Cancer Biology Essay: Hallmarks of Cancer and Their Impact
VerifiedAdded on 2021/04/16
|16
|4400
|99
Essay
AI Summary
This essay provides a comprehensive overview of cancer biology, focusing on the ten hallmarks of cancer development, with a detailed examination of sustaining proliferative signaling and resisting cell death. The essay explores the mechanisms underlying these hallmarks, including the role of growth factors, signaling pathways like PI3K/Akt, and the evasion of growth suppressors. Furthermore, it discusses the importance of angiogenesis, genome instability, and tumor-promoting inflammation in cancer progression. The essay also highlights the role of specific chemotherapy drugs, such as Cisplatin, in targeting these hallmarks and the challenges of drug resistance. Overall, the essay offers a deep dive into the complexities of cancer biology, providing insights into the mechanisms driving tumor development and potential therapeutic interventions.

Running head: HALLMARKS OF CANCER BIOLOGY
Identifying Hallmarks of Cancer Biology with a detailed insight of Sustaining Proliferative
Thinking and Resisting Cell Death
Name of the Student
Name of the University
Author Note
Identifying Hallmarks of Cancer Biology with a detailed insight of Sustaining Proliferative
Thinking and Resisting Cell Death
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1HALLMARKS OF CANCER BIOLOGY
Metastatic cancer is a type of cancer in which cancer cells transfer to different organs
in contrast to the site where it was first formed. The other part of the body in which it forms
the new tumor is called as metastatic tumors. Metastatic of cancer takes place in a complex
system of heterogeneous cell population. One of the hallmarks of metastatic cancer is the
invasion of cell from local areas to distance tissues. There are many other mechanism
underlying development of metastatic cancer such as sustaining proliferative signaling,
evading growth suppressors, overcoming immune destruction, tumor promoting
inflammation, invasion, angiogenesis, mutation, resisting cell death and deregulation
energetic (1). This essay gives more insight into each hallmark of cancer and its contribution
to cancer development. Furthermore, the essay also discusses in more detail about the
hallmarks of sustaining proliferative signaling and resisting cell death and the role of specific
chemotherapy drugs targeting these hallmarks.
Hallmarks of cancer development:
The 10 hallmarks underlying metastatic cancer development and their role in
metastatic dissemination are as follows:
1. Sustaining proliferative signaling: The most fundamental mechanism for cancer cell
development is their ability to proliferate constantly. Compared to normal cells,
cancer cells deregulate the growth promoting signals resulting in unlimited growth as
they are no more dependent on proliferation signals. Tumor cells acquire the property
to sustain proliferative signaling by producing their own growth factors causing
autocrine stimulation. The production of paracrine signal also enhances growth of
normal cells. In addition, their reliance on growth factors are reduced by the
constitutive activation of downstream signaling pathways (2). The downstream
signaling pathway is activated by somatic mutations in the catalytic subunit of
Metastatic cancer is a type of cancer in which cancer cells transfer to different organs
in contrast to the site where it was first formed. The other part of the body in which it forms
the new tumor is called as metastatic tumors. Metastatic of cancer takes place in a complex
system of heterogeneous cell population. One of the hallmarks of metastatic cancer is the
invasion of cell from local areas to distance tissues. There are many other mechanism
underlying development of metastatic cancer such as sustaining proliferative signaling,
evading growth suppressors, overcoming immune destruction, tumor promoting
inflammation, invasion, angiogenesis, mutation, resisting cell death and deregulation
energetic (1). This essay gives more insight into each hallmark of cancer and its contribution
to cancer development. Furthermore, the essay also discusses in more detail about the
hallmarks of sustaining proliferative signaling and resisting cell death and the role of specific
chemotherapy drugs targeting these hallmarks.
Hallmarks of cancer development:
The 10 hallmarks underlying metastatic cancer development and their role in
metastatic dissemination are as follows:
1. Sustaining proliferative signaling: The most fundamental mechanism for cancer cell
development is their ability to proliferate constantly. Compared to normal cells,
cancer cells deregulate the growth promoting signals resulting in unlimited growth as
they are no more dependent on proliferation signals. Tumor cells acquire the property
to sustain proliferative signaling by producing their own growth factors causing
autocrine stimulation. The production of paracrine signal also enhances growth of
normal cells. In addition, their reliance on growth factors are reduced by the
constitutive activation of downstream signaling pathways (2). The downstream
signaling pathway is activated by somatic mutations in the catalytic subunit of

2HALLMARKS OF CANCER BIOLOGY
phosphoinositide 3-kinase (PI3-kinase) (3). This indicates the mechanism by which
cancerous cell grow and develop.
2. Evading growth suppressors- Evading growth suppressors is that hallmarks of cancer
cells that complements the process of sustaining proliferative signaling. The ability to
evade growth suppression is a necessary process to sustain growth signal as this
mechanism acts to prevent all those pathways that negatively influence cell
proliferation. TP53 and RB are some tumor suppressive protein coding gene involves
in inhibiting cell growth and mutation or deletion of these genes results in the
developments of cancerous tumors in patient (3). Another mechanism by which
cancer cells inhibits the function of tumor suppressor genes includes interaction of the
ncRNA fragments with tumor suppressor proteins. This results in release of high
levels of PSF-binding RNAs from tumor cell line (4). Hence, role of ncRNAs in
evading growth suppressors gives clear insight into mechanism behind cancer
development.
