Scientific Report: Comparing Boiling Points of Butanoic Acid/Pentanal
VerifiedAdded on 2023/06/08
|6
|826
|252
Report
AI Summary
This scientific report elucidates the difference in boiling points between butanoic acid and pentanal by examining their molecular structures and intermolecular forces. Butanoic acid, a carboxylic acid, exhibits a higher boiling point (164.30C) compared to pentanal (103.70C), an aldehyde. The report attributes this difference to the presence of hydrogen bonds in butanoic acid, which require more energy to break than the dipole-dipole moments present in pentanal. The discussion covers the influence of Van der Waals forces, molecular surface area, and the exposure of polar functional groups on boiling points, referencing key studies in the field. The conclusion emphasizes that the hydrogen bonding in butanoic acid is the primary factor causing its higher boiling point relative to pentanal. Desklib provides access to similar reports and study resources for students.

SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
NAME OF THE STUDENT.
SCHOOL AFFILIATION.
DATE.
BUTANOIC ACID AND PENTANAL.
NAME OF THE STUDENT.
SCHOOL AFFILIATION.
DATE.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
Boiling point is the transition phase where the attractive forces in liquids are completely
broken and the liquid is changed into a gaseous state. According to Damaceno, Jesus, Ceriani
(2018 p.89), organic substances are held by four types of intermolecular forces: Ionic forces,
hydrogen bonding, dipole-dipole moments and Van der Waals dispersion forces. Ionic bonds
impart the highest boiling points in organic compounds followed by those with hydrogen bonds
then dipole-dipole moments and lastly are the Van der Waals dispersion forces that have the
lowest boiling points. Depending on the functional group on the organic compound, the boiling
points differ. An example is compounds with OH groups like the carboxylic acids have higher
boiling points than the aldehydes with the COH group that contains dipole-dipole moments.
According to Damaceno et al (2018 p.121), molecular structure of the molecules affects the
surface area of the molecules which in turn affects the Van der Waals dispersion forces and
hence, the boiling points of the molecules. A long chain organic compound increases the surface
area of the molecule and the molecular weight of the molecule. Hence, the ability of the
individual molecules to attract each other is also increased. The interactions result in increased
dispersion forces and subsequently higher boiling points.
BUTANOIC ACID AND PENTANAL.
Boiling point is the transition phase where the attractive forces in liquids are completely
broken and the liquid is changed into a gaseous state. According to Damaceno, Jesus, Ceriani
(2018 p.89), organic substances are held by four types of intermolecular forces: Ionic forces,
hydrogen bonding, dipole-dipole moments and Van der Waals dispersion forces. Ionic bonds
impart the highest boiling points in organic compounds followed by those with hydrogen bonds
then dipole-dipole moments and lastly are the Van der Waals dispersion forces that have the
lowest boiling points. Depending on the functional group on the organic compound, the boiling
points differ. An example is compounds with OH groups like the carboxylic acids have higher
boiling points than the aldehydes with the COH group that contains dipole-dipole moments.
According to Damaceno et al (2018 p.121), molecular structure of the molecules affects the
surface area of the molecules which in turn affects the Van der Waals dispersion forces and
hence, the boiling points of the molecules. A long chain organic compound increases the surface
area of the molecule and the molecular weight of the molecule. Hence, the ability of the
individual molecules to attract each other is also increased. The interactions result in increased
dispersion forces and subsequently higher boiling points.
