated with cell-surface sialylation that results in cell–to-cell
interactions. This mechanism is directly responsible for the over-expression of
sialyltransferase in the breast cancer cells that are located in the brain, thereby highlighting
the implication of cell-surface glycosylation in metastatic interactions between different
organs (Vajaria et al. 2016).
(Freag 2017) The primary aim of treatment is to regulate and reduce down the spread of
cancerous cells to the brain.
Leone (2015) highlights breast cancer as the common cause of brain metastases and it
is a catastrophic event for patients as it is associated with poor prognosis and survival of
patient. Investigations regarding prognostic factors indicate that tumour subtypes is a
prognostic factor that influences overall survival rate. Another prognostic factor is the
performance status of patients at the time of diagnosis of brain metastases. Some of the local
therapy modalities available for the treatment of brain metastasis include surgical resection,
stereotatic radiosurgery and the whole brain radiation therapy. The advantage of surgical
therapy is that it is associated with improvements in focal deficits and decrease in intracranial
hypertension. In contrast, whole brain radiation therapy plays a role in controlling
macroscopic metastases. However, as brain metastases represent at unmet need in breast
cancer patient, a better understanding of the molecular underpinnings of CNS progression is
needed in future.
According to the "seed and soil" hypothesis particular organs trigger metastases from
one kind of cancer by promoting their proliferation and growth, when compared to other
cancer types. This interaction is generally found to be reciprocal and dynamic, owing to the
capability of the cancer cells to transform the environment where they are present (Liu et al.
2017). Hence, this hypothesis considers tumour embolus as the seed and suggests that the
target organs that are affected by cancer act as the substratum or soil. Secondary breast cancer
in the brain has also been associated with cell-surface sialylation that results in cell–to-cell
interactions. This mechanism is directly responsible for the over-expression of
sialyltransferase in the breast cancer cells that are located in the brain, thereby highlighting
the implication of cell-surface glycosylation in metastatic interactions between different
organs (Vajaria et al. 2016).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
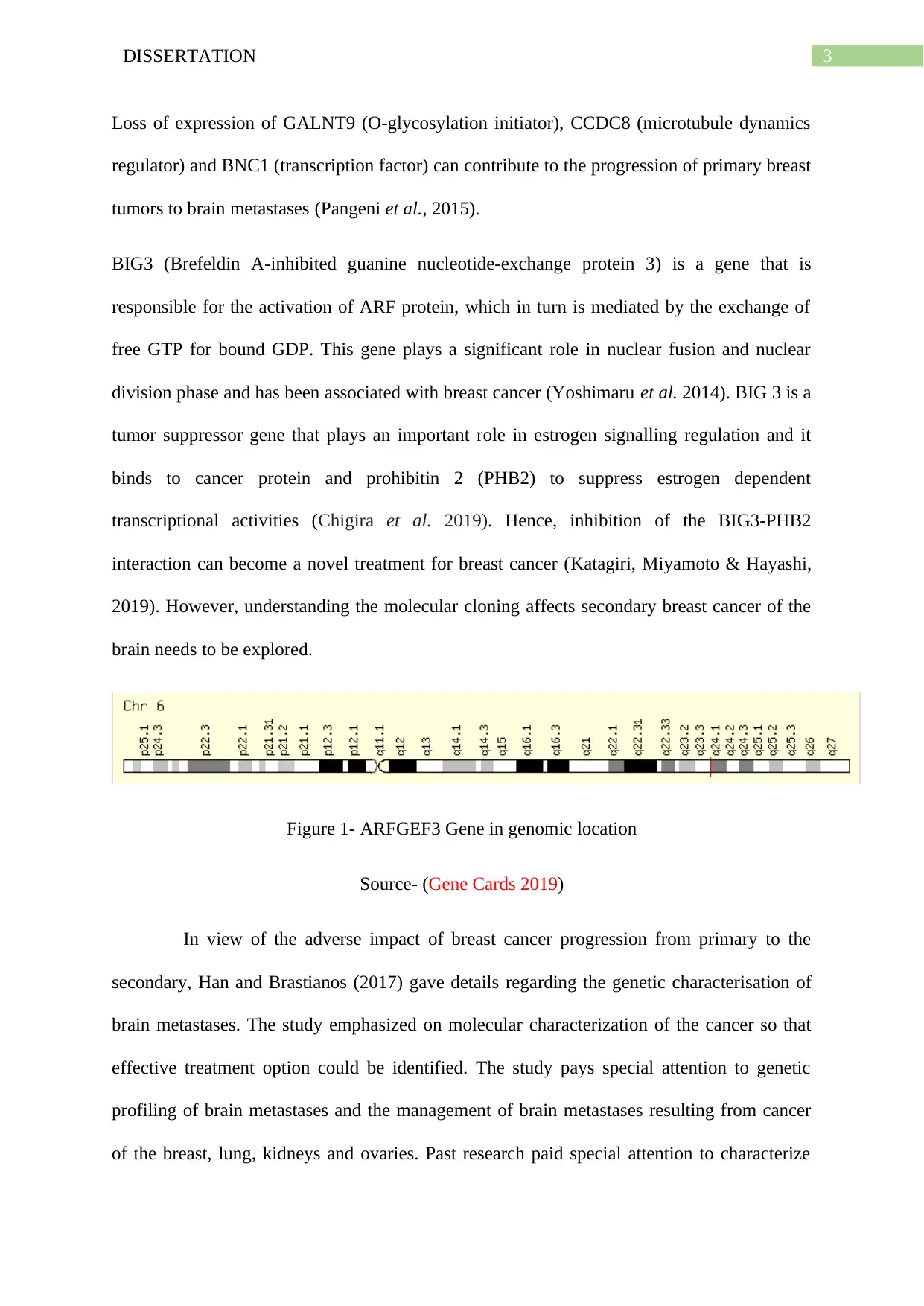
3DISSERTATION
Loss of expression of GALNT9 (O-glycosylation initiator), CCDC8 (microtubule dynamics
regulator) and BNC1 (transcription factor) can contribute to the progression of primary breast
tumors to brain metastases (Pangeni et al., 2015).