3. Avoiding immune destruction: Immune evasion or avoiding immune destruction is
also one of the hallmarks of cancer development. This process also acts as a major
barrier in designing anti-cancer therapeutic strategies. The evasion of immune attack
occurs by creating an immune suppressive environment by means of tumor variants
resistant to immune effectors. Cytotoxic T cells and CD4+ and T helper cells produce
interferon and cytotoxin to inhibit the development of cancer cells, however the
process of chronic inflammation counteracts immune response and promotes cancer
development. The tumor cells also exploit regulatory T cells (Tregs), defective
antigen presentation and immune suppressive mediators and apaptosis mechanism to
evade immune response. In the case of cancer metastasis also, the mechanism of
phosphoinositide 3-kinase (PI3-kinase) (3). This indicates the mechanism by which
cancerous cell grow and develop.
2. Evading growth suppressors- Evading growth suppressors is that hallmarks of cancer
cells that complements the process of sustaining proliferative signaling. The ability to
evade growth suppression is a necessary process to sustain growth signal as this
mechanism acts to prevent all those pathways that negatively influence cell
proliferation. TP53 and RB are some tumor suppressive protein coding gene involves
in inhibiting cell growth and mutation or deletion of these genes results in the
developments of cancerous tumors in patient (3). Another mechanism by which
cancer cells inhibits the function of tumor suppressor genes includes interaction of the
ncRNA fragments with tumor suppressor proteins. This results in release of high
levels of PSF-binding RNAs from tumor cell line (4). Hence, role of ncRNAs in
evading growth suppressors gives clear insight into mechanism behind cancer
development.
3. Avoiding immune destruction: Immune evasion or avoiding immune destruction is
also one of the hallmarks of cancer development. This process also acts as a major
barrier in designing anti-cancer therapeutic strategies. The evasion of immune attack
occurs by creating an immune suppressive environment by means of tumor variants
resistant to immune effectors. Cytotoxic T cells and CD4+ and T helper cells produce
interferon and cytotoxin to inhibit the development of cancer cells, however the
process of chronic inflammation counteracts immune response and promotes cancer
development. The tumor cells also exploit regulatory T cells (Tregs), defective
antigen presentation and immune suppressive mediators and apaptosis mechanism to
evade immune response. In the case of cancer metastasis also, the mechanism of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3HALLMARKS OF CANCER BIOLOGY
detaching from primary tumor and travelling through the surroundings tissues occurs
by avoiding immune destruction (5).
4. Enabling replicative immortality: Enabling replicative immortality is the third trait of
cancer which indicates the potential of cancer cells towards unlimited replication.
Normal cells cannot pass through large number of cell division cycles; however tumor
cells possess the potential to unlimited replication. Tumor cells possess unlimited
replication potential by way of circumventing the loss of telomeres that determines
the number of cell division cycles. Tumor cells are able to control the loss of
telomeres by the expression of telomerase enzyme and the activation of telomere
tandem lengthening pathway. Long ncRNAs also plays a role in replicative
immortality as it acts as the regulator of genome stability and replication (3).
5. Tumor promoting inflammation: In the process of cancer development, it has been
found that the negative effect of the immune system results in cancer development.
The presence of white blood cells in tumor cells gives indication of the relation
between inflammation and cancer development. The complex interplay between
immunity and inflammation causes the development of cancer cells. Tumor
promoting inflammation (TAM) and anti-tumor immunity regulates the pathway for
formation of tumor. Tumor associated macrophases also provides the environment for
tumor growth, invasion and metastasis (6). From this evidence, it can be said that
TAM cells plays a major role in tumor promoting inflammation and cancer
development. This knowledge can be effectively utilized to design cancer treatment
options.
6. Activating invasion and metastasis: Another mechanism that is regarded as a hallmark
of cancer includes their ability to invade and form distant metastases. The step
towards invasion and metastatis initiates when morphological changes occur in cancer
detaching from primary tumor and travelling through the surroundings tissues occurs
by avoiding immune destruction (5).
4. Enabling replicative immortality: Enabling replicative immortality is the third trait of
cancer which indicates the potential of cancer cells towards unlimited replication.
Normal cells cannot pass through large number of cell division cycles; however tumor
cells possess the potential to unlimited replication. Tumor cells possess unlimited
replication potential by way of circumventing the loss of telomeres that determines
the number of cell division cycles. Tumor cells are able to control the loss of
telomeres by the expression of telomerase enzyme and the activation of telomere
tandem lengthening pathway. Long ncRNAs also plays a role in replicative
immortality as it acts as the regulator of genome stability and replication (3).
5. Tumor promoting inflammation: In the process of cancer development, it has been
found that the negative effect of the immune system results in cancer development.
The presence of white blood cells in tumor cells gives indication of the relation
between inflammation and cancer development. The complex interplay between
immunity and inflammation causes the development of cancer cells. Tumor
promoting inflammation (TAM) and anti-tumor immunity regulates the pathway for
formation of tumor. Tumor associated macrophases also provides the environment for
tumor growth, invasion and metastasis (6). From this evidence, it can be said that
TAM cells plays a major role in tumor promoting inflammation and cancer
development. This knowledge can be effectively utilized to design cancer treatment
options.
6. Activating invasion and metastasis: Another mechanism that is regarded as a hallmark
of cancer includes their ability to invade and form distant metastases. The step
towards invasion and metastatis initiates when morphological changes occur in cancer
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4HALLMARKS OF CANCER BIOLOGY
cells. The invasion-metastasis reaction occurs when cancer cells are able to escape
immune surveillance and move from primary regions to target tissues to form
micromestastases. The expression of E-cadherin, a cell-to-cell adhesion molecule is
also one of the important factor of the invasion-metastasis cascade. In contrast to
down regulation of E-cadherin in human carcinomas, N-Cadherin is upregulated in
invasive tumors (7).