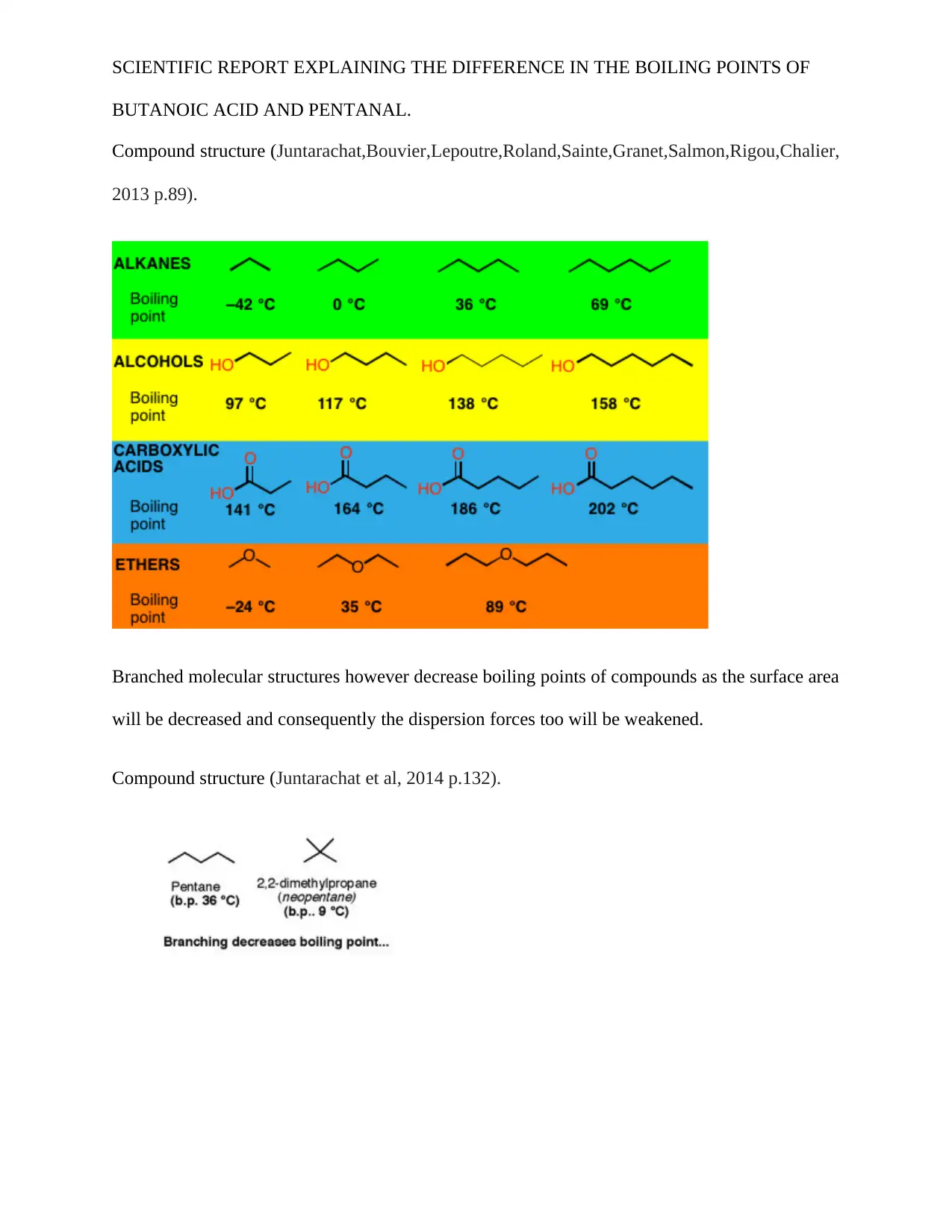
SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
Compound structure (Juntarachat,Bouvier,Lepoutre,Roland,Sainte,Granet,Salmon,Rigou,Chalier,
2013 p.89).
Branched molecular structures however decrease boiling points of compounds as the surface area
will be decreased and consequently the dispersion forces too will be weakened.
Compound structure (Juntarachat et al, 2014 p.132).
BUTANOIC ACID AND PENTANAL.
Compound structure (Juntarachat,Bouvier,Lepoutre,Roland,Sainte,Granet,Salmon,Rigou,Chalier,
2013 p.89).
Branched molecular structures however decrease boiling points of compounds as the surface area
will be decreased and consequently the dispersion forces too will be weakened.