BIG3 (Brefeldin A-inhibited guanine nucleotide-exchange protein 3) is a gene that is
responsible for the activation of ARF protein, which in turn is mediated by the exchange of
free GTP for bound GDP. This gene plays a significant role in nuclear fusion and nuclear
division phase and has been associated with breast cancer (Yoshimaru et al. 2014). BIG 3 is a
tumor suppressor gene that plays an important role in estrogen signalling regulation and it
binds to cancer protein and prohibitin 2 (PHB2) to suppress estrogen dependent
transcriptional activities (Chigira et al. 2019). Hence, inhibition of the BIG3-PHB2
interaction can become a novel treatment for breast cancer (Katagiri, Miyamoto & Hayashi,
2019). However, understanding the molecular cloning affects secondary breast cancer of the
brain needs to be explored.
Figure 1- ARFGEF3 Gene in genomic location
Source- (Gene Cards 2019)
In view of the adverse impact of breast cancer progression from primary to the
secondary, Han and Brastianos (2017) gave details regarding the genetic characterisation of
brain metastases. The study emphasized on molecular characterization of the cancer so that
effective treatment option could be identified. The study pays special attention to genetic
profiling of brain metastases and the management of brain metastases resulting from cancer
of the breast, lung, kidneys and ovaries. Past research paid special attention to characterize
Loss of expression of GALNT9 (O-glycosylation initiator), CCDC8 (microtubule dynamics
regulator) and BNC1 (transcription factor) can contribute to the progression of primary breast
tumors to brain metastases (Pangeni et al., 2015).
BIG3 (Brefeldin A-inhibited guanine nucleotide-exchange protein 3) is a gene that is
responsible for the activation of ARF protein, which in turn is mediated by the exchange of
free GTP for bound GDP. This gene plays a significant role in nuclear fusion and nuclear
division phase and has been associated with breast cancer (Yoshimaru et al. 2014). BIG 3 is a
tumor suppressor gene that plays an important role in estrogen signalling regulation and it
binds to cancer protein and prohibitin 2 (PHB2) to suppress estrogen dependent
transcriptional activities (Chigira et al. 2019). Hence, inhibition of the BIG3-PHB2
interaction can become a novel treatment for breast cancer (Katagiri, Miyamoto & Hayashi,
2019). However, understanding the molecular cloning affects secondary breast cancer of the
brain needs to be explored.
Figure 1- ARFGEF3 Gene in genomic location
Source- (Gene Cards 2019)
In view of the adverse impact of breast cancer progression from primary to the
secondary, Han and Brastianos (2017) gave details regarding the genetic characterisation of
brain metastases. The study emphasized on molecular characterization of the cancer so that
effective treatment option could be identified. The study pays special attention to genetic
profiling of brain metastases and the management of brain metastases resulting from cancer
of the breast, lung, kidneys and ovaries. Past research paid special attention to characterize
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4DISSERTATION
genetic drivers in the brain. However, research in this area is still going on and better
understanding of the molecular changes associated with secondary breast can help in better
evaluation of brain metastases and knowledge of targeted therapies. Breast cancer is the most
common form of cancer and the exact mechanism leading to brain metastases is unknown.
One study evaluating the cumulative incidence of CNS relapse revealed human epidermal
growth factor receptor 2 (HER2) positive breast cancers as a major risk factor behind brain
metastases (Han and Brastianos 2017). Early occurrence of brain metastases from breast
cancer is associated with early onset tumor. Survival of patient is also found to be dependent
on presence of ER positive tumor types Hence, this finding gives the implication that survival
outcomes for breast cancer patient is affected by different subtypes of breast cancer.
