CBMS 825 Chemical Analysis II: GC-MS Analysis of Fat in Snack Foods
VerifiedAdded on 2023/04/11
|26
|2660
|70
Practical Assignment
AI Summary
This experiment focuses on analyzing the types and amounts of fat present in snack foods using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). The experiment involves the preparation of fatty acid methyl esters (FAMEs) from snack foods like Twisties and Pretzels through a derivatization process. The FAMEs are then analyzed using both GC and GC-MS to identify and quantify different fatty acids. The report includes a comparison of the two methods, calculations of relative response factors (RRF), and statistical analysis to determine significant differences in saturated FAMEs. The limit of detection (LOD) for C12:0 is also calculated for both GC and GC-MS. The results provide insights into the fat composition of the selected snack foods, highlighting the levels of saturated, monounsaturated, and polyunsaturated fats.

Lab Demonstrator- Ben Ford
Presented by:
Abrar Taif (44182562)
CBMS 825 Chemical Analysis II
Analysis of Fat in Snack Foods by Gas Chromatography and
Gas
Chromatography / Mass Spectrometry
Experiment 3
Presented by:
Abrar Taif (44182562)
CBMS 825 Chemical Analysis II
Analysis of Fat in Snack Foods by Gas Chromatography and
Gas
Chromatography / Mass Spectrometry
Experiment 3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Experimental
Outline• Introduction
• Experimental Principals
• Procedure
• Results and Discussion
• Conclusion
• Learning Outcomes
• References
Outline• Introduction
• Experimental Principals
• Procedure
• Results and Discussion
• Conclusion
• Learning Outcomes
• References

Introduction
Snack foods that contain large amount of fat is of great significant health concern
Saturated fats increases blood cholesterol in humans1
High LDL level associated with increased risk of heart disease and stroke in humans1
Perception of Saturated and Unsaturated fats2
Saturated Fats have single C-C bond2
Unsaturated Fats have one more more double C=C bond2
The principles involving the application of gas chromatography (GC) and gas
chromatography/mass spectrometry (GC-MS)
The investigation of operational differences between flame ionisation detector that
implements gas chromatography and mass spectrometer detector that implements gas
chromatography/mass spectrometer.
The significance of derivation technique of fatty acid in snacks while applying gas
chromatography (GC)
Snack foods that contain large amount of fat is of great significant health concern
Saturated fats increases blood cholesterol in humans1
High LDL level associated with increased risk of heart disease and stroke in humans1
Perception of Saturated and Unsaturated fats2
Saturated Fats have single C-C bond2
Unsaturated Fats have one more more double C=C bond2
The principles involving the application of gas chromatography (GC) and gas
chromatography/mass spectrometry (GC-MS)
The investigation of operational differences between flame ionisation detector that
implements gas chromatography and mass spectrometer detector that implements gas
chromatography/mass spectrometer.
The significance of derivation technique of fatty acid in snacks while applying gas
chromatography (GC)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

AIM
The aim of the experiment is to analyze
types of fat in snack foods by using gas
chromatography and gas chromatography/
mass spectrometry.
