Ethics Approval Application Form for Business Development Project
VerifiedAdded on 2023/01/23
|7
|2156
|84
Homework Assignment
AI Summary
This assignment presents a completed ethics approval application form for a business development proposal. The form outlines the project's rationale, which involves gathering primary data to identify business concepts and target markets using various models. The ethical considerations section details how informed consent will be obtained, ensuring participant confidentiality. The application addresses ethical concerns, including data security through secure storage and deletion within four months, and clarifies that no collaboration with other researchers will occur. The form also confirms that no vulnerable participants will be involved and emphasizes adherence to ethical guidelines. The application covers aspects like project start and end dates, potential conflicts of interest, primary data collection, participant recruitment, research location, and funding. It also includes an ethical checklist addressing issues like site permission, collaboration, volition, informed consent, data security, and debriefing. Furthermore, it addresses risks to participants and researchers, and it provides a statement of ethical considerations, assuring confidentiality through secure data storage and destruction protocols.

Ethical Approval Application Form, modified Spring 2012 TPM
Approval Code:
E1- APPLICATION FORMFOR APPROVAL TO CONDUCT RESEARCH
BUSINESS SCHOOL ETHICS COMMITTEE
Dept/School
and unit
code:
Please select Postgraduate Project Undergraduate Project
(Double click on the box then click ‘Checked’ for a cross to appear in the box)
Project Title:
Researchers
Name(s):
Supervisor(s):
Date:
Applications should be submitted electronically to the Secretary of the School Research Ethics Committee as one single
file.One original hard copy must also be submitted with the signatures of all applicants and Supervisors.
Rationale: Please give a BRIEF description of the project in ‘lay language’.This summary will be reviewedby UREC and
may be published as part of its reporting procedures.
Ethical Considerations:Please give a BRIEF description of the ethical considerations of this project. Please mention
questions raised specifically in the form and, where appropriate, show that the basic ethical criteria have been met in
any use of (a) participant information sheets (b) consent forms (c) debriefing procedures.
This summary will be forwarded to and reviewed by UREC and may be published as part of its reporting procedures.
Page 1
Business development proposal
75 words max.-approx 5 lines (for database reasons). Detail should be provided in Q. 27
A research is been conducted on a new business plan development idea. For this study is been conducted and primary data is
gathered from people. The main aim of project is to identify concept of business and target market. In this different models will be
used to determine market, products, etc. moreover, data will be analyzed that what opportunities exists in market for business
growth.
75 words - approx 5 lines (for database reasons). Elucidation, if required, can be given in Q. 27.
In this various ethics will be followed. The participation consent will be taken in written. This will enable in maintaining
confidentiality of data and information. Besides this, consent form will be filled with participants. They will be given a briefing about
what procedure is followed. It will enable in making taking their views and opinions openly. By this authenticity of study will be
maintained. It will be easy to conduct research in ethical way.
Approval Code:
E1- APPLICATION FORMFOR APPROVAL TO CONDUCT RESEARCH
BUSINESS SCHOOL ETHICS COMMITTEE
Dept/School
and unit
code:
Please select Postgraduate Project Undergraduate Project
(Double click on the box then click ‘Checked’ for a cross to appear in the box)
Project Title:
Researchers
Name(s):
Supervisor(s):
Date:
Applications should be submitted electronically to the Secretary of the School Research Ethics Committee as one single
file.One original hard copy must also be submitted with the signatures of all applicants and Supervisors.
Rationale: Please give a BRIEF description of the project in ‘lay language’.This summary will be reviewedby UREC and
may be published as part of its reporting procedures.
Ethical Considerations:Please give a BRIEF description of the ethical considerations of this project. Please mention
questions raised specifically in the form and, where appropriate, show that the basic ethical criteria have been met in
any use of (a) participant information sheets (b) consent forms (c) debriefing procedures.
This summary will be forwarded to and reviewed by UREC and may be published as part of its reporting procedures.