Another molecular study highlights the role of epidermal growth factor (EGFR) mutations on
cumulative incidence of brain metastases too. The study compared cases or incidence of brain
metastases in patients with EGFR compared to patients with EGFR wild types. The study
revealed that mutation in the EGFR gene renders the tumor sensitive to the EGFR tyrosine
kinase inhibitor (Helena et al., 2013). The ultimate impact of such mutation is that it
enhances the chance of survival for patients. Due to positive CNS activity, including EGFR
TKIs in the treatment of brain metastases can be a novel treatment options in the future
(Helena et al., 2013). However, before these molecular mutation are confirmed as a pathway
for effective treatment, there is a needed for prospective investigations of the role of EGFR
TKIs in active brain metastases particularly for primary breast cancer transitioning to
secondary breast cancer to the brain. This research is focused on evaluating the impact of
molecular cloning of BIG3 (ARFGEF3) Gene associate with secondary Breast Cancer in the
Brain.
genetic drivers in the brain. However, research in this area is still going on and better
understanding of the molecular changes associated with secondary breast can help in better
evaluation of brain metastases and knowledge of targeted therapies. Breast cancer is the most
common form of cancer and the exact mechanism leading to brain metastases is unknown.
One study evaluating the cumulative incidence of CNS relapse revealed human epidermal
growth factor receptor 2 (HER2) positive breast cancers as a major risk factor behind brain
metastases (Han and Brastianos 2017). Early occurrence of brain metastases from breast
cancer is associated with early onset tumor. Survival of patient is also found to be dependent
on presence of ER positive tumor types Hence, this finding gives the implication that survival
outcomes for breast cancer patient is affected by different subtypes of breast cancer.
Another molecular study highlights the role of epidermal growth factor (EGFR) mutations on
cumulative incidence of brain metastases too. The study compared cases or incidence of brain
metastases in patients with EGFR compared to patients with EGFR wild types. The study
revealed that mutation in the EGFR gene renders the tumor sensitive to the EGFR tyrosine
kinase inhibitor (Helena et al., 2013). The ultimate impact of such mutation is that it
enhances the chance of survival for patients. Due to positive CNS activity, including EGFR
TKIs in the treatment of brain metastases can be a novel treatment options in the future
(Helena et al., 2013). However, before these molecular mutation are confirmed as a pathway
for effective treatment, there is a needed for prospective investigations of the role of EGFR
TKIs in active brain metastases particularly for primary breast cancer transitioning to
secondary breast cancer to the brain. This research is focused on evaluating the impact of
molecular cloning of BIG3 (ARFGEF3) Gene associate with secondary Breast Cancer in the
Brain.

5DISSERTATION
BIG3 gene in breast cancer
According to Chigira et al. (2019) Brefeldin A-inhibited guanine nucleotide-exchange
protein 3 (BIG3) has been found to directly interact with and bring about inhibition of
prohibitin-2 (PHB2) and specifically its tumour suppressing function. Furthermore, the
researchers also highlighted the fact that interaction between BIG3-PHB2 has been identified
as a favourable target for implementation of breast cancer therapy. On comparing the 8-class
secondary structure related to the N-terminal domain present in the BIG family proteins, the
researchers were able to state that a loop region that is present in BIG3 creates a significant
impact on the thermodynamic and colloidal stability of the gene, thus affecting the kinetic
and thermodynamic profile of its PHB2 interaction. Hence, the researchers were able to
effectively establish a model that elucidated the interaction between BIG3-PHB2, thereby
characterising it as a potential site for breast cancer related drug discovery.
Figure 2- Interaction between BIG3-PHB2
Source- (Chigira et al. 2019)
It has been argued by Yoshimaru et al. (2015) that particular inhibition of complex
formed between BIG3‐PHB2, which acts in the form of a crucial modulator in signalling of
oestrogen (E2), through the use of ERAP, which is a central negative peptide inhibitor,
BIG3 gene in breast cancer
According to Chigira et al. (2019) Brefeldin A-inhibited guanine nucleotide-exchange
protein 3 (BIG3) has been found to directly interact with and bring about inhibition of
prohibitin-2 (PHB2) and specifically its tumour suppressing function. Furthermore, the
researchers also highlighted the fact that interaction between BIG3-PHB2 has been identified
as a favourable target for implementation of breast cancer therapy. On comparing the 8-class
secondary structure related to the N-terminal domain present in the BIG family proteins, the
researchers were able to state that a loop region that is present in BIG3 creates a significant
impact on the thermodynamic and colloidal stability of the gene, thus affecting the kinetic
and thermodynamic profile of its PHB2 interaction. Hence, the researchers were able to
effectively establish a model that elucidated the interaction between BIG3-PHB2, thereby
characterising it as a potential site for breast cancer related drug discovery.