The aim of the experiment is to analyze
types of fat in snack foods by using gas
chromatography and gas chromatography/
mass spectrometry.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
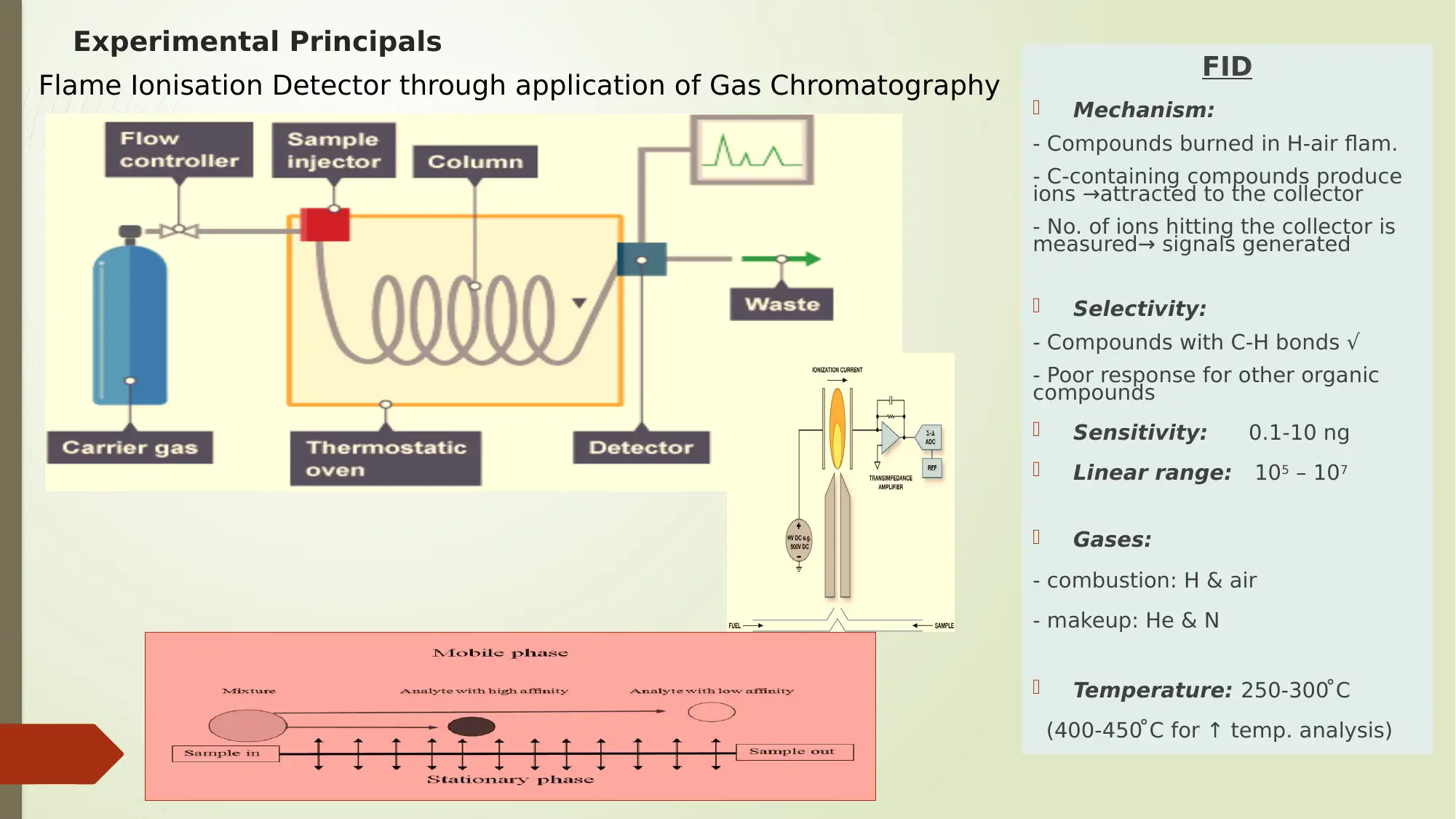
Experimental Principals
Flame Ionisation Detector through application of Gas Chromatography FID
Mechanism:
- Compounds burned in H-air flam.
- C-containing compounds produce
ions →attracted to the collector
- No. of ions hitting the collector is
measured→ signals generated
Selectivity:
- Compounds with C-H bonds √
- Poor response for other organic
compounds
Sensitivity: 0.1-10 ng
Linear range: 105 – 107
Gases:
- combustion: H & air
- makeup: He & N
Temperature: 250-300 ̊ C
(400-450 ̊ C for ↑ temp. analysis)
Flame Ionisation Detector through application of Gas Chromatography FID
Mechanism:
- Compounds burned in H-air flam.
