Cancer Treatment: Selective Toxicity, Chemotherapy, and Limitations
VerifiedAdded on 2022/08/23
|12
|3009
|13
Essay
AI Summary
This essay delves into the complexities of cancer treatment, beginning with an exploration of selective toxicity, a crucial principle in targeting cancerous cells while minimizing harm to healthy ones. It examines the mechanisms of action and limitations of chemotherapy, including the challenges of drug resistance and associated side effects. The essay provides detailed insights into various classes of chemotherapeutic agents such as antitumor antibiotics, alkylating agents, and antimetabolites, discussing their specific modes of action and the limitations they present. Furthermore, it highlights emerging treatment strategies like molecular targeted therapies, nano medicines, and gene therapy, which aim to overcome the limitations of traditional chemotherapy and offer more precise and effective cancer treatments. The essay also includes a discussion of drug resistance and the need for new technologies and combination therapies to enhance treatment efficacy and improve patient outcomes.

Running head: CANCER AND IT’S CURE
CANCER AND IT’S CURE
Name of the Student
Name of the University
Author Note
CANCER AND IT’S CURE
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1CANCER AND IT’S CURE
a) SELECTIVE TOXICITY OF ANTICANCER MEDICINES
Selective Toxicity can be defined as the potential of a medicine or drug to recognize
the specific site in a microorganism or cell and inhibit its critical action on a living organism
(Biology LibreTexts, 2020). In this case, of anti-cancerous drugs, selective toxicity of a drug
is its ability to target cancerous cells at the specific site and inhibit its growth as well as
metastasizing capability that is, spread to other sites, and keeping the normal cells safe from
the action of toxic effects of the drug (Yun et al. 2015). The resistance grows when the cells
undergo different genetic changes thus, modifying the target sites on which the drugs acted
(Wei et al. 2015). There are quite a few mechanisms including selective toxicity. While
prescribing these drugs to the cancer patients, the dosage should also be decided after critical
thinking as it might exert various side effects, which includes the action of alkylating agents,
such as melphalan and cyclophosphamide that can destroy the cancer cells by inhibiting DNA
synthesis but also affects the bone marrow leading to leukaemia (Singh et al. 2015). There
are a few anticancer antibiotics as well which inhibits the DNA replication altogether but also
exerts a negative effect on the heart when prescribed in higher dosage (Agudelo et al. 2016).
The drugs which are tested on animal models in every aspect and ratio possible, when shows
positive results can be recommended to human (Salzano et al. 2015). However, one
significant advantage of selective toxicity is that it targets only the affected cells and does no
harm to healthy cells, which is essential for normal. Thus, it can be concluded that the ability
of selective toxicity to target specific targets sites is used in cancer therapy to inhibit the
cancerous cell growth and spread.
b) Limitations of Chemotherapy
Chemotherapy is an essential procedure of treating people with cancer and has proved
its efficiency over the time. There are a few positive points that make the usage of
a) SELECTIVE TOXICITY OF ANTICANCER MEDICINES
Selective Toxicity can be defined as the potential of a medicine or drug to recognize
the specific site in a microorganism or cell and inhibit its critical action on a living organism
(Biology LibreTexts, 2020). In this case, of anti-cancerous drugs, selective toxicity of a drug
is its ability to target cancerous cells at the specific site and inhibit its growth as well as
metastasizing capability that is, spread to other sites, and keeping the normal cells safe from
the action of toxic effects of the drug (Yun et al. 2015). The resistance grows when the cells
undergo different genetic changes thus, modifying the target sites on which the drugs acted
(Wei et al. 2015). There are quite a few mechanisms including selective toxicity. While
prescribing these drugs to the cancer patients, the dosage should also be decided after critical
thinking as it might exert various side effects, which includes the action of alkylating agents,
such as melphalan and cyclophosphamide that can destroy the cancer cells by inhibiting DNA
synthesis but also affects the bone marrow leading to leukaemia (Singh et al. 2015). There
are a few anticancer antibiotics as well which inhibits the DNA replication altogether but also
exerts a negative effect on the heart when prescribed in higher dosage (Agudelo et al. 2016).
The drugs which are tested on animal models in every aspect and ratio possible, when shows
positive results can be recommended to human (Salzano et al. 2015). However, one
significant advantage of selective toxicity is that it targets only the affected cells and does no
harm to healthy cells, which is essential for normal. Thus, it can be concluded that the ability
of selective toxicity to target specific targets sites is used in cancer therapy to inhibit the
cancerous cell growth and spread.
b) Limitations of Chemotherapy
Chemotherapy is an essential procedure of treating people with cancer and has proved
its efficiency over the time. There are a few positive points that make the usage of

2CANCER AND IT’S CURE
chemotherapy much advantageous as it can spread throughout the body destroying the cancer
cells. However, it has some disadvantages as well which include resistance towards the
treatment and many derogatory effects on the body (Wagner et al. 2017). The side effects
caused by chemotherapeutic agents vary among various types of cancer. Further side effects
include alopecia, vomiting, headache, fatigue, diarrhoea and might even lead to memory loss
in severe cases (Pearce et al. 2017). The cancerous cells in the body gradually develop
various mechanisms in which it can inhibit or bypass the action of chemotherapeutic drugs on
them. These mechanisms include the altering the target sites of the drugs, increase in the rate
of repairing the DNA replication altered by the drug, reducing the build-up or collection of
drug at a specific point and its increased exportation and avoiding the apoptosis or
programmed cell death mechanism (Wei et al. 2015). Chemotherapeutic drugs like antitumor
antibiotics, alkylating agents and antimetabolites exhibit quite a few limitations and leads to
side effects.