Figure 2- Interaction between BIG3-PHB2
Source- (Chigira et al. 2019)
It has been argued by Yoshimaru et al. (2015) that particular inhibition of complex
formed between BIG3‐PHB2, which acts in the form of a crucial modulator in signalling of
oestrogen (E2), through the use of ERAP, which is a central negative peptide inhibitor,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6DISSERTATION
results in supressing alpha activation of E2‐dependent oestrogen receptor (ER). This
suppression is generally mediated by reactivating the tumour suppressive activity of the inner
mitochondrial receptor PHB2. The researchers reported that ERAP creates noteworthy
suppressive impact on synergistic activation. This activation is generally brought about by
crosstalk between growth factors that are related with acquired or intrinsic resistance to anti‐
oestrogen tamoxifen and E2 present in breast cancer cells. Intrinsic release of PHB2 from
BIG3 by the ERAP was also found to successfully disrupt every single interaction of the
membrane‐related ERα with EGFR, PI3K, insulin‐like growth factor 1 receptor beta (IGF‐
1Rβ), or human epidermal growth factor 2 (HER2). This inhibition occurs in the presence of
growth factors EGF or IGF and E2, and was succeeded by the inhibition of EGFR, IGF‐1Rβ,
or HER2 activation. Hence, it was found that PHB2 release was responsible for a reduction of
MAPK, Akt, and ERα levels of phosphorylation, this leading to substantial inhibition of
proliferation of the breast cancer cells that were ERα‐positive, both in vitro and in vivo.
Hence, the researchers were able to highlight the fact that BIG3‐PHB2 inhibition must be
identified as a novel possible therapeutic method for treating breast cancers that have been
activated due to crosstalk.
It has also been postulated by Yoshimaru et al. (2017) that an estimated 70% of all
breast cancer cells generally express the oestrogen receptor alpha (ERα). However, the
researchers recognised the lack of evidence on regulation of PHB2 suppressive activity by
BIG3. They provided evidence for the fact that BIG3 acts in the form of A-kinase anchoring
protein, and this protein is able to effectively develop association with protein kinase A
(PKA), in addition to the α isoform present in protein phosphatase 1 (PP1Cα) catalytic
subunit. Hence, this binding is responsible for the dephosphorylation and subsequent
inactivation of PHB2. Furthermore, it was also proposed that PKA-governed phosphorylation
of BIG3-S1208 and -S305, which in turn is E2-induced, plays an important role in enhancing
results in supressing alpha activation of E2‐dependent oestrogen receptor (ER). This
suppression is generally mediated by reactivating the tumour suppressive activity of the inner
mitochondrial receptor PHB2. The researchers reported that ERAP creates noteworthy
suppressive impact on synergistic activation. This activation is generally brought about by
crosstalk between growth factors that are related with acquired or intrinsic resistance to anti‐
oestrogen tamoxifen and E2 present in breast cancer cells. Intrinsic release of PHB2 from
BIG3 by the ERAP was also found to successfully disrupt every single interaction of the
membrane‐related ERα with EGFR, PI3K, insulin‐like growth factor 1 receptor beta (IGF‐
1Rβ), or human epidermal growth factor 2 (HER2). This inhibition occurs in the presence of
growth factors EGF or IGF and E2, and was succeeded by the inhibition of EGFR, IGF‐1Rβ,
or HER2 activation. Hence, it was found that PHB2 release was responsible for a reduction of
MAPK, Akt, and ERα levels of phosphorylation, this leading to substantial inhibition of
proliferation of the breast cancer cells that were ERα‐positive, both in vitro and in vivo.
Hence, the researchers were able to highlight the fact that BIG3‐PHB2 inhibition must be
identified as a novel possible therapeutic method for treating breast cancers that have been
activated due to crosstalk.
It has also been postulated by Yoshimaru et al. (2017) that an estimated 70% of all
breast cancer cells generally express the oestrogen receptor alpha (ERα). However, the
researchers recognised the lack of evidence on regulation of PHB2 suppressive activity by
BIG3. They provided evidence for the fact that BIG3 acts in the form of A-kinase anchoring
protein, and this protein is able to effectively develop association with protein kinase A
(PKA), in addition to the α isoform present in protein phosphatase 1 (PP1Cα) catalytic
subunit. Hence, this binding is responsible for the dephosphorylation and subsequent
inactivation of PHB2. Furthermore, it was also proposed that PKA-governed phosphorylation
of BIG3-S1208 and -S305, which in turn is E2-induced, plays an important role in enhancing
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7DISSERTATION
PP1Cα activity, thus leading to activation of E2/ERα signalling via inactivation of PHB2, due
to dephosphorylation of PHB2-S39. In addition, the researchers also analysed cohort that
comprised of ERα-positive breast cancers survivors and found the presence of significant
association of poor cancer prognosis with PHB2-S39 dephosphorylation and overexpression
of BIG3. This was in accordance to the findings presented by Yoshimaru et al. (2017) who
identified the pivotal role played by E2 and ERα signalling pathway in breast cancer cell
proliferation. The researchers took efforts to utilise stapled ERAP method for development of
ERAP that was chemically altered, with the aim of improving antitumor effect duration. It
was found that stapled ERAP was successful in precisely inhibiting interaction between
BIG3-PHB2, besides demonstrating long-term suppressive activity. Furthermore, intracellular
localization of stapled ERAP, in absence of polyarginine sequence that was membrane-
permeable was conceivable owing to the development of α-helix stable structure. The
researchers established that tumour bearing-mice, which were subjected to regular or weekly
treatment with stapled ERAP, were able to successfully prevent interaction between BIG3-
PHB2, thus promoting comprehensive E2-dependent tumour regression. Taking into
consideration the synergistic inhibitory action that was demonstrated on breast cancer cell
growth by combination of stapled ERAP with fulvestrant, tamoxifen, and everolimus, the
researchers were able to elucidate the role of stapled ERAP as a favourable anti-tumour drug.