Page 1
Business development proposal
75 words max.-approx 5 lines (for database reasons). Detail should be provided in Q. 27
A research is been conducted on a new business plan development idea. For this study is been conducted and primary data is
gathered from people. The main aim of project is to identify concept of business and target market. In this different models will be
used to determine market, products, etc. moreover, data will be analyzed that what opportunities exists in market for business
growth.
75 words - approx 5 lines (for database reasons). Elucidation, if required, can be given in Q. 27.
In this various ethics will be followed. The participation consent will be taken in written. This will enable in maintaining
confidentiality of data and information. Besides this, consent form will be filled with participants. They will be given a briefing about
what procedure is followed. It will enable in making taking their views and opinions openly. By this authenticity of study will be
maintained. It will be easy to conduct research in ethical way.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Ethical Approval Application Form, modified Spring 2012 TPM
Page 2
Page 2
Ethical Approval Application Form, modified Spring 2012 TPM
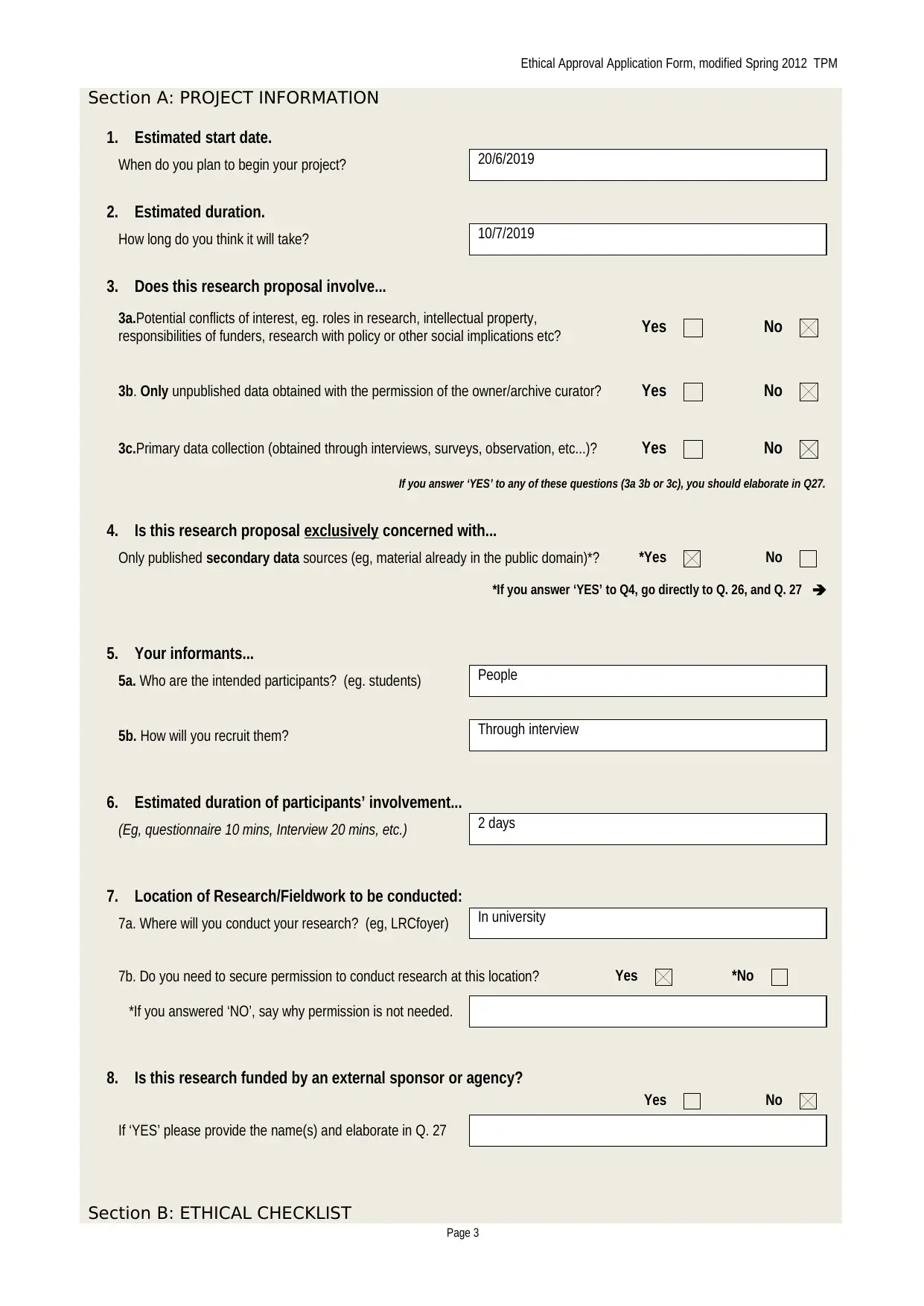
Section A: PROJECT INFORMATION
1. Estimated start date.
When do you plan to begin your project? 20/6/2019
2. Estimated duration.
How long do you think it will take? 10/7/2019
3. Does this research proposal involve...
3a.Potential conflicts of interest, eg. roles in research, intellectual property,
responsibilities of funders, research with policy or other social implications etc? Yes No
3b. Only unpublished data obtained with the permission of the owner/archive curator? Yes No
3c.Primary data collection (obtained through interviews, surveys, observation, etc...)? Yes No
If you answer ‘YES’ to any of these questions (3a 3b or 3c), you should elaborate in Q27.
4. Is this research proposal exclusively concerned with...
Only published secondary data sources (eg, material already in the public domain)*? *Yes No