The antitumor antibiotics act by blocking the growth of cell by interfering with the
DNA. It also attack the cells during various phases of the cell cycle thus preventing the
uncontrolled cell division. Examples include anthracycline such as doxorubicine and
chromomycin. The mechanism of action of antitumor antibiotics occurs in three steps.
Initially, the drug interferes with the DNA replication and mRNA transcription by getting
intercalated within the DNA. Following this, the free radicals are generated by the action of
the antitumor antibiotics and the DNA strand gets broken. Lastly, the functions of DNA
Topoisomerase, an enzyme required to maintain the DNA replication is inhibited by the
antitumor antibiotic thus rendering the DNA non-functional (Zhang et al. 2016). There are
also a few limitations of using these antitumor antibiotics as it cause various critical effects
on human which includes vomiting, headache, fever, reduced white blood cell, red blood cell
and platelet count, damage of the neural system and the most important side effect of using
chemotherapy much advantageous as it can spread throughout the body destroying the cancer
cells. However, it has some disadvantages as well which include resistance towards the
treatment and many derogatory effects on the body (Wagner et al. 2017). The side effects
caused by chemotherapeutic agents vary among various types of cancer. Further side effects
include alopecia, vomiting, headache, fatigue, diarrhoea and might even lead to memory loss
in severe cases (Pearce et al. 2017). The cancerous cells in the body gradually develop
various mechanisms in which it can inhibit or bypass the action of chemotherapeutic drugs on
them. These mechanisms include the altering the target sites of the drugs, increase in the rate
of repairing the DNA replication altered by the drug, reducing the build-up or collection of
drug at a specific point and its increased exportation and avoiding the apoptosis or
programmed cell death mechanism (Wei et al. 2015). Chemotherapeutic drugs like antitumor
antibiotics, alkylating agents and antimetabolites exhibit quite a few limitations and leads to
side effects.
The antitumor antibiotics act by blocking the growth of cell by interfering with the
DNA. It also attack the cells during various phases of the cell cycle thus preventing the
uncontrolled cell division. Examples include anthracycline such as doxorubicine and
chromomycin. The mechanism of action of antitumor antibiotics occurs in three steps.
Initially, the drug interferes with the DNA replication and mRNA transcription by getting
intercalated within the DNA. Following this, the free radicals are generated by the action of
the antitumor antibiotics and the DNA strand gets broken. Lastly, the functions of DNA
Topoisomerase, an enzyme required to maintain the DNA replication is inhibited by the
antitumor antibiotic thus rendering the DNA non-functional (Zhang et al. 2016). There are
also a few limitations of using these antitumor antibiotics as it cause various critical effects
on human which includes vomiting, headache, fever, reduced white blood cell, red blood cell
and platelet count, damage of the neural system and the most important side effect of using
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3CANCER AND IT’S CURE
this drug is it causes heart diseases and might lead to heart failure (Bhattacharya and
Mukherjee 2015). To overcome these side-effects, the patient can be recommended to check
temperature every day, decrease roughage and fiber, eating small & frequent meals, increase
fluids and take prescribed medications regularly.
Fig.1: Action of Doxorubicin. It is getting intercalated within the minor groove of DNA.
Free radicals such as superoxide ion generated and function of topoisomerase inhibited.
(Image retrieved from Meredith and Dass 2016)
Another class of drugs is of the alkylating agents, which prevent the cancer cells from
replicating by destroying the DNA. It can act at during any of the phases of cell cycle and
thus prevents the uncontrolled cell growth. For example, nitrogen mustard and busulfan. The
alkylating agents exhibit electrophilic behaviour by which it alkylates various nucleophiles
including the seventh nitrogen in guanine base of the DNA leading to the death of the cell
and proteins. The alkylation of DNA leads to various abnormal conditions in the DNA that
include abnormal base pairing of guanine with thymine, cross linking within the strand,
imidazole ring cleavage and depurination of the purine residue (Sing et al. 2018) However,
there are various limitations of using alkylating agents as the cure for cancer. The reasons are,
it causes toxicity of the bone marrow, toxicity of the mucosal system, toxicity of the neural
this drug is it causes heart diseases and might lead to heart failure (Bhattacharya and
Mukherjee 2015). To overcome these side-effects, the patient can be recommended to check
temperature every day, decrease roughage and fiber, eating small & frequent meals, increase
fluids and take prescribed medications regularly.
Fig.1: Action of Doxorubicin. It is getting intercalated within the minor groove of DNA.
Free radicals such as superoxide ion generated and function of topoisomerase inhibited.