According to Kim et al. (2015) BIG3 forms an association with PHB2 present in the
cytoplasm, thus leading to PHB2 tumour suppressor loss of function in breast cancer cell
nuclei. The researchers highlighted that BIG3 blocks the nuclear import of PHB2 that is
oestrogen (E2)-dependent, through the help of karyopherin alpha (KPNA) family, located in
the breast cancer cells. The research findings provided evidence for the interaction of
overexpressed PHB2 interacted with KPNA5, KPNA1, and KPNA6, which in turn resulted in
translocation of PHB2 in an E2-dependent manner, inside the nuclei of the target breast
PP1Cα activity, thus leading to activation of E2/ERα signalling via inactivation of PHB2, due
to dephosphorylation of PHB2-S39. In addition, the researchers also analysed cohort that
comprised of ERα-positive breast cancers survivors and found the presence of significant
association of poor cancer prognosis with PHB2-S39 dephosphorylation and overexpression
of BIG3. This was in accordance to the findings presented by Yoshimaru et al. (2017) who
identified the pivotal role played by E2 and ERα signalling pathway in breast cancer cell
proliferation. The researchers took efforts to utilise stapled ERAP method for development of
ERAP that was chemically altered, with the aim of improving antitumor effect duration. It
was found that stapled ERAP was successful in precisely inhibiting interaction between
BIG3-PHB2, besides demonstrating long-term suppressive activity. Furthermore, intracellular
localization of stapled ERAP, in absence of polyarginine sequence that was membrane-
permeable was conceivable owing to the development of α-helix stable structure. The
researchers established that tumour bearing-mice, which were subjected to regular or weekly
treatment with stapled ERAP, were able to successfully prevent interaction between BIG3-
PHB2, thus promoting comprehensive E2-dependent tumour regression. Taking into
consideration the synergistic inhibitory action that was demonstrated on breast cancer cell
growth by combination of stapled ERAP with fulvestrant, tamoxifen, and everolimus, the
researchers were able to elucidate the role of stapled ERAP as a favourable anti-tumour drug.
According to Kim et al. (2015) BIG3 forms an association with PHB2 present in the
cytoplasm, thus leading to PHB2 tumour suppressor loss of function in breast cancer cell
nuclei. The researchers highlighted that BIG3 blocks the nuclear import of PHB2 that is
oestrogen (E2)-dependent, through the help of karyopherin alpha (KPNA) family, located in
the breast cancer cells. The research findings provided evidence for the interaction of
overexpressed PHB2 interacted with KPNA5, KPNA1, and KPNA6, which in turn resulted in
translocation of PHB2 in an E2-dependent manner, inside the nuclei of the target breast

8DISSERTATION
cancer cells. Furthermore, the findings suggested that endogenous KPNA knockdown that
was mediated by siRNA brought about a substantial suppression of E2-dependent PHB2
translocation in breast cancer cells that were categorised as BIG3-depleted, thereby
augmenting oestrogen receptor alpha (ERα) activation. Hence, the research was able to
highlight the role of BIG3 in blocking KPNAs binding region present in PHB2, thus
facilitating identification of potential therapeutic targets for breast cancer treatment.
It has been argued by Katagiri et al. (2014) that as much as 70% of primary breast
cancer cells have been categorised as oestrogen receptor alpha (ERα)-positive. Furthermore,
the researchers also affirmed the fact that interactions between ERα and oestrogen (E2)
affectedly boost the metastatic and proliferative action of the breast tumour cells. However,
they recognised the lack of evidence on inactivation mechanism of endogenous PHB2. They
reported that BIG3 protein develops an interaction with PP1α, followed by the
dephosphorylation of E2-dependent PHB2 phosphorylation. This in turn was recognised
crucial for tumour suppressive capability, thus triggering an outward “loss-of-function” of the
PHB2 protein. This “loss-of-function” eventually resulted in constitutive activation of
signalling pathways that were related to ERα. Therefore, findings from the research
demonstrated that release of intrinsic PHB2 from BIG3 by the peptide inhibitor, which is
cell-permeable, directly forms a bond with both membrane- and nuclear-associated ERα. This
in turn triggers the inhibition of manifold ERα-signalling pathways that directly leads to the
growth and proliferation of breast cancer cells that are ERα-positive, both in vivo and in
vitro. Taking into consideration the efficacy of this treatment in suppressing tamoxifen
resistance, besides enhancing its responsiveness in breast cancer cells that were ERα-positive,
the findings helped in uncovering novel therapeutic approaches for treatment of E2/ ERα-
positive breast cancer.