*If you answer ‘YES’ to Q4, go directly to Q. 26, and Q. 27
5. Your informants...
5a. Who are the intended participants? (eg. students) People
5b. How will you recruit them? Through interview
6. Estimated duration of participants’ involvement...
(Eg, questionnaire 10 mins, Interview 20 mins, etc.) 2 days
7. Location of Research/Fieldwork to be conducted:
7a. Where will you conduct your research? (eg, LRCfoyer) In university
7b. Do you need to secure permission to conduct research at this location? Yes *No
*If you answered ‘NO’, say why permission is not needed.
8. Is this research funded by an external sponsor or agency?
Yes No
If ‘YES’ please provide the name(s) and elaborate in Q. 27
Section B: ETHICAL CHECKLIST
Page 3
Section A: PROJECT INFORMATION
1. Estimated start date.
When do you plan to begin your project? 20/6/2019
2. Estimated duration.
How long do you think it will take? 10/7/2019
3. Does this research proposal involve...
3a.Potential conflicts of interest, eg. roles in research, intellectual property,
responsibilities of funders, research with policy or other social implications etc? Yes No
3b. Only unpublished data obtained with the permission of the owner/archive curator? Yes No
3c.Primary data collection (obtained through interviews, surveys, observation, etc...)? Yes No
If you answer ‘YES’ to any of these questions (3a 3b or 3c), you should elaborate in Q27.
4. Is this research proposal exclusively concerned with...
Only published secondary data sources (eg, material already in the public domain)*? *Yes No
*If you answer ‘YES’ to Q4, go directly to Q. 26, and Q. 27
5. Your informants...
5a. Who are the intended participants? (eg. students) People
5b. How will you recruit them? Through interview
6. Estimated duration of participants’ involvement...
(Eg, questionnaire 10 mins, Interview 20 mins, etc.) 2 days
7. Location of Research/Fieldwork to be conducted:
7a. Where will you conduct your research? (eg, LRCfoyer) In university
7b. Do you need to secure permission to conduct research at this location? Yes *No
*If you answered ‘NO’, say why permission is not needed.