7. Inducing angiogenesis: Inducing angiogenesis is also a trait found in cancer cells. The
main advantage of this trait is that the process of angiogenesis prevents the natural
diffusion limit of oxygen and nutrients. The process of angiogenesis is necessary for
wound healing and tumor progression turns on the angiogenic switch thus helping to
sustain tumor growth. The mechanism behind angiogenesis includes binding of the
angiogenic regulators to receptors on the endothelial cells (8). Certain evidence has
also given indication about the role of ncRNAs in facilitating the angiogenic process
(3).
8. Genome instability and mutation: Genome instability is also one of the properties of
cancer cell. Genome instability is the increased likelihood of genomic alterations
during cell division. Genome stability is necessary for cellular integrity, however the
opposite process of genome instability leads to the progression and development of
tumor. The presence of genetic unstability factor in cancer cells results in a shorter
cell cycle and evasion of immunological control mechanism. This provides cancer
cells the advantage of proliferation and transforming into a malignant cell. The
process of genetic instability is associated with structural changes like variations in
base pair mutation and function of microsatellite. There various contrasting evidence
regarding the mechanism underlying genetic instability. One hypothesis is that occurs
cells. The invasion-metastasis reaction occurs when cancer cells are able to escape
immune surveillance and move from primary regions to target tissues to form
micromestastases. The expression of E-cadherin, a cell-to-cell adhesion molecule is
also one of the important factor of the invasion-metastasis cascade. In contrast to
down regulation of E-cadherin in human carcinomas, N-Cadherin is upregulated in
invasive tumors (7).
7. Inducing angiogenesis: Inducing angiogenesis is also a trait found in cancer cells. The
main advantage of this trait is that the process of angiogenesis prevents the natural
diffusion limit of oxygen and nutrients. The process of angiogenesis is necessary for
wound healing and tumor progression turns on the angiogenic switch thus helping to
sustain tumor growth. The mechanism behind angiogenesis includes binding of the
angiogenic regulators to receptors on the endothelial cells (8). Certain evidence has
also given indication about the role of ncRNAs in facilitating the angiogenic process
(3).
8. Genome instability and mutation: Genome instability is also one of the properties of
cancer cell. Genome instability is the increased likelihood of genomic alterations
during cell division. Genome stability is necessary for cellular integrity, however the
opposite process of genome instability leads to the progression and development of
tumor. The presence of genetic unstability factor in cancer cells results in a shorter
cell cycle and evasion of immunological control mechanism. This provides cancer
cells the advantage of proliferation and transforming into a malignant cell. The
process of genetic instability is associated with structural changes like variations in
base pair mutation and function of microsatellite. There various contrasting evidence
regarding the mechanism underlying genetic instability. One hypothesis is that occurs
5HALLMARKS OF CANCER BIOLOGY
by the loss of gene function (9). More research in this area may help to identify
chemotherapeutic drugs that target this hallmark.
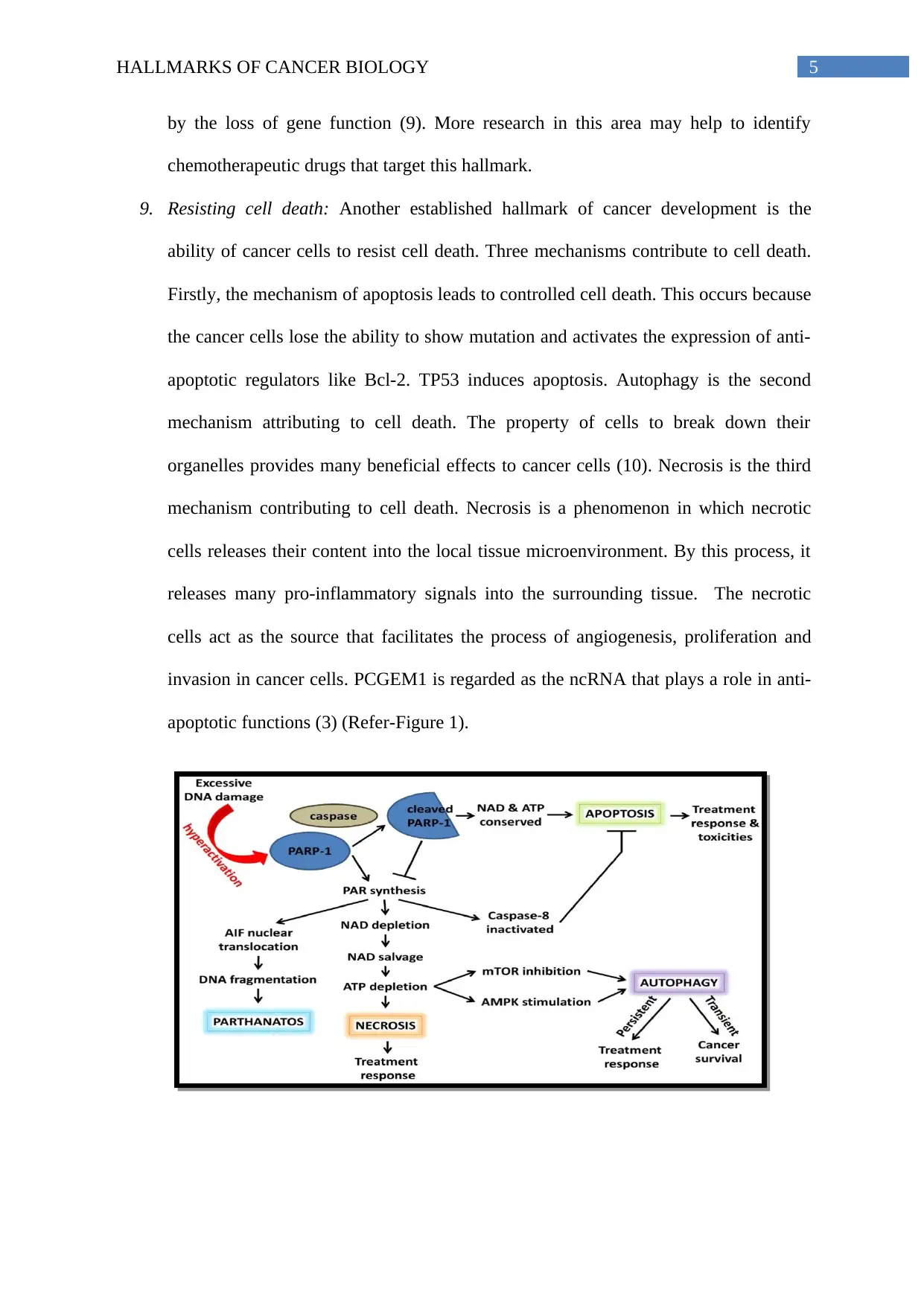
9. Resisting cell death: Another established hallmark of cancer development is the
ability of cancer cells to resist cell death. Three mechanisms contribute to cell death.