(Image retrieved from Meredith and Dass 2016)
Another class of drugs is of the alkylating agents, which prevent the cancer cells from
replicating by destroying the DNA. It can act at during any of the phases of cell cycle and
thus prevents the uncontrolled cell growth. For example, nitrogen mustard and busulfan. The
alkylating agents exhibit electrophilic behaviour by which it alkylates various nucleophiles
including the seventh nitrogen in guanine base of the DNA leading to the death of the cell
and proteins. The alkylation of DNA leads to various abnormal conditions in the DNA that
include abnormal base pairing of guanine with thymine, cross linking within the strand,
imidazole ring cleavage and depurination of the purine residue (Sing et al. 2018) However,
there are various limitations of using alkylating agents as the cure for cancer. The reasons are,
it causes toxicity of the bone marrow, toxicity of the mucosal system, toxicity of the neural
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
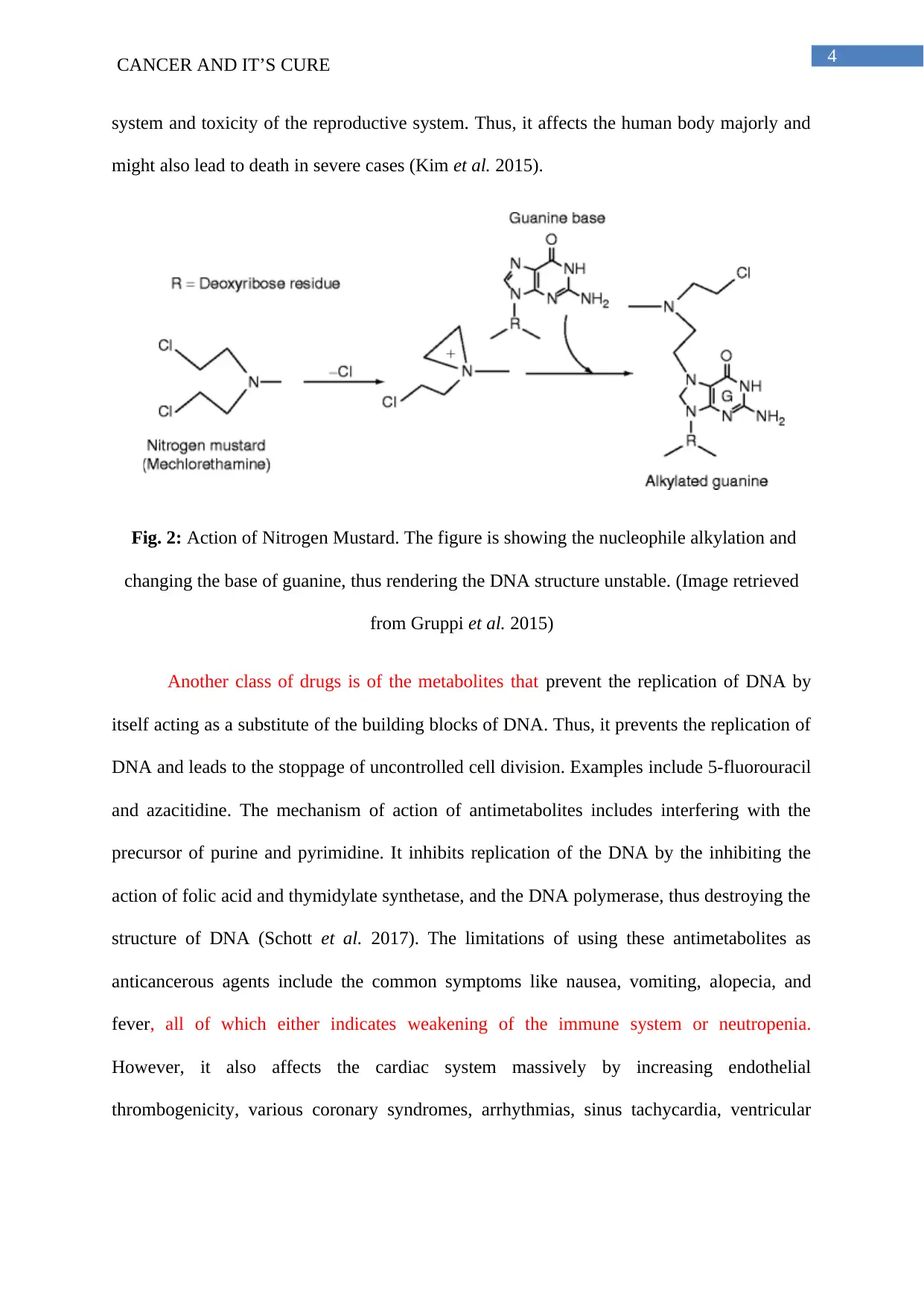
4CANCER AND IT’S CURE
system and toxicity of the reproductive system. Thus, it affects the human body majorly and
might also lead to death in severe cases (Kim et al. 2015).
Fig. 2: Action of Nitrogen Mustard. The figure is showing the nucleophile alkylation and
changing the base of guanine, thus rendering the DNA structure unstable. (Image retrieved
from Gruppi et al. 2015)
Another class of drugs is of the metabolites that prevent the replication of DNA by
itself acting as a substitute of the building blocks of DNA. Thus, it prevents the replication of
DNA and leads to the stoppage of uncontrolled cell division. Examples include 5-fluorouracil
and azacitidine. The mechanism of action of antimetabolites includes interfering with the
precursor of purine and pyrimidine. It inhibits replication of the DNA by the inhibiting the
action of folic acid and thymidylate synthetase, and the DNA polymerase, thus destroying the
structure of DNA (Schott et al. 2017). The limitations of using these antimetabolites as
anticancerous agents include the common symptoms like nausea, vomiting, alopecia, and
fever, all of which either indicates weakening of the immune system or neutropenia.
However, it also affects the cardiac system massively by increasing endothelial
thrombogenicity, various coronary syndromes, arrhythmias, sinus tachycardia, ventricular
system and toxicity of the reproductive system. Thus, it affects the human body majorly and
might also lead to death in severe cases (Kim et al. 2015).