Compound structure (Juntarachat et al, 2014 p.132).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
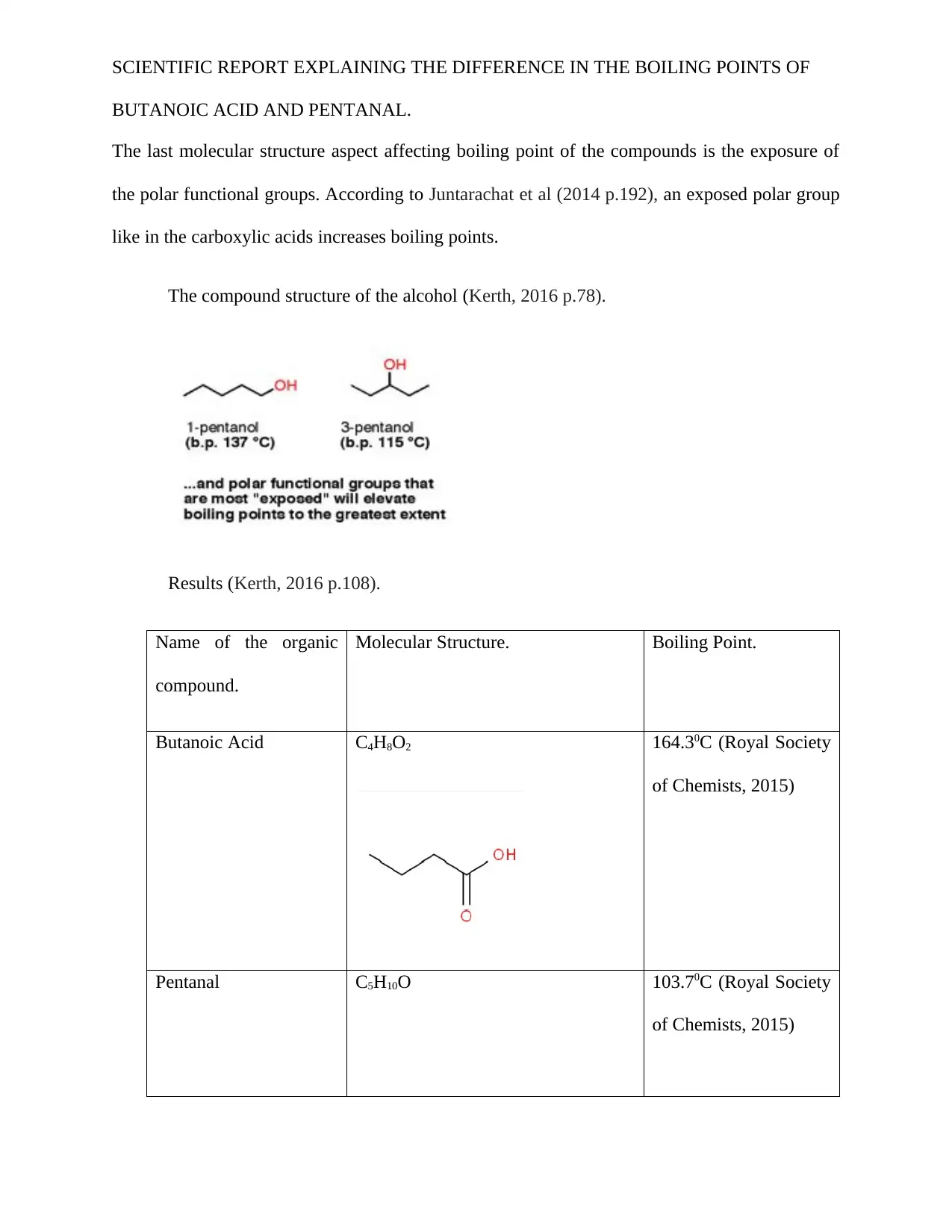
SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
The last molecular structure aspect affecting boiling point of the compounds is the exposure of
the polar functional groups. According to Juntarachat et al (2014 p.192), an exposed polar group
like in the carboxylic acids increases boiling points.
The compound structure of the alcohol (Kerth, 2016 p.78).
Results (Kerth, 2016 p.108).
Name of the organic
compound.
Molecular Structure. Boiling Point.
Butanoic Acid C4H8O2 164.30C (Royal Society
of Chemists, 2015)
Pentanal C5H10O 103.70C (Royal Society
of Chemists, 2015)
BUTANOIC ACID AND PENTANAL.
The last molecular structure aspect affecting boiling point of the compounds is the exposure of
the polar functional groups. According to Juntarachat et al (2014 p.192), an exposed polar group
like in the carboxylic acids increases boiling points.
The compound structure of the alcohol (Kerth, 2016 p.78).
Results (Kerth, 2016 p.108).
Name of the organic
compound.
Molecular Structure. Boiling Point.