8. Is this research funded by an external sponsor or agency?
Yes No
If ‘YES’ please provide the name(s) and elaborate in Q. 27
Section B: ETHICAL CHECKLIST
Page 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Ethical Approval Application Form, modified Spring 2012 TPM
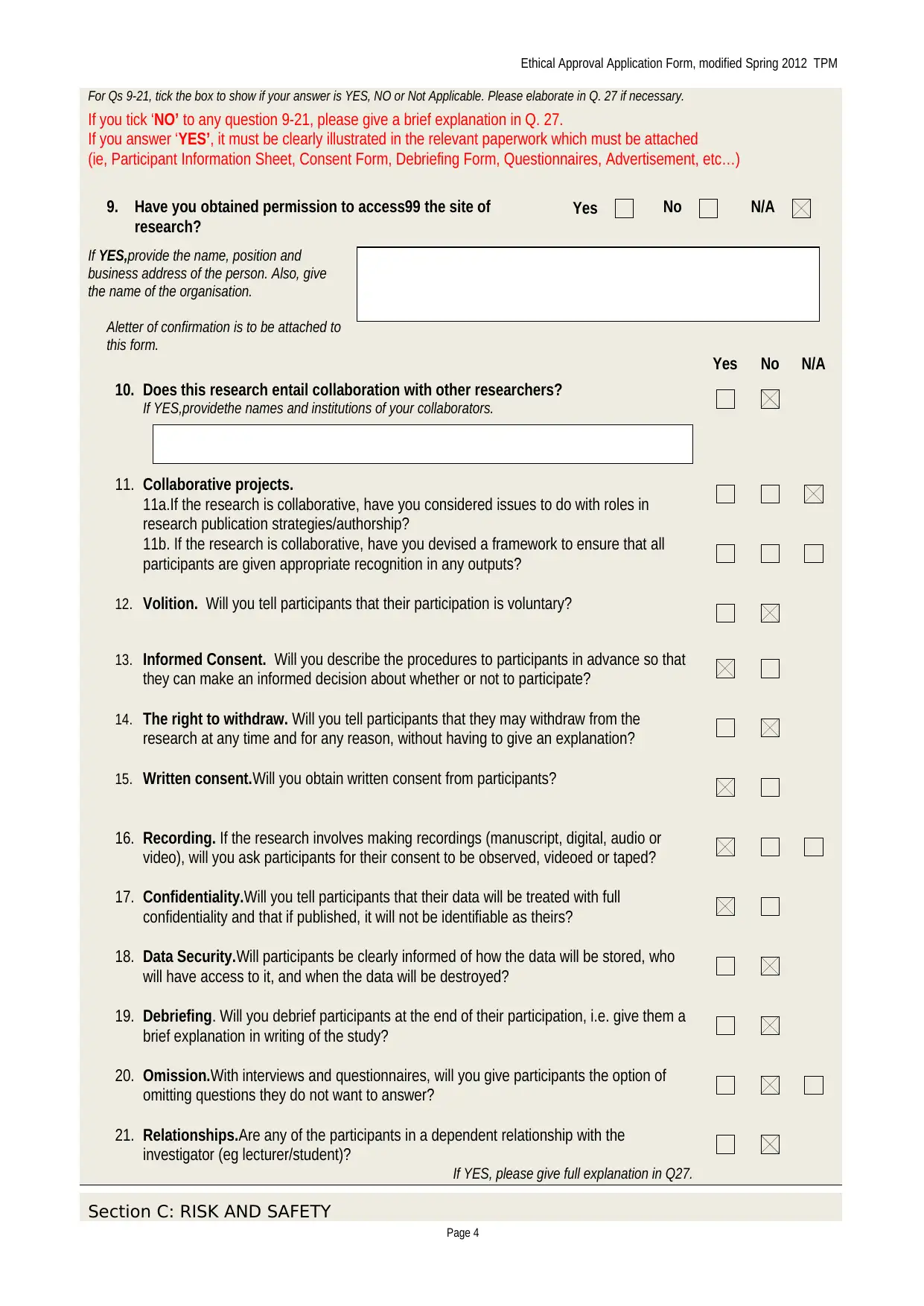
For Qs 9-21, tick the box to show if your answer is YES, NO or Not Applicable. Please elaborate in Q. 27 if necessary.
If you tick ‘NO’ to any question 9-21, please give a brief explanation in Q. 27.