Fig. 2: Action of Nitrogen Mustard. The figure is showing the nucleophile alkylation and
changing the base of guanine, thus rendering the DNA structure unstable. (Image retrieved
from Gruppi et al. 2015)
Another class of drugs is of the metabolites that prevent the replication of DNA by
itself acting as a substitute of the building blocks of DNA. Thus, it prevents the replication of
DNA and leads to the stoppage of uncontrolled cell division. Examples include 5-fluorouracil
and azacitidine. The mechanism of action of antimetabolites includes interfering with the
precursor of purine and pyrimidine. It inhibits replication of the DNA by the inhibiting the
action of folic acid and thymidylate synthetase, and the DNA polymerase, thus destroying the
structure of DNA (Schott et al. 2017). The limitations of using these antimetabolites as
anticancerous agents include the common symptoms like nausea, vomiting, alopecia, and
fever, all of which either indicates weakening of the immune system or neutropenia.
However, it also affects the cardiac system massively by increasing endothelial
thrombogenicity, various coronary syndromes, arrhythmias, sinus tachycardia, ventricular
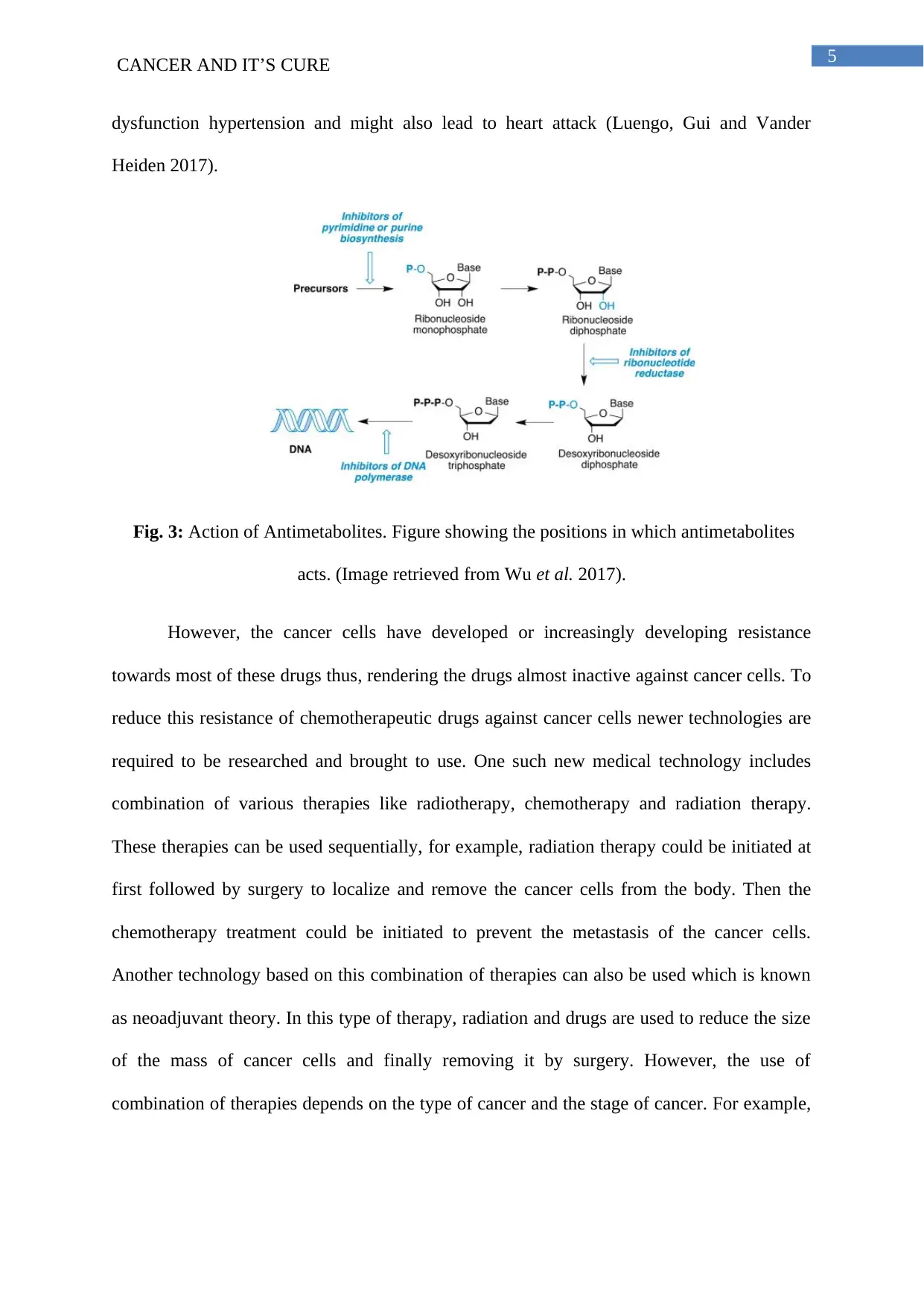
5CANCER AND IT’S CURE
dysfunction hypertension and might also lead to heart attack (Luengo, Gui and Vander
Heiden 2017).
Fig. 3: Action of Antimetabolites. Figure showing the positions in which antimetabolites
acts. (Image retrieved from Wu et al. 2017).
However, the cancer cells have developed or increasingly developing resistance
towards most of these drugs thus, rendering the drugs almost inactive against cancer cells. To
reduce this resistance of chemotherapeutic drugs against cancer cells newer technologies are
required to be researched and brought to use. One such new medical technology includes
combination of various therapies like radiotherapy, chemotherapy and radiation therapy.