Firstly, the mechanism of apoptosis leads to controlled cell death. This occurs because
the cancer cells lose the ability to show mutation and activates the expression of anti-
apoptotic regulators like Bcl-2. TP53 induces apoptosis. Autophagy is the second
mechanism attributing to cell death. The property of cells to break down their
organelles provides many beneficial effects to cancer cells (10). Necrosis is the third
mechanism contributing to cell death. Necrosis is a phenomenon in which necrotic
cells releases their content into the local tissue microenvironment. By this process, it
releases many pro-inflammatory signals into the surrounding tissue. The necrotic
cells act as the source that facilitates the process of angiogenesis, proliferation and
invasion in cancer cells. PCGEM1 is regarded as the ncRNA that plays a role in anti-
apoptotic functions (3) (Refer-Figure 1).
by the loss of gene function (9). More research in this area may help to identify
chemotherapeutic drugs that target this hallmark.
9. Resisting cell death: Another established hallmark of cancer development is the
ability of cancer cells to resist cell death. Three mechanisms contribute to cell death.
Firstly, the mechanism of apoptosis leads to controlled cell death. This occurs because
the cancer cells lose the ability to show mutation and activates the expression of anti-
apoptotic regulators like Bcl-2. TP53 induces apoptosis. Autophagy is the second
mechanism attributing to cell death. The property of cells to break down their
organelles provides many beneficial effects to cancer cells (10). Necrosis is the third
mechanism contributing to cell death. Necrosis is a phenomenon in which necrotic
cells releases their content into the local tissue microenvironment. By this process, it
releases many pro-inflammatory signals into the surrounding tissue. The necrotic
cells act as the source that facilitates the process of angiogenesis, proliferation and
invasion in cancer cells. PCGEM1 is regarded as the ncRNA that plays a role in anti-
apoptotic functions (3) (Refer-Figure 1).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6HALLMARKS OF CANCER BIOLOGY
Figure 1: Mechanism behind hallmarks of resisting cell death. The diagram above depicts
the three mechanisms contributing to survival of cancer cells and resisting cell death.
Source: (Weaver and Yang 2012)
10. Deregulating cellular energetic: Deregulating cellular energetics is also one of the
attributes of cancer cells. Normal cells produce energy the process of glycolysis,
whereas the malignant cells increase their source of energy by the upregulation of
glycolysis. This phenomenon of increases utilization of glucose is known as the
Warburg effect. Hence. The continuous activation of gylcolysis in cancer cells leads
to activation of oncogenes and progression of cancer. The tumor suppressor genes and
the mutated oncogenes plays a major role in deregulating cellular energetic. Normal
cells have several signaling networks that activates metabolic pathways for cell
reproduction. However, many byproducts produced during aerobic metabolism such
as reactive oxygen species result in DNA mutation and cell damage. This change in
cell metabolism is the factor resulting in tumorigenesis. The mutations in the tumor
suppressor genes and oncogenes change many signally pathways thus triggering the
process of tumor development (11).
Hence, from the above evidence, it can be said that the Warburg effect is the mechanism
that results in metabolism of tumor. Cancer cells finds glycolysis as a source of energy.
Hence, it can be said that there is direct relations between tumor malignancy and glycolytic
ATP production. By understanding this phenomenon behind cancer cell development and the
dependence of cancer cells on glucose utilization, many therapeutic interventions can be
designed.
Figure 1: Mechanism behind hallmarks of resisting cell death. The diagram above depicts
the three mechanisms contributing to survival of cancer cells and resisting cell death.