If you answer ‘YES’, it must be clearly illustrated in the relevant paperwork which must be attached
(ie, Participant Information Sheet, Consent Form, Debriefing Form, Questionnaires, Advertisement, etc…)
9. Have you obtained permission to access99 the site of
research?
If YES,provide the name, position and
business address of the person. Also, give
the name of the organisation.
Aletter of confirmation is to be attached to
this form.
Yes No N/A
10. Does this research entail collaboration with other researchers?
If YES,providethe names and institutions of your collaborators.
11. Collaborative projects.
11a.If the research is collaborative, have you considered issues to do with roles in
research publication strategies/authorship?
11b. If the research is collaborative, have you devised a framework to ensure that all
participants are given appropriate recognition in any outputs?
12. Volition. Will you tell participants that their participation is voluntary?
13. Informed Consent. Will you describe the procedures to participants in advance so that
they can make an informed decision about whether or not to participate?
14. The right to withdraw. Will you tell participants that they may withdraw from the
research at any time and for any reason, without having to give an explanation?
15. Written consent.Will you obtain written consent from participants?
16. Recording. If the research involves making recordings (manuscript, digital, audio or
video), will you ask participants for their consent to be observed, videoed or taped?
17. Confidentiality.Will you tell participants that their data will be treated with full
confidentiality and that if published, it will not be identifiable as theirs?
18. Data Security.Will participants be clearly informed of how the data will be stored, who
will have access to it, and when the data will be destroyed?
19. Debriefing. Will you debrief participants at the end of their participation, i.e. give them a
brief explanation in writing of the study?
20. Omission.With interviews and questionnaires, will you give participants the option of
omitting questions they do not want to answer?
21. Relationships.Are any of the participants in a dependent relationship with the
investigator (eg lecturer/student)?
If YES, please give full explanation in Q27.
Section C: RISK AND SAFETY
Page 4
Yes No N/A
For Qs 9-21, tick the box to show if your answer is YES, NO or Not Applicable. Please elaborate in Q. 27 if necessary.
If you tick ‘NO’ to any question 9-21, please give a brief explanation in Q. 27.
If you answer ‘YES’, it must be clearly illustrated in the relevant paperwork which must be attached
(ie, Participant Information Sheet, Consent Form, Debriefing Form, Questionnaires, Advertisement, etc…)
9. Have you obtained permission to access99 the site of
research?
If YES,provide the name, position and
business address of the person. Also, give
the name of the organisation.
Aletter of confirmation is to be attached to
this form.
Yes No N/A
10. Does this research entail collaboration with other researchers?
If YES,providethe names and institutions of your collaborators.
11. Collaborative projects.
11a.If the research is collaborative, have you considered issues to do with roles in
research publication strategies/authorship?
11b. If the research is collaborative, have you devised a framework to ensure that all
participants are given appropriate recognition in any outputs?
12. Volition. Will you tell participants that their participation is voluntary?
13. Informed Consent. Will you describe the procedures to participants in advance so that
they can make an informed decision about whether or not to participate?
14. The right to withdraw. Will you tell participants that they may withdraw from the
research at any time and for any reason, without having to give an explanation?
15. Written consent.Will you obtain written consent from participants?
16. Recording. If the research involves making recordings (manuscript, digital, audio or
video), will you ask participants for their consent to be observed, videoed or taped?
17. Confidentiality.Will you tell participants that their data will be treated with full
confidentiality and that if published, it will not be identifiable as theirs?
18. Data Security.Will participants be clearly informed of how the data will be stored, who
will have access to it, and when the data will be destroyed?
19. Debriefing. Will you debrief participants at the end of their participation, i.e. give them a
brief explanation in writing of the study?
20. Omission.With interviews and questionnaires, will you give participants the option of
omitting questions they do not want to answer?
21. Relationships.Are any of the participants in a dependent relationship with the
investigator (eg lecturer/student)?
If YES, please give full explanation in Q27.