cancer cells. Furthermore, the findings suggested that endogenous KPNA knockdown that
was mediated by siRNA brought about a substantial suppression of E2-dependent PHB2
translocation in breast cancer cells that were categorised as BIG3-depleted, thereby
augmenting oestrogen receptor alpha (ERα) activation. Hence, the research was able to
highlight the role of BIG3 in blocking KPNAs binding region present in PHB2, thus
facilitating identification of potential therapeutic targets for breast cancer treatment.
It has been argued by Katagiri et al. (2014) that as much as 70% of primary breast
cancer cells have been categorised as oestrogen receptor alpha (ERα)-positive. Furthermore,
the researchers also affirmed the fact that interactions between ERα and oestrogen (E2)
affectedly boost the metastatic and proliferative action of the breast tumour cells. However,
they recognised the lack of evidence on inactivation mechanism of endogenous PHB2. They
reported that BIG3 protein develops an interaction with PP1α, followed by the
dephosphorylation of E2-dependent PHB2 phosphorylation. This in turn was recognised
crucial for tumour suppressive capability, thus triggering an outward “loss-of-function” of the
PHB2 protein. This “loss-of-function” eventually resulted in constitutive activation of
signalling pathways that were related to ERα. Therefore, findings from the research
demonstrated that release of intrinsic PHB2 from BIG3 by the peptide inhibitor, which is
cell-permeable, directly forms a bond with both membrane- and nuclear-associated ERα. This
in turn triggers the inhibition of manifold ERα-signalling pathways that directly leads to the
growth and proliferation of breast cancer cells that are ERα-positive, both in vivo and in
vitro. Taking into consideration the efficacy of this treatment in suppressing tamoxifen
resistance, besides enhancing its responsiveness in breast cancer cells that were ERα-positive,
the findings helped in uncovering novel therapeutic approaches for treatment of E2/ ERα-
positive breast cancer.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9DISSERTATION
In the words of Yoshimaru et al. (2014) xanthohumol (XN) has been identified as a
natural anticarcignogenic compound that brings about an inhibition of ERα-positive breast
cancer cell proliferation. The researchers recognised inadequate information on mechanism
of XN anticancer effects and tried to investigate its association to PHB2. It was confirmed in
the research findings that treatment of breast cancer through the administration of XN
successfully prohibited the interaction between BIG3-PHB2. This inhibition was found to
stimulate the release of PHB2 to unswervingly form association with both cytoplasmic and
nuclear ERα. This binding was found to result in comprehensive suppression of the signalling
pathways that were related to E2, and subsequent growth of ERα-positive breast cancer cells.
However, they stated that XN was not able to inhibit the growth and development of normal
mammary epithelial cells. Thus, the research findings were significant since it helped in
classification of XN as a promising natural compound that could be used for the suppression
of luminal-type breast cancer cell growth.
Research has recently conducted by Viale et al. (2018) in order to draw a comparison
between molecular (MS) (MammaPrint and BluePrint) and immunohistochemical (IHC)
subtyping among breast cancer patients who had been recruited to EORTC 10041/BIG 3-
04 MINDACT clinical trial. Following the classification of patients into different subtypes
namely, HER-2-, luminal B, luminal A, and basal-type, the patients were centrally assessed,
followed by adjustment of hazard ratio. It was found that there was no significant difference
in DMFS between luminal type and the other two types of breast cancer (95.9%): HR = 1.40,
95% CI 0.75–2.60 (p = 0.294).
It has been contended by Tryfonidis et al. (2017) that implementation of adjuvant
systemic therapy for the treatment of pT1abN0 breast cancer is contentious, owing to the fact
that the tumours generally demonstrate a low reversion risk. The researchers conducted the
study amid pT1abN0 tumour containing patients from EORTC 10041/BIG 3-04 (MINDACT)
In the words of Yoshimaru et al. (2014) xanthohumol (XN) has been identified as a
natural anticarcignogenic compound that brings about an inhibition of ERα-positive breast
cancer cell proliferation. The researchers recognised inadequate information on mechanism
of XN anticancer effects and tried to investigate its association to PHB2. It was confirmed in
the research findings that treatment of breast cancer through the administration of XN
successfully prohibited the interaction between BIG3-PHB2. This inhibition was found to
stimulate the release of PHB2 to unswervingly form association with both cytoplasmic and
nuclear ERα. This binding was found to result in comprehensive suppression of the signalling
pathways that were related to E2, and subsequent growth of ERα-positive breast cancer cells.