Source: (Weaver and Yang 2012)
10. Deregulating cellular energetic: Deregulating cellular energetics is also one of the
attributes of cancer cells. Normal cells produce energy the process of glycolysis,
whereas the malignant cells increase their source of energy by the upregulation of
glycolysis. This phenomenon of increases utilization of glucose is known as the
Warburg effect. Hence. The continuous activation of gylcolysis in cancer cells leads
to activation of oncogenes and progression of cancer. The tumor suppressor genes and
the mutated oncogenes plays a major role in deregulating cellular energetic. Normal
cells have several signaling networks that activates metabolic pathways for cell
reproduction. However, many byproducts produced during aerobic metabolism such
as reactive oxygen species result in DNA mutation and cell damage. This change in
cell metabolism is the factor resulting in tumorigenesis. The mutations in the tumor
suppressor genes and oncogenes change many signally pathways thus triggering the
process of tumor development (11).
Hence, from the above evidence, it can be said that the Warburg effect is the mechanism
that results in metabolism of tumor. Cancer cells finds glycolysis as a source of energy.
Hence, it can be said that there is direct relations between tumor malignancy and glycolytic
ATP production. By understanding this phenomenon behind cancer cell development and the
dependence of cancer cells on glucose utilization, many therapeutic interventions can be
designed.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7HALLMARKS OF CANCER BIOLOGY
Part 2
Sustaining Proliferative Signalling
One of the major hallmarks of cancer is sustaining proliferative signalling (13). One
of the major characteristic of cancer cells is their ability to proliferate at a constant rate even
in the absence stimuli coming from the external growth factors (13). Normal cells strictly
manipulate the production of the growth initiating and inhibiting factors in order to ensure a
tight control of the tissues and cell number, integrity of the tissue and architecture. However,
tumour cell physiology is completely different from the normal cell lines. They showcase
deregulated signalling cascades that promote them to be more or less free from the effect of
the signals of proliferation which results in the unlimited cycles of proliferation. In order to
attain this immortal capability, the tumours cells attain the capability of sustain proliferative
signalling. This power of sustaining proliferative signalling is acquired by the tumour cells in
different ways for example they generate their own growth factors and their complementary
receptor molecules thereby resulting in the process of autocrine stimulation (14). Other
pathways which are undertaken by the tumour cells in order to attain sustaining proliferative
signalling include paracrine signalling which help in the production of numerous growth
factors that support the development of cancer cells. The growth factor receptors are also
constantly expressed in the tumour cells and this help in sustained proliferation via
continuous binding of the growth factors with its receptors on the tumour. In extreme cases,
the cancer cells become totally independent from the effect of the exogenous growth factors
because of constitutive activation of the downstream signalling pathways or other disruption
of negative-feedback mechanism (14).
In order target the cancerous cell in the ground of sustained proliferative signalling,
PI3K/Akt pathway is being targeted by the chemotherapeutic drugs. This is because,
PI3L/Akt signalling pathways plays an important role not only towards the growth of tumour
Part 2
Sustaining Proliferative Signalling
One of the major hallmarks of cancer is sustaining proliferative signalling (13). One
of the major characteristic of cancer cells is their ability to proliferate at a constant rate even
in the absence stimuli coming from the external growth factors (13). Normal cells strictly
manipulate the production of the growth initiating and inhibiting factors in order to ensure a
tight control of the tissues and cell number, integrity of the tissue and architecture. However,
tumour cell physiology is completely different from the normal cell lines. They showcase
deregulated signalling cascades that promote them to be more or less free from the effect of
the signals of proliferation which results in the unlimited cycles of proliferation. In order to
attain this immortal capability, the tumours cells attain the capability of sustain proliferative
signalling. This power of sustaining proliferative signalling is acquired by the tumour cells in
different ways for example they generate their own growth factors and their complementary
receptor molecules thereby resulting in the process of autocrine stimulation (14). Other
pathways which are undertaken by the tumour cells in order to attain sustaining proliferative
signalling include paracrine signalling which help in the production of numerous growth
factors that support the development of cancer cells. The growth factor receptors are also
constantly expressed in the tumour cells and this help in sustained proliferation via
continuous binding of the growth factors with its receptors on the tumour. In extreme cases,
the cancer cells become totally independent from the effect of the exogenous growth factors
because of constitutive activation of the downstream signalling pathways or other disruption
of negative-feedback mechanism (14).