These therapies can be used sequentially, for example, radiation therapy could be initiated at
first followed by surgery to localize and remove the cancer cells from the body. Then the
chemotherapy treatment could be initiated to prevent the metastasis of the cancer cells.
Another technology based on this combination of therapies can also be used which is known
as neoadjuvant theory. In this type of therapy, radiation and drugs are used to reduce the size
of the mass of cancer cells and finally removing it by surgery. However, the use of
combination of therapies depends on the type of cancer and the stage of cancer. For example,
dysfunction hypertension and might also lead to heart attack (Luengo, Gui and Vander
Heiden 2017).
Fig. 3: Action of Antimetabolites. Figure showing the positions in which antimetabolites
acts. (Image retrieved from Wu et al. 2017).
However, the cancer cells have developed or increasingly developing resistance
towards most of these drugs thus, rendering the drugs almost inactive against cancer cells. To
reduce this resistance of chemotherapeutic drugs against cancer cells newer technologies are
required to be researched and brought to use. One such new medical technology includes
combination of various therapies like radiotherapy, chemotherapy and radiation therapy.
These therapies can be used sequentially, for example, radiation therapy could be initiated at
first followed by surgery to localize and remove the cancer cells from the body. Then the
chemotherapy treatment could be initiated to prevent the metastasis of the cancer cells.
Another technology based on this combination of therapies can also be used which is known
as neoadjuvant theory. In this type of therapy, radiation and drugs are used to reduce the size
of the mass of cancer cells and finally removing it by surgery. However, the use of
combination of therapies depends on the type of cancer and the stage of cancer. For example,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
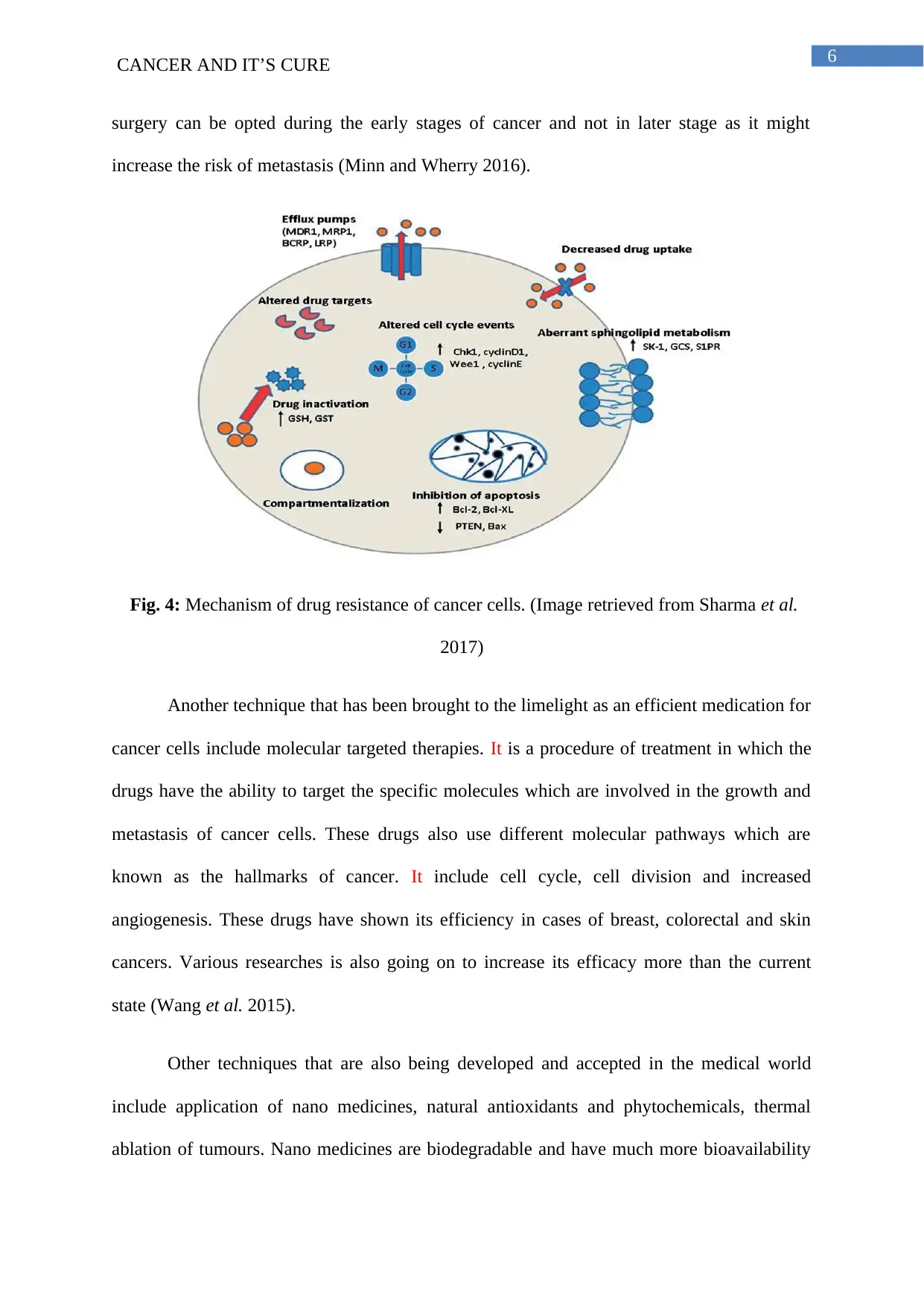
6CANCER AND IT’S CURE
surgery can be opted during the early stages of cancer and not in later stage as it might
increase the risk of metastasis (Minn and Wherry 2016).