Section C: RISK AND SAFETY
Page 4
Yes No N/A
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Ethical Approval Application Form, modified Spring 2012 TPM
For Qs 22-25, tick the box to show if your answer is YES, or NO. You should elaborate in Q. 27 where appropriate.
Yes No
22. Deception.Will your project involve deliberately misleading participants in any way?
If YES, explain in Q. 27 why it is necessary and how debriefing will occur.
23. Risk to Participant(s). Is there any realistic risk to any paid or unpaid participant, field
assistant, helper or student involved in the project? (Nb,‘risk’ means experiencing any
physical, psychological distress or discomfort)
If YES, please explain in Q.27, saying what you will do if they should
experience any problems, eg who to contact for help.
24. Risk to Researcher(s) Is there any realistic risk to the investigator?
If YES, have the appropriate risk assessment forms been submitted to
the appropriate Safety Committee(s)?
25. Risk of Harm or Damage.Do you think the results of your research have the potential to
cause any damage, harm or other problems for people in your study area?
If YES, explain in Q. 27 why it is necessary and how debriefing will occur
Section D: WORKING WITH CHILDREN/VULNERABLE PEOPLE
For Qs 26a-g, tick the box to show if your answer is YES, or NO. You must elaborate in Q. 27 any ‘YES response in this section.
26. Vulnerable individuals. Do participants in this study fall into any of the following special groups?
If they do, please tick the appropriate answer, refer to the relevant guidelines and complete Q.27.
Yes No
a. Children (under 18 years of age).
b. People with learning or communication difficulties.
c. Patients (including careers of NHS patients).
d. People in custody.
e. Institutionalised persons.
f. People engaged in illegal activities, eg drug-taking.
g. Other vulnerable groups. (please specify)
There is an obligation on the Lead Researcher & Supervisor to bring to the attention of the School Ethics Committee
(S.E.C.) any issues with ethical implications not clearly covered by the above checklist.
Page 5
For Qs 22-25, tick the box to show if your answer is YES, or NO. You should elaborate in Q. 27 where appropriate.
Yes No
22. Deception.Will your project involve deliberately misleading participants in any way?
If YES, explain in Q. 27 why it is necessary and how debriefing will occur.
23. Risk to Participant(s). Is there any realistic risk to any paid or unpaid participant, field
assistant, helper or student involved in the project? (Nb,‘risk’ means experiencing any
physical, psychological distress or discomfort)
If YES, please explain in Q.27, saying what you will do if they should
experience any problems, eg who to contact for help.
24. Risk to Researcher(s) Is there any realistic risk to the investigator?
If YES, have the appropriate risk assessment forms been submitted to
the appropriate Safety Committee(s)?
25. Risk of Harm or Damage.Do you think the results of your research have the potential to
cause any damage, harm or other problems for people in your study area?
If YES, explain in Q. 27 why it is necessary and how debriefing will occur
Section D: WORKING WITH CHILDREN/VULNERABLE PEOPLE
For Qs 26a-g, tick the box to show if your answer is YES, or NO. You must elaborate in Q. 27 any ‘YES response in this section.
26. Vulnerable individuals. Do participants in this study fall into any of the following special groups?
If they do, please tick the appropriate answer, refer to the relevant guidelines and complete Q.27.
Yes No
a. Children (under 18 years of age).
b. People with learning or communication difficulties.
c. Patients (including careers of NHS patients).
d. People in custody.
e. Institutionalised persons.
f. People engaged in illegal activities, eg drug-taking.
g. Other vulnerable groups. (please specify)
There is an obligation on the Lead Researcher & Supervisor to bring to the attention of the School Ethics Committee
(S.E.C.) any issues with ethical implications not clearly covered by the above checklist.
Page 5

Ethical Approval Application Form, modified Spring 2012 TPM
Section E: ETHICAL STATEMENT
27. Write a clear but concise statement of the ethical considerations of this project,clearly stating how you intend
to addresseachof them.