In order target the cancerous cell in the ground of sustained proliferative signalling,
PI3K/Akt pathway is being targeted by the chemotherapeutic drugs. This is because,
PI3L/Akt signalling pathways plays an important role not only towards the growth of tumour
8HALLMARKS OF CANCER BIOLOGY
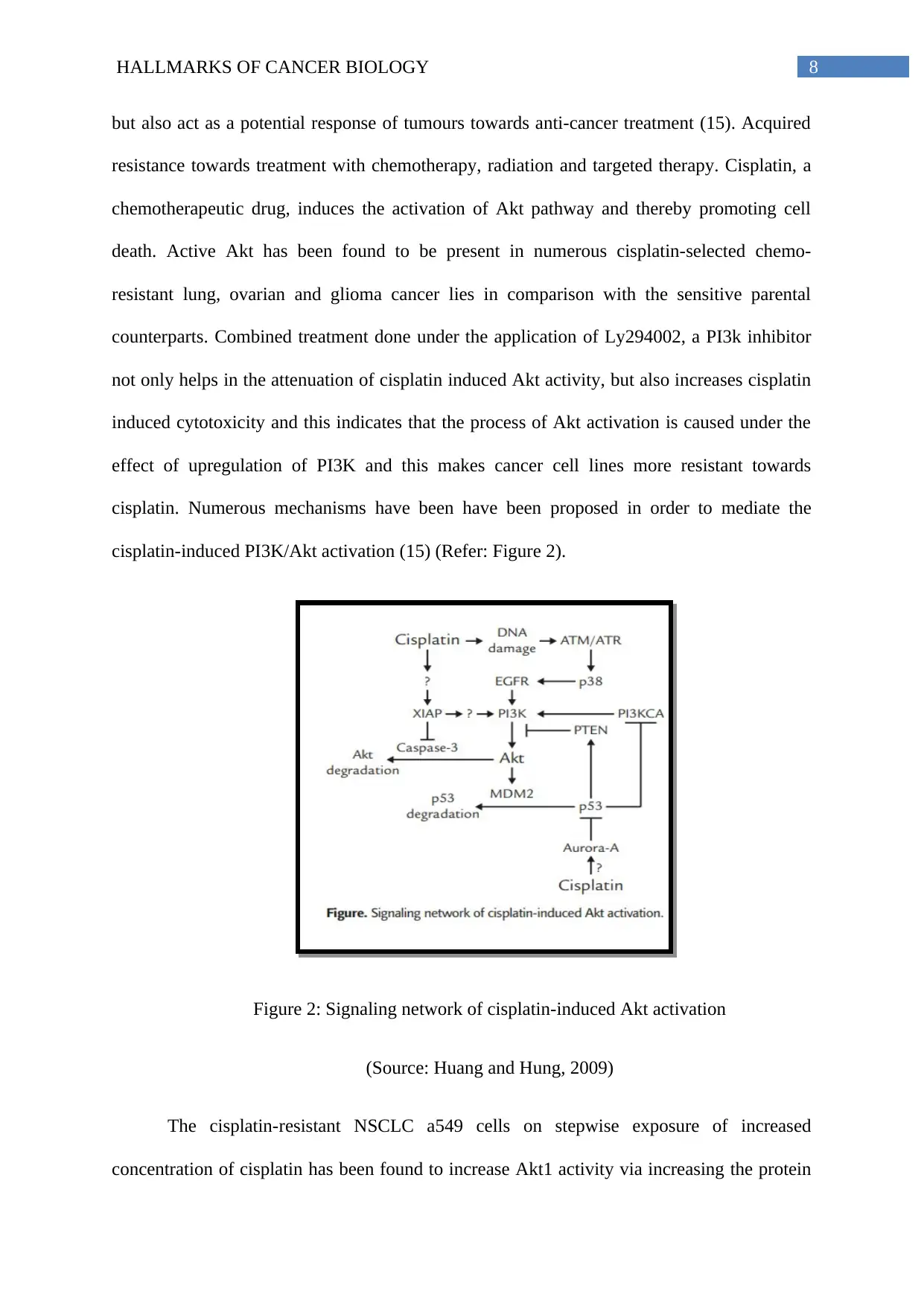
but also act as a potential response of tumours towards anti-cancer treatment (15). Acquired
resistance towards treatment with chemotherapy, radiation and targeted therapy. Cisplatin, a
chemotherapeutic drug, induces the activation of Akt pathway and thereby promoting cell
death. Active Akt has been found to be present in numerous cisplatin-selected chemo-
resistant lung, ovarian and glioma cancer lies in comparison with the sensitive parental
counterparts. Combined treatment done under the application of Ly294002, a PI3k inhibitor
not only helps in the attenuation of cisplatin induced Akt activity, but also increases cisplatin
induced cytotoxicity and this indicates that the process of Akt activation is caused under the
effect of upregulation of PI3K and this makes cancer cell lines more resistant towards
cisplatin. Numerous mechanisms have been have been proposed in order to mediate the
cisplatin-induced PI3K/Akt activation (15) (Refer: Figure 2).
Figure 2: Signaling network of cisplatin-induced Akt activation
(Source: Huang and Hung, 2009)
The cisplatin-resistant NSCLC a549 cells on stepwise exposure of increased
concentration of cisplatin has been found to increase Akt1 activity via increasing the protein
but also act as a potential response of tumours towards anti-cancer treatment (15). Acquired
resistance towards treatment with chemotherapy, radiation and targeted therapy. Cisplatin, a
chemotherapeutic drug, induces the activation of Akt pathway and thereby promoting cell
death. Active Akt has been found to be present in numerous cisplatin-selected chemo-
resistant lung, ovarian and glioma cancer lies in comparison with the sensitive parental
counterparts. Combined treatment done under the application of Ly294002, a PI3k inhibitor
not only helps in the attenuation of cisplatin induced Akt activity, but also increases cisplatin
induced cytotoxicity and this indicates that the process of Akt activation is caused under the
effect of upregulation of PI3K and this makes cancer cell lines more resistant towards
cisplatin. Numerous mechanisms have been have been proposed in order to mediate the
cisplatin-induced PI3K/Akt activation (15) (Refer: Figure 2).
Figure 2: Signaling network of cisplatin-induced Akt activation
(Source: Huang and Hung, 2009)
The cisplatin-resistant NSCLC a549 cells on stepwise exposure of increased
concentration of cisplatin has been found to increase Akt1 activity via increasing the protein
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9HALLMARKS OF CANCER BIOLOGY
level and increased gene expression in comparison to that of the parental cells. On the other
hand, the levels of pAkt signals in the lung cancer tumour are inversely proportional towards
the cisplatin sensitivity towards the primary cultured cancer cell lines of the lung from
identical tumour tissues. The Cisplatin-induced Akt activation is dependent on the EGFR
activity which lies upstream of PI3K. Cisplatin-induced phosphorylation of EGFR is
associated with EGFR internalization along with ATM and ATR-dependent activation of p38
and this occurs as a result of cisplatin-induced DNA damage. This activated p38 causes the
internalization of epidermal growth factor receptor (EGFR). Internalized EGFR causes
downstream phosphorylation of the tyrosine residues and thereby active further downstream
tumour suppressor protein p85. This p85 is another important component of PI3K and thus
help in the prevention of sustained signalling and thereby promoting cell death (16).