Fig. 4: Mechanism of drug resistance of cancer cells. (Image retrieved from Sharma et al.
2017)
Another technique that has been brought to the limelight as an efficient medication for
cancer cells include molecular targeted therapies. It is a procedure of treatment in which the
drugs have the ability to target the specific molecules which are involved in the growth and
metastasis of cancer cells. These drugs also use different molecular pathways which are
known as the hallmarks of cancer. It include cell cycle, cell division and increased
angiogenesis. These drugs have shown its efficiency in cases of breast, colorectal and skin
cancers. Various researches is also going on to increase its efficacy more than the current
state (Wang et al. 2015).
Other techniques that are also being developed and accepted in the medical world
include application of nano medicines, natural antioxidants and phytochemicals, thermal
ablation of tumours. Nano medicines are biodegradable and have much more bioavailability
surgery can be opted during the early stages of cancer and not in later stage as it might
increase the risk of metastasis (Minn and Wherry 2016).
Fig. 4: Mechanism of drug resistance of cancer cells. (Image retrieved from Sharma et al.
2017)
Another technique that has been brought to the limelight as an efficient medication for
cancer cells include molecular targeted therapies. It is a procedure of treatment in which the
drugs have the ability to target the specific molecules which are involved in the growth and
metastasis of cancer cells. These drugs also use different molecular pathways which are
known as the hallmarks of cancer. It include cell cycle, cell division and increased
angiogenesis. These drugs have shown its efficiency in cases of breast, colorectal and skin
cancers. Various researches is also going on to increase its efficacy more than the current
state (Wang et al. 2015).
Other techniques that are also being developed and accepted in the medical world
include application of nano medicines, natural antioxidants and phytochemicals, thermal
ablation of tumours. Nano medicines are biodegradable and have much more bioavailability
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7CANCER AND IT’S CURE
and gets released at the site of cancer cells decreasing the side effects and increasing the site
specificity. Natural antioxidants and phytochemical shave also proven to exhibit anti-
proliferative and anticancer capabilities that can trigger apoptosis of the cancer cells. Thermal
ablation of tumours is the process in which the treatment of cancer cells can be made very
localized within a narrow region thus, the further treatment would be easier. Gene therapy is
also a procedure in which the genes for tumour suppression and apoptosis are activated or
triggered (Pucci, Martinelli and Ciofan 2019).
Thus, it can be concluded that there are different class of drugs used for chemotherapy
with varied targeted mechanisms. However, each drug has several side-effects associated
with its administration, which commonly include gastro-intestinal issues, fever and alopecia.
The patient should be educated to adhere to medications, eat small and frequent meals,
reduced intake of fibers and roughage and increase the intake of fluid to keep the signs in
check.
and gets released at the site of cancer cells decreasing the side effects and increasing the site
specificity. Natural antioxidants and phytochemical shave also proven to exhibit anti-
proliferative and anticancer capabilities that can trigger apoptosis of the cancer cells. Thermal
ablation of tumours is the process in which the treatment of cancer cells can be made very
localized within a narrow region thus, the further treatment would be easier. Gene therapy is
also a procedure in which the genes for tumour suppression and apoptosis are activated or
triggered (Pucci, Martinelli and Ciofan 2019).
Thus, it can be concluded that there are different class of drugs used for chemotherapy
with varied targeted mechanisms. However, each drug has several side-effects associated
with its administration, which commonly include gastro-intestinal issues, fever and alopecia.
The patient should be educated to adhere to medications, eat small and frequent meals,
reduced intake of fibers and roughage and increase the intake of fluid to keep the signs in
check.

8CANCER AND IT’S CURE
REFERENCES
Agudelo, D., Bourassa, P., Bérubé, G. and Tajmir-Riahi, H.A., 2016. Review on the binding
of anticancer drug doxorubicin with DNA and tRNA: Structural models and antitumor
activity. Journal of Photochemistry and Photobiology B: Biology, 158, pp.274-279.
Bhattacharya, B. and Mukherjee, S., 2015. Cancer therapy using antibiotics. Journal of
Cancer Therapy, 6(10), p.849.
Biology LibreTexts, 2020. 13.1C: Antibiotics And Selective Toxicity. [online] Available at:
<https://bio.libretexts.org/Bookshelves/Microbiology/Book
%3A_Microbiology_(Boundless)/13%3A_Antimicrobial_Drugs/
13.1%3A_Overview_of_Antimicrobial_Therapy/13.1C
%3A_Antibiotics_and_Selective_Toxicity> [Accessed 23 March 2020].
Gruppi, F., Hejazi, L., Christov, P.P., Krishnamachari, S., Turesky, R.J. and Rizzo, C.J.,
2015. Characterization of nitrogen mustard formamidopyrimidine adduct formation of bis (2-
chloroethyl) ethylamine with calf thymus DNA and a human mammary cancer cell
line. Chemical research in toxicology, 28(9), pp.1850-1860.
Kim, S.H., Shin, K.H., Seok, S.O., Cho, Y.J., Noh, J.K., Suh, J.S. and Yang, W.I., 2015.