There will be various ethical consideration followed. It will help in proceeding in ethical way and maintaining confidentiality of data
and information. Here, first is data will be store in server and database. This will be done to protect it from misuse or
unauthorised access. Also, data authenticity will be maintained. Second is no other researcher will be collaborated with study.
There will be no recording of data obtained from participants. Data security is ensured by storing data and deleting it within 4
months. The risk of researcher will be ensured as no participants will be involved deliberately in study. The student, professor,
etc. will not be involved in study. There will be no vulnerable candidate chosen in participant. This will ensure that there is no
external element involved in study.
28. Assuring Confidentiality. As we regard informants’ consent to be finite, please say how and where you will
store your data (to guard against accidental data loss) and the date by which your data will be destroyed
(usually, within 6 months after the completion of your course).
The data will be stored in database and server. It will be stored via hard disk or taking help of expert or professional. The data will
be destroyed after 4 months.
Page 6
Section E: ETHICAL STATEMENT
27. Write a clear but concise statement of the ethical considerations of this project,clearly stating how you intend
to addresseachof them.
There will be various ethical consideration followed. It will help in proceeding in ethical way and maintaining confidentiality of data
and information. Here, first is data will be store in server and database. This will be done to protect it from misuse or
unauthorised access. Also, data authenticity will be maintained. Second is no other researcher will be collaborated with study.
There will be no recording of data obtained from participants. Data security is ensured by storing data and deleting it within 4
months. The risk of researcher will be ensured as no participants will be involved deliberately in study. The student, professor,
etc. will not be involved in study. There will be no vulnerable candidate chosen in participant. This will ensure that there is no
external element involved in study.
28. Assuring Confidentiality. As we regard informants’ consent to be finite, please say how and where you will
store your data (to guard against accidental data loss) and the date by which your data will be destroyed
(usually, within 6 months after the completion of your course).