Another chemotherapeutic drug that is found to prevent sustained signalling in
cancerous cell line is Etoposide. Etoposide is a podophyllotoxin which is kown to casr
peliotropic actions within the cells including inhibition of the action of topoisomerase II,
production of reactive oxygen species (ROS) and subsequent induction of DNA damage. This
DNa damage actives PIK3-Akt kinase signalling pathway which in turn found to cast an
effective impact towards the treatment of gastric cancer (17)(18).
Resisting Cell Death
The aim of immortality for the cancer cells can be easily achieved if the cancerous
cell acquires the hallmark of resting cell death. There are three important pathways that
promotes cell death and careful regulation of three of these pathways help in the achievement
of the immortality of the cancerous cell. The first mechanisms that promote towards
controlled cell death include apoptosis. The process of apoptosis is initiated via numerous
external and internal stimuli and numerous studies have highlighted that cancerous cell which
level and increased gene expression in comparison to that of the parental cells. On the other
hand, the levels of pAkt signals in the lung cancer tumour are inversely proportional towards
the cisplatin sensitivity towards the primary cultured cancer cell lines of the lung from
identical tumour tissues. The Cisplatin-induced Akt activation is dependent on the EGFR
activity which lies upstream of PI3K. Cisplatin-induced phosphorylation of EGFR is
associated with EGFR internalization along with ATM and ATR-dependent activation of p38
and this occurs as a result of cisplatin-induced DNA damage. This activated p38 causes the
internalization of epidermal growth factor receptor (EGFR). Internalized EGFR causes
downstream phosphorylation of the tyrosine residues and thereby active further downstream
tumour suppressor protein p85. This p85 is another important component of PI3K and thus
help in the prevention of sustained signalling and thereby promoting cell death (16).
Another chemotherapeutic drug that is found to prevent sustained signalling in
cancerous cell line is Etoposide. Etoposide is a podophyllotoxin which is kown to casr
peliotropic actions within the cells including inhibition of the action of topoisomerase II,
production of reactive oxygen species (ROS) and subsequent induction of DNA damage. This
DNa damage actives PIK3-Akt kinase signalling pathway which in turn found to cast an
effective impact towards the treatment of gastric cancer (17)(18).
Resisting Cell Death
The aim of immortality for the cancer cells can be easily achieved if the cancerous
cell acquires the hallmark of resting cell death. There are three important pathways that
promotes cell death and careful regulation of three of these pathways help in the achievement
of the immortality of the cancerous cell. The first mechanisms that promote towards
controlled cell death include apoptosis. The process of apoptosis is initiated via numerous
external and internal stimuli and numerous studies have highlighted that cancerous cell which
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10HALLMARKS OF CANCER BIOLOGY
are malignant in nature can attenuate the process of apoptosis and thereby becoming resistant
towards cell death. In normal cell, the induction of DNA damage is one of the main markers
towards the initiation of apoptosis. DNA damage, promotes the expression of the pro-
apoptotic proteins Noxa and Puma (upregulated modulator p53, apoptotic gene) causing cell
death. However, more than 50% of all human cancers have lost the signalling pathways
mediated by p53 gene or p53 gene remain mutated and this lead to termination of the process
of apoptosis even during the cell damage. Alternatively the tumours represents show an
increased level of expression of numerous survival factors or other anti-apoptotic regulators
like Bcl-2 and Bcl-xL. This can be regarded as one of the second important mechanism that
repress controlled cell death or autophage. The cellular mechanism of autophagy operates at
low levels within the cell line. However, it can be alternatively activated via numerous kind
of cellular stress factors like nutrient deficiency. Autophagy is considered as a cell-recycling
program that enables the cell to break down into their organelles and then to employ the
degradation of the products towards the process of fuel biosynthesis pathways or for re-usual
for subsequent energy production within the body. However, autophagy can act both as
strength and weakness of cancerous cell. This weakness comes in the form of blocking the
pathway for carcinogenesis. The last mode of cell death that is negatively impaired in cancer
cell line is necrosis. Necrosis is defined as a process of un-controlled cell-death that occurs
mostly with the damaged or injured cell lines. Similar to that of autophagy, necrosis can also
serve to be beneficial as well as harmful for the cancerous cell lines. In the beneficial
grounds, the negative regulation of necrosis in cancer cell lead to the uncontrolled cell
proliferation and the other hand, necrotic cell lines have also found to attract the
proinflammatory cell mediators causing death of the cancer cells (14).