Secondary malignant neoplasms after osteosarcoma: early onset and cumulative alkylating
agent dose dependency. Annals of surgical oncology, 22(3), pp.859-865.
Luengo, A., Gui, D.Y. and Vander Heiden, M.G., 2017. Targeting metabolism for cancer
therapy. Cell chemical biology, 24(9), pp.1161-1180.
Meredith, A.M. and Dass, C.R., 2016. Increasing role of the cancer chemotherapeutic
doxorubicin in cellular metabolism. Journal of Pharmacy and Pharmacology, 68(6), pp.729-
741.
REFERENCES
Agudelo, D., Bourassa, P., Bérubé, G. and Tajmir-Riahi, H.A., 2016. Review on the binding
of anticancer drug doxorubicin with DNA and tRNA: Structural models and antitumor
activity. Journal of Photochemistry and Photobiology B: Biology, 158, pp.274-279.
Bhattacharya, B. and Mukherjee, S., 2015. Cancer therapy using antibiotics. Journal of
Cancer Therapy, 6(10), p.849.
Biology LibreTexts, 2020. 13.1C: Antibiotics And Selective Toxicity. [online] Available at:
<https://bio.libretexts.org/Bookshelves/Microbiology/Book
%3A_Microbiology_(Boundless)/13%3A_Antimicrobial_Drugs/
13.1%3A_Overview_of_Antimicrobial_Therapy/13.1C
%3A_Antibiotics_and_Selective_Toxicity> [Accessed 23 March 2020].
Gruppi, F., Hejazi, L., Christov, P.P., Krishnamachari, S., Turesky, R.J. and Rizzo, C.J.,
2015. Characterization of nitrogen mustard formamidopyrimidine adduct formation of bis (2-
chloroethyl) ethylamine with calf thymus DNA and a human mammary cancer cell
line. Chemical research in toxicology, 28(9), pp.1850-1860.
Kim, S.H., Shin, K.H., Seok, S.O., Cho, Y.J., Noh, J.K., Suh, J.S. and Yang, W.I., 2015.
Secondary malignant neoplasms after osteosarcoma: early onset and cumulative alkylating
agent dose dependency. Annals of surgical oncology, 22(3), pp.859-865.
Luengo, A., Gui, D.Y. and Vander Heiden, M.G., 2017. Targeting metabolism for cancer
therapy. Cell chemical biology, 24(9), pp.1161-1180.
Meredith, A.M. and Dass, C.R., 2016. Increasing role of the cancer chemotherapeutic
doxorubicin in cellular metabolism. Journal of Pharmacy and Pharmacology, 68(6), pp.729-
741.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9CANCER AND IT’S CURE
Minn, A.J. and Wherry, E.J., 2016. Combination cancer therapies with immune checkpoint
blockade: convergence on interferon signaling. Cell, 165(2), pp.272-275.
Pearce, A., Haas, M., Viney, R., Pearson, S.A., Haywood, P., Brown, C. and Ward, R., 2017.
Incidence and severity of self-reported chemotherapy side effects in routine care: A
prospective cohort study. PloS one, 12(10).
Pucci, C., Martinelli, C. and Ciofani, G., 2019. Innovative approaches for cancer treatment:
current perspectives and new challenges. Ecancermedicalscience, 13.
Salzano, G., Navarro, G., Trivedi, M.S., De Rosa, G. and Torchilin, V.P., 2015.
Multifunctional polymeric micelles co-loaded with anti–survivin siRNA and paclitaxel
overcome drug resistance in an animal model of ovarian cancer. Molecular cancer
therapeutics, 14(4), pp.1075-1084.
Schott, S., Wimberger, P., Klink, B., Grützmann, K., Puppe, J., Wauer, U.S., Klotz, D.M.,
Schröck, E. and Kuhlmann, J.D., 2017. The conjugated antimetabolite 5-FdU-ECyd and its
cellular and molecular effects on platinum-sensitive vs.-resistant ovarian cancer cells in
vitro. Oncotarget, 8(44), p.76935.
Sharma, P., Hu-Lieskovan, S., Wargo, J.A. and Ribas, A., 2017. Primary, adaptive, and
acquired resistance to cancer immunotherapy. Cell, 168(4), pp.707-723.
Singh, R.K., Kumar, S., Prasad, D.N. and Bhardwaj, T.R., 2018. Therapeutic journery of
nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. European
journal of medicinal chemistry, 151, pp.401-433.
Singh, R.K., Kumar, S., Prasad, D.N. and Bhardwaj, T.R., 2018. Therapeutic journery of
nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. European
journal of medicinal chemistry, 151, pp.401-433.
Minn, A.J. and Wherry, E.J., 2016. Combination cancer therapies with immune checkpoint
blockade: convergence on interferon signaling. Cell, 165(2), pp.272-275.
Pearce, A., Haas, M., Viney, R., Pearson, S.A., Haywood, P., Brown, C. and Ward, R., 2017.
Incidence and severity of self-reported chemotherapy side effects in routine care: A
prospective cohort study. PloS one, 12(10).
Pucci, C., Martinelli, C. and Ciofani, G., 2019. Innovative approaches for cancer treatment:
current perspectives and new challenges. Ecancermedicalscience, 13.
Salzano, G., Navarro, G., Trivedi, M.S., De Rosa, G. and Torchilin, V.P., 2015.
Multifunctional polymeric micelles co-loaded with anti–survivin siRNA and paclitaxel
overcome drug resistance in an animal model of ovarian cancer. Molecular cancer
therapeutics, 14(4), pp.1075-1084.