The data will be stored in database and server. It will be stored via hard disk or taking help of expert or professional. The data will
be destroyed after 4 months.
Page 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Ethical Approval Application Form, modified Spring 2012 TPM
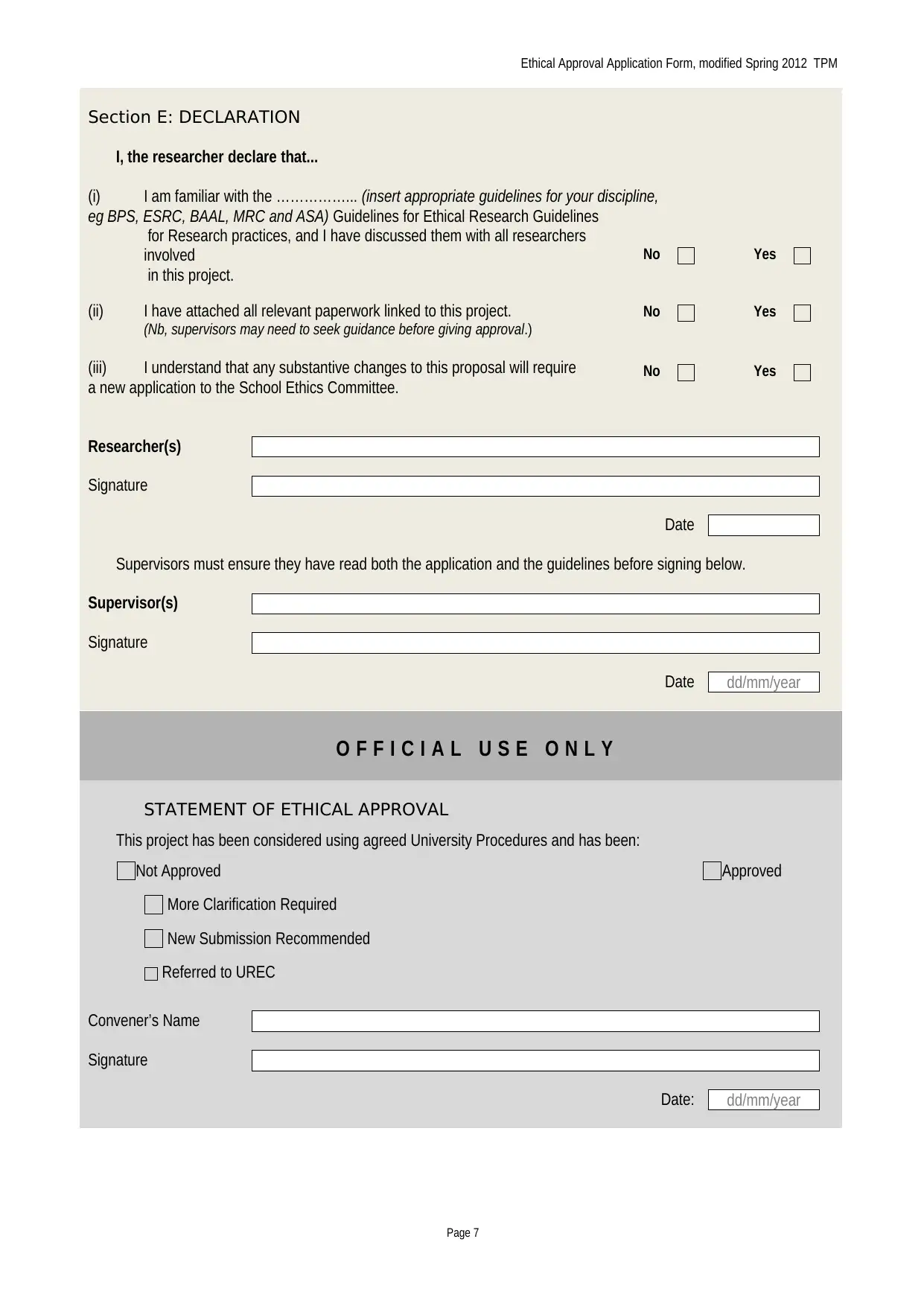
Section E: DECLARATION
I, the researcher declare that...
(i) I am familiar with the ……………... (insert appropriate guidelines for your discipline,
eg BPS, ESRC, BAAL, MRC and ASA) Guidelines for Ethical Research Guidelines
for Research practices, and I have discussed them with all researchers
involved
in this project.
(ii) I have attached all relevant paperwork linked to this project.
(Nb, supervisors may need to seek guidance before giving approval.)
(iii) I understand that any substantive changes to this proposal will require
a new application to the School Ethics Committee.
Researcher(s)
Signature
Date
Supervisors must ensure they have read both the application and the guidelines before signing below.
Supervisor(s)
Signature
Date
O F F I C I A L U S E O N L Y
STATEMENT OF ETHICAL APPROVAL
This project has been considered using agreed University Procedures and has been:
Not Approved Approved
More Clarification Required
New Submission Recommended
Referred to UREC
Convener’s Name
Signature
Date:
Page 7
No Yes
No Yes
dd/mm/year
No Yes
dd/mm/year
Section E: DECLARATION
I, the researcher declare that...
(i) I am familiar with the ……………... (insert appropriate guidelines for your discipline,
eg BPS, ESRC, BAAL, MRC and ASA) Guidelines for Ethical Research Guidelines
for Research practices, and I have discussed them with all researchers
involved
in this project.
(ii) I have attached all relevant paperwork linked to this project.
(Nb, supervisors may need to seek guidance before giving approval.)
(iii) I understand that any substantive changes to this proposal will require
a new application to the School Ethics Committee.
Researcher(s)
Signature
Date
Supervisors must ensure they have read both the application and the guidelines before signing below.
Supervisor(s)
Signature
Date
O F F I C I A L U S E O N L Y
STATEMENT OF ETHICAL APPROVAL
This project has been considered using agreed University Procedures and has been:
Not Approved Approved
More Clarification Required
New Submission Recommended
Referred to UREC
Convener’s Name
Signature
Date:
Page 7
No Yes
No Yes
dd/mm/year
No Yes
dd/mm/year
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