In order to induce apoptosis in cancerous cell, the chemotherapeutic drugs like the
cisplatimum or etoposide triggers apoptosis via the activation of TP53 pathways (2).
are malignant in nature can attenuate the process of apoptosis and thereby becoming resistant
towards cell death. In normal cell, the induction of DNA damage is one of the main markers
towards the initiation of apoptosis. DNA damage, promotes the expression of the pro-
apoptotic proteins Noxa and Puma (upregulated modulator p53, apoptotic gene) causing cell
death. However, more than 50% of all human cancers have lost the signalling pathways
mediated by p53 gene or p53 gene remain mutated and this lead to termination of the process
of apoptosis even during the cell damage. Alternatively the tumours represents show an
increased level of expression of numerous survival factors or other anti-apoptotic regulators
like Bcl-2 and Bcl-xL. This can be regarded as one of the second important mechanism that
repress controlled cell death or autophage. The cellular mechanism of autophagy operates at
low levels within the cell line. However, it can be alternatively activated via numerous kind
of cellular stress factors like nutrient deficiency. Autophagy is considered as a cell-recycling
program that enables the cell to break down into their organelles and then to employ the
degradation of the products towards the process of fuel biosynthesis pathways or for re-usual
for subsequent energy production within the body. However, autophagy can act both as
strength and weakness of cancerous cell. This weakness comes in the form of blocking the
pathway for carcinogenesis. The last mode of cell death that is negatively impaired in cancer
cell line is necrosis. Necrosis is defined as a process of un-controlled cell-death that occurs
mostly with the damaged or injured cell lines. Similar to that of autophagy, necrosis can also
serve to be beneficial as well as harmful for the cancerous cell lines. In the beneficial
grounds, the negative regulation of necrosis in cancer cell lead to the uncontrolled cell
proliferation and the other hand, necrotic cell lines have also found to attract the
proinflammatory cell mediators causing death of the cancer cells (14).
In order to induce apoptosis in cancerous cell, the chemotherapeutic drugs like the
cisplatimum or etoposide triggers apoptosis via the activation of TP53 pathways (2).

11HALLMARKS OF CANCER BIOLOGY
Alterative research has showed that over-expression of the anti-apoptotic protein from the
Bcl-2 family like Bcl-2, Bcl-xl is found to contribute chemotherapeutic resistance in cancers
cell. One of the strategy that is used to destroy this anti-apoptotic protein include application
of interfering oligonucleotide that downregulate the expression of the Bcl2 family of proteins.
Controlled expression of Bax protein or application of BH-3 peptide has been found to
abrogate protection againt the antiapoptotic protein in cancerous cell. One agent that is
present gaining importance in the clinical trials is oblimersen. It is a nuclease-resistant
antisense oligonucleaotide that targets Bcl2 mRNA. However, Oblimersen is still in Phase II
and II clinical trials and is used to treat a wide range of adult and childhood tumours (19)
(20). Oblimersen is still not approved for the treatment of melanoma this is because the
results published from the phase III trials failed to show any extended survivals of the
patients. On the other hand, oblimersen has been shown to produce favourable outcome when
it is combined and injected along with docetaxel in the patients who are suffering from
hormone-refractory prostate cancer (19)(20).
Another drug that is used to induce aopotosis is 5-Fluorouracil (5-FU). It is mainly
used for the treatment of colorectal and breast cancer. 5-FU mainly targets p53 mediated cell
apoptosis. However, one of the major disadvantage of 5-FU is, it becomes non-functional
among the p53 independent cells (21). 5-FU is an uracil analogue. It has fluorine atom
located at the C5 position of the pyrimidine ring. Once 5-FU is transmitted inside the cell, it
gets converted into active metabolites like, fluorodeoxyuridine triphosphate (FdUTP),
fluorodeoxyuridine monophosphate (FdUMP) and fluorouridine triphosphate (FUTP). These
metabolites promotes global RNA metabolism via incorporating FUMP ribonucleotide into
RNA as well as DNA. This incorporation either ocuurs directly or occurs via thymidylate
synthase (TS) inhibition leading to a wide range of abnormal biological effects which trigger
controlled cell death or apoptosis (21).
Alterative research has showed that over-expression of the anti-apoptotic protein from the
Bcl-2 family like Bcl-2, Bcl-xl is found to contribute chemotherapeutic resistance in cancers
cell. One of the strategy that is used to destroy this anti-apoptotic protein include application
of interfering oligonucleotide that downregulate the expression of the Bcl2 family of proteins.
Controlled expression of Bax protein or application of BH-3 peptide has been found to
abrogate protection againt the antiapoptotic protein in cancerous cell. One agent that is
present gaining importance in the clinical trials is oblimersen. It is a nuclease-resistant
antisense oligonucleaotide that targets Bcl2 mRNA. However, Oblimersen is still in Phase II
and II clinical trials and is used to treat a wide range of adult and childhood tumours (19)
(20). Oblimersen is still not approved for the treatment of melanoma this is because the
results published from the phase III trials failed to show any extended survivals of the
patients. On the other hand, oblimersen has been shown to produce favourable outcome when
it is combined and injected along with docetaxel in the patients who are suffering from
hormone-refractory prostate cancer (19)(20).
Another drug that is used to induce aopotosis is 5-Fluorouracil (5-FU). It is mainly
used for the treatment of colorectal and breast cancer. 5-FU mainly targets p53 mediated cell
apoptosis. However, one of the major disadvantage of 5-FU is, it becomes non-functional
among the p53 independent cells (21). 5-FU is an uracil analogue. It has fluorine atom
located at the C5 position of the pyrimidine ring. Once 5-FU is transmitted inside the cell, it
gets converted into active metabolites like, fluorodeoxyuridine triphosphate (FdUTP),
fluorodeoxyuridine monophosphate (FdUMP) and fluorouridine triphosphate (FUTP). These
metabolites promotes global RNA metabolism via incorporating FUMP ribonucleotide into
RNA as well as DNA. This incorporation either ocuurs directly or occurs via thymidylate
synthase (TS) inhibition leading to a wide range of abnormal biological effects which trigger
controlled cell death or apoptosis (21).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