Schott, S., Wimberger, P., Klink, B., Grützmann, K., Puppe, J., Wauer, U.S., Klotz, D.M.,
Schröck, E. and Kuhlmann, J.D., 2017. The conjugated antimetabolite 5-FdU-ECyd and its
cellular and molecular effects on platinum-sensitive vs.-resistant ovarian cancer cells in
vitro. Oncotarget, 8(44), p.76935.
Sharma, P., Hu-Lieskovan, S., Wargo, J.A. and Ribas, A., 2017. Primary, adaptive, and
acquired resistance to cancer immunotherapy. Cell, 168(4), pp.707-723.
Singh, R.K., Kumar, S., Prasad, D.N. and Bhardwaj, T.R., 2018. Therapeutic journery of
nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. European
journal of medicinal chemistry, 151, pp.401-433.
Singh, R.K., Kumar, S., Prasad, D.N. and Bhardwaj, T.R., 2018. Therapeutic journery of
nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. European
journal of medicinal chemistry, 151, pp.401-433.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10CANCER AND IT’S CURE
Wagner, A.D., Syn, N.L., Moehler, M., Grothe, W., Yong, W.P., Tai, B.C., Ho, J. and
Unverzagt, S., 2017. Chemotherapy for advanced gastric cancer. Cochrane database of
systematic reviews, (8).
Wang, H., Xu, T., Jiang, Y., Xu, H., Yan, Y., Fu, D. and Chen, J., 2015. The challenges and
the promise of molecular targeted therapy in malignant gliomas. Neoplasia, 17(3), pp.239-
255.
Wei, T., Chen, C., Liu, J., Liu, C., Posocco, P., Liu, X., Cheng, Q., Huo, S., Liang, Z.,
Fermeglia, M. and Pricl, S., 2015. Anticancer drug nanomicelles formed by self-assembling
amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National
Academy of Sciences, 112(10), pp.2978-2983.
Wei, T., Chen, C., Liu, J., Liu, C., Posocco, P., Liu, X., Cheng, Q., Huo, S., Liang, Z.,
Fermeglia, M. and Pricl, S., 2015. Anticancer drug nanomicelles formed by self-assembling
amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National
Academy of Sciences, 112(10), pp.2978-2983.
Wu, Y., Zhang, D., Wu, B., Quan, Y., Liu, D., Li, Y. and Zhang, X., 2017. Synergistic
activity of an antimetabolite drug and tyrosine kinase inhibitors against breast cancer
cells. Chemical and Pharmaceutical Bulletin, pp.c17-00261.
Yun, J., Mullarky, E., Lu, C., Bosch, K.N., Kavalier, A., Rivera, K., Roper, J., Chio, I.I.C.,
Giannopoulou, E.G., Rago, C. and Muley, A., 2015. Vitamin C selectively kills KRAS and
BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350(6266), pp.1391-
1396.
Wagner, A.D., Syn, N.L., Moehler, M., Grothe, W., Yong, W.P., Tai, B.C., Ho, J. and
Unverzagt, S., 2017. Chemotherapy for advanced gastric cancer. Cochrane database of
systematic reviews, (8).
Wang, H., Xu, T., Jiang, Y., Xu, H., Yan, Y., Fu, D. and Chen, J., 2015. The challenges and
the promise of molecular targeted therapy in malignant gliomas. Neoplasia, 17(3), pp.239-
255.
Wei, T., Chen, C., Liu, J., Liu, C., Posocco, P., Liu, X., Cheng, Q., Huo, S., Liang, Z.,
Fermeglia, M. and Pricl, S., 2015. Anticancer drug nanomicelles formed by self-assembling
amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National
Academy of Sciences, 112(10), pp.2978-2983.
Wei, T., Chen, C., Liu, J., Liu, C., Posocco, P., Liu, X., Cheng, Q., Huo, S., Liang, Z.,
Fermeglia, M. and Pricl, S., 2015. Anticancer drug nanomicelles formed by self-assembling
amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National
Academy of Sciences, 112(10), pp.2978-2983.
Wu, Y., Zhang, D., Wu, B., Quan, Y., Liu, D., Li, Y. and Zhang, X., 2017. Synergistic
activity of an antimetabolite drug and tyrosine kinase inhibitors against breast cancer
cells. Chemical and Pharmaceutical Bulletin, pp.c17-00261.
Yun, J., Mullarky, E., Lu, C., Bosch, K.N., Kavalier, A., Rivera, K., Roper, J., Chio, I.I.C.,
Giannopoulou, E.G., Rago, C. and Muley, A., 2015. Vitamin C selectively kills KRAS and
BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350(6266), pp.1391-
1396.

11CANCER AND IT’S CURE
Zhang, X.F., Sun, R.Q., Jia, Y.F., Chen, Q., Tu, R.F., Li, K.K., Zhang, X.D., Du, R.L. and
Cao, R.H., 2016. Synthesis and mechanisms of action of novel harmine derivatives as
potential antitumor agents. Scientific reports, 6, p.33204.
Zhang, X.F., Sun, R.Q., Jia, Y.F., Chen, Q., Tu, R.F., Li, K.K., Zhang, X.D., Du, R.L. and
Cao, R.H., 2016. Synthesis and mechanisms of action of novel harmine derivatives as
potential antitumor agents. Scientific reports, 6, p.33204.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