However, they stated that XN was not able to inhibit the growth and development of normal
mammary epithelial cells. Thus, the research findings were significant since it helped in
classification of XN as a promising natural compound that could be used for the suppression
of luminal-type breast cancer cell growth.
Research has recently conducted by Viale et al. (2018) in order to draw a comparison
between molecular (MS) (MammaPrint and BluePrint) and immunohistochemical (IHC)
subtyping among breast cancer patients who had been recruited to EORTC 10041/BIG 3-
04 MINDACT clinical trial. Following the classification of patients into different subtypes
namely, HER-2-, luminal B, luminal A, and basal-type, the patients were centrally assessed,
followed by adjustment of hazard ratio. It was found that there was no significant difference
in DMFS between luminal type and the other two types of breast cancer (95.9%): HR = 1.40,
95% CI 0.75–2.60 (p = 0.294).
It has been contended by Tryfonidis et al. (2017) that implementation of adjuvant
systemic therapy for the treatment of pT1abN0 breast cancer is contentious, owing to the fact
that the tumours generally demonstrate a low reversion risk. The researchers conducted the
study amid pT1abN0 tumour containing patients from EORTC 10041/BIG 3-04 (MINDACT)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10DISSERTATION
trial, who had been classified as low-risk. The primary aim of the investigation was to report
the 5-year DMFS rate, overall survival (OS) rate, and distant metastases-free interval (DMFI)
rate. It was found that of the 826/6693 patients who had been enrolled in the trial, 37.5%
were aged more than 60 years and 63.6% were postmenopausal. The findings suggested that
5-year DMFS for all the patients having CL/GH pT1abN0 tumours, who had been subjected
to CT was an estimated 97.3% (95% CI, 89.4-99.3), in comparison to 91.4% (95% CI, 82.6-
95.9) for the patients who were not subjected to CT. 5-years DMFI & OS for the
aforementioned patients, subjected to CT were 98.8% (95% CI, 91.9-99.8) & 98.5% (95% CI,
89.6- 99.8), compared to 91.4% (95% CI, 82.6- 95.9) and 95.8% (89.1- 98.4%) respectively,
for patients not receiving CT. This made the researchers conclude that it is feasible to use
biological characteristics as major determinants for adjuvant administration of CT for
treatment of T1abN0 tumours.
In contrast, findings from the phase III MINDACT trial conducted by Cardoso et al.
(2017) suggested that there was no significant impact of docetaxel-capecitabine on OS or
DFS, when compared with the mainstay anthracycline-based CT. This research was based on
the premise that 46% breast cancer patients were found to be low genomic but high clinical
risk, according to MammaPrint, and might be subjected to adjuvant CT. This research was
conducted in order to determine the effects of experimental docetaxel versus anthracycline-
based treatment among breast cancer patients. It was found that the overall compliance rate
was 97% for R-C. In addition, at a median follow-up of five years, there was no significant
difference in DFS between DC (652 pts) and AT (649 pts) [HR = 0.83 (0.60- 1.15, p =
0.263]. Furthermore, there was similarity in OS in both the arms (HR 0.91, 95% CI, 0.54-
1.53).
Metzger Filho et al. (2015) also tried to assess the efficacy of letrozole, in comparison
to tamoxifen for patients who had been diagnosed with lobular or invasive ductal carcinoma.
trial, who had been classified as low-risk. The primary aim of the investigation was to report
the 5-year DMFS rate, overall survival (OS) rate, and distant metastases-free interval (DMFI)
rate. It was found that of the 826/6693 patients who had been enrolled in the trial, 37.5%
were aged more than 60 years and 63.6% were postmenopausal. The findings suggested that
5-year DMFS for all the patients having CL/GH pT1abN0 tumours, who had been subjected
to CT was an estimated 97.3% (95% CI, 89.4-99.3), in comparison to 91.4% (95% CI, 82.6-
95.9) for the patients who were not subjected to CT. 5-years DMFI & OS for the
aforementioned patients, subjected to CT were 98.8% (95% CI, 91.9-99.8) & 98.5% (95% CI,
89.6- 99.8), compared to 91.4% (95% CI, 82.6- 95.9) and 95.8% (89.1- 98.4%) respectively,
for patients not receiving CT. This made the researchers conclude that it is feasible to use
biological characteristics as major determinants for adjuvant administration of CT for
treatment of T1abN0 tumours.
In contrast, findings from the phase III MINDACT trial conducted by Cardoso et al.