Butanoic Acid C4H8O2 164.30C (Royal Society
of Chemists, 2015)
Pentanal C5H10O 103.70C (Royal Society
of Chemists, 2015)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
Discussion and Conclusion
Discussion
Botanic acid is a carboxylic acid with forces comprising of Van der Waals dispersion
forces, dipole-dipole moments and hydrogen bonds that require to be broken at the boiling point
while Pentanal is an aldehyde comprising of Van der Waals dispersion forces and dipole-dipole
moments that require to be broken at the boiling point (Ni, Robarge, Xiao, Heber, 2012 p.86).
The hydrogen bonds found in the botanic acid are responsible for the higher boiling point
observed since these forces require high amount s of energy to break them as compared to the
dipole-dipole moments found in the pentanal.
Conclusion
The hydrogen bonding in the botanic acid molecule results in the observed difference
between the boiling points of the botanic acid and the pentanal since the bond requires more
energy to break as compared to the dipole-dipole bonds found in the pentanal molecule.
BUTANOIC ACID AND PENTANAL.
Discussion and Conclusion
Discussion
Botanic acid is a carboxylic acid with forces comprising of Van der Waals dispersion
forces, dipole-dipole moments and hydrogen bonds that require to be broken at the boiling point
while Pentanal is an aldehyde comprising of Van der Waals dispersion forces and dipole-dipole
moments that require to be broken at the boiling point (Ni, Robarge, Xiao, Heber, 2012 p.86).
The hydrogen bonds found in the botanic acid are responsible for the higher boiling point
observed since these forces require high amount s of energy to break them as compared to the
dipole-dipole moments found in the pentanal.
Conclusion
The hydrogen bonding in the botanic acid molecule results in the observed difference
between the boiling points of the botanic acid and the pentanal since the bond requires more
energy to break as compared to the dipole-dipole bonds found in the pentanal molecule.

SCIENTIFIC REPORT EXPLAINING THE DIFFERENCE IN THE BOILING POINTS OF
BUTANOIC ACID AND PENTANAL.
References.
Damaceno, D.S., Jesus, E.P. and Ceriani, R., 2018. Experimental data and prediction of normal
boiling points of partial acylglycerols. Fuel, 232, pp.470-475.
https://www.sciencedirect.com/science/article/pii/S0016236118310056
Juntarachat, N., Bouvier, N., Lepoutre, J.P., Roland, A., Sainte‐Beuve, J., Granet, F., Salmon,
J.M., Rigou, P. and Chalier, P., 2013. Identification by GC‐O and GC‐MS of new odorous
compounds in natural rubber. Journal of Applied Polymer Science, 130(3), pp.1863-1872.
https://onlinelibrary.wiley.com/doi/abs/10.1002/app.39356
Kerth, C., 2016. Determination of volatile aroma compounds in beef using differences in steak
thickness and cook surface temperature. Meat science, 117, pp.27-35.
https://www.sciencedirect.com/science/article/pii/S0309174016300432
Ni, J.Q., Robarge, W.P., Xiao, C. and Heber, A.J., 2012. Volatile organic compounds at swine
facilities: A critical review. Chemosphere, 89(7), pp.769-788.
https://www.sciencedirect.com/science/article/pii/S0045653512005930
BUTANOIC ACID AND PENTANAL.
References.
Damaceno, D.S., Jesus, E.P. and Ceriani, R., 2018. Experimental data and prediction of normal
boiling points of partial acylglycerols. Fuel, 232, pp.470-475.
https://www.sciencedirect.com/science/article/pii/S0016236118310056
Juntarachat, N., Bouvier, N., Lepoutre, J.P., Roland, A., Sainte‐Beuve, J., Granet, F., Salmon,
J.M., Rigou, P. and Chalier, P., 2013. Identification by GC‐O and GC‐MS of new odorous
compounds in natural rubber. Journal of Applied Polymer Science, 130(3), pp.1863-1872.
https://onlinelibrary.wiley.com/doi/abs/10.1002/app.39356
Kerth, C., 2016. Determination of volatile aroma compounds in beef using differences in steak
thickness and cook surface temperature. Meat science, 117, pp.27-35.
https://www.sciencedirect.com/science/article/pii/S0309174016300432
Ni, J.Q., Robarge, W.P., Xiao, C. and Heber, A.J., 2012. Volatile organic compounds at swine
facilities: A critical review. Chemosphere, 89(7), pp.769-788.
https://www.sciencedirect.com/science/article/pii/S0045653512005930
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