(2017) suggested that there was no significant impact of docetaxel-capecitabine on OS or
DFS, when compared with the mainstay anthracycline-based CT. This research was based on
the premise that 46% breast cancer patients were found to be low genomic but high clinical
risk, according to MammaPrint, and might be subjected to adjuvant CT. This research was
conducted in order to determine the effects of experimental docetaxel versus anthracycline-
based treatment among breast cancer patients. It was found that the overall compliance rate
was 97% for R-C. In addition, at a median follow-up of five years, there was no significant
difference in DFS between DC (652 pts) and AT (649 pts) [HR = 0.83 (0.60- 1.15, p =
0.263]. Furthermore, there was similarity in OS in both the arms (HR 0.91, 95% CI, 0.54-
1.53).
Metzger Filho et al. (2015) also tried to assess the efficacy of letrozole, in comparison
to tamoxifen for patients who had been diagnosed with lobular or invasive ductal carcinoma.

11DISSERTATION
On classifying ILC and HER2-negative IDC as hormone receptor positive with low or high
proliferative activity, the researchers also performed survival analysis. It was found that there
was noteworthy interaction between histology and treatment (ILC or IDC; P = .006) and
subgroup and treatment (LB like or LA like; P = .01). Furthermore, the ILS group
demonstrated a 50% decrease for LA subtype (HR, 0.50; 95% CI, 0.32 to 0.78) and 35%
decrease in DFS event hazard with letrozole administration for LB subtype (HR, 0.65; 95%
CI, 0.53 to 0.79). Hence, the findings helped the researchers confirm that adjuvant
letrozole exerted greater benefit on patients suffering from lobular carcinoma, in comparison
to ductal carcinoma, who had been recruited from BIG 1-98 Trial. Katagiri and Yoshimaru
(2017) stated that roughly 70% of the breast cancer cells generally express ERα, and
demonstrate a reliance on oestrogen (E2) for their metastatic and proliferative activity. The
contemporary endocrine treatment regimen for breast cancer are largely founded on targeting
ERα signalling by means of selective ERα down regulators, ERα modulators, and aromatase
inhibitors. Nonetheless, as much as 50% of the patients reporting the presence of ERα-
positive tumours either originally do not show response or demonstrate resistance to these
drugs. The researchers stated that stapled ERAP is able to specifically inhibit BIG3-PHB2
interaction, thus displaying the long-lasting repressive activity, in addition to their
intracellular localization without the presence of polyarginine sequence that is membrane-
permeable. This is generally mediated through the development of stable α-helix structure,.
Prominently, a combination of tamoxifen and stapled ERAP also resulted in a synergistic
inhibitory impact on the growth of breast cancer cells. The researchers stated that on treating
tumour bearing mice with stapled ERAP, for every 7 days, they were able to inhibit BIG3-
PHB2 interaction, thereby leading to the complete E2-dependent tumour regression. Hence,
the findings confirmed that stapled ERAP holds promise for the therapeutic suppression of
luminal type growth of breast cancer.
On classifying ILC and HER2-negative IDC as hormone receptor positive with low or high
proliferative activity, the researchers also performed survival analysis. It was found that there
was noteworthy interaction between histology and treatment (ILC or IDC; P = .006) and
subgroup and treatment (LB like or LA like; P = .01). Furthermore, the ILS group
demonstrated a 50% decrease for LA subtype (HR, 0.50; 95% CI, 0.32 to 0.78) and 35%
decrease in DFS event hazard with letrozole administration for LB subtype (HR, 0.65; 95%
CI, 0.53 to 0.79). Hence, the findings helped the researchers confirm that adjuvant
letrozole exerted greater benefit on patients suffering from lobular carcinoma, in comparison
to ductal carcinoma, who had been recruited from BIG 1-98 Trial. Katagiri and Yoshimaru
(2017) stated that roughly 70% of the breast cancer cells generally express ERα, and
demonstrate a reliance on oestrogen (E2) for their metastatic and proliferative activity. The
contemporary endocrine treatment regimen for breast cancer are largely founded on targeting
ERα signalling by means of selective ERα down regulators, ERα modulators, and aromatase
inhibitors. Nonetheless, as much as 50% of the patients reporting the presence of ERα-
positive tumours either originally do not show response or demonstrate resistance to these
drugs. The researchers stated that stapled ERAP is able to specifically inhibit BIG3-PHB2
interaction, thus displaying the long-lasting repressive activity, in addition to their
intracellular localization without the presence of polyarginine sequence that is membrane-
permeable. This is generally mediated through the development of stable α-helix structure,.
Prominently, a combination of tamoxifen and stapled ERAP also resulted in a synergistic
inhibitory impact on the growth of breast cancer cells. The researchers stated that on treating
tumour bearing mice with stapled ERAP, for every 7 days, they were able to inhibit BIG3-
PHB2 interaction, thereby leading to the complete E2-dependent tumour regression. Hence,
the findings confirmed that stapled ERAP holds promise for the therapeutic suppression of
luminal type growth of breast cancer.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