- C-containing compounds produce
ions →attracted to the collector
- No. of ions hitting the collector is
measured→ signals generated
Selectivity:
- Compounds with C-H bonds √
- Poor response for other organic
compounds
Sensitivity: 0.1-10 ng
Linear range: 105 – 107
Gases:
- combustion: H & air
- makeup: He & N
Temperature: 250-300 ̊ C
(400-450 ̊ C for ↑ temp. analysis)
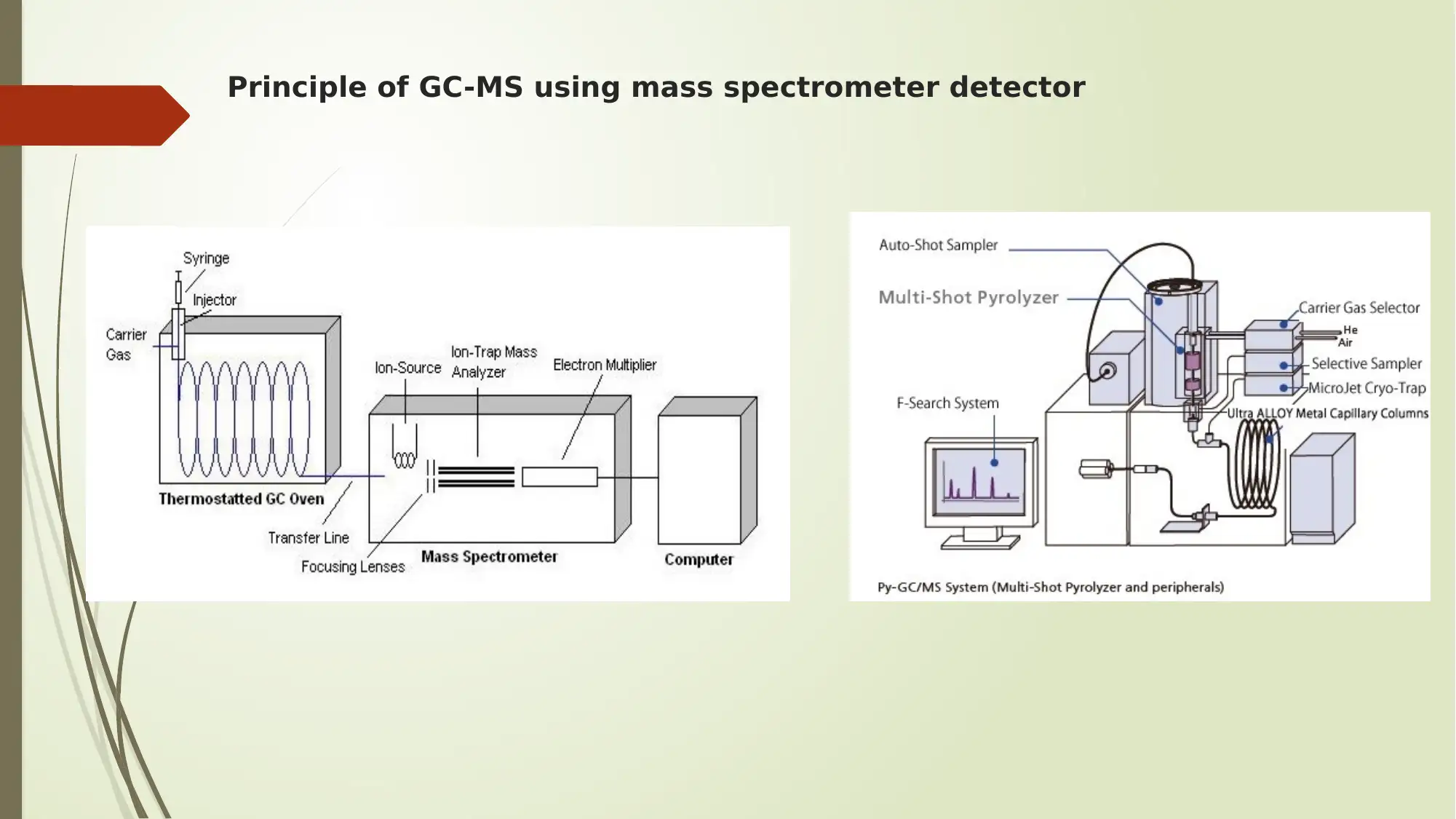
Principle of GC-MS using mass spectrometer detector
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
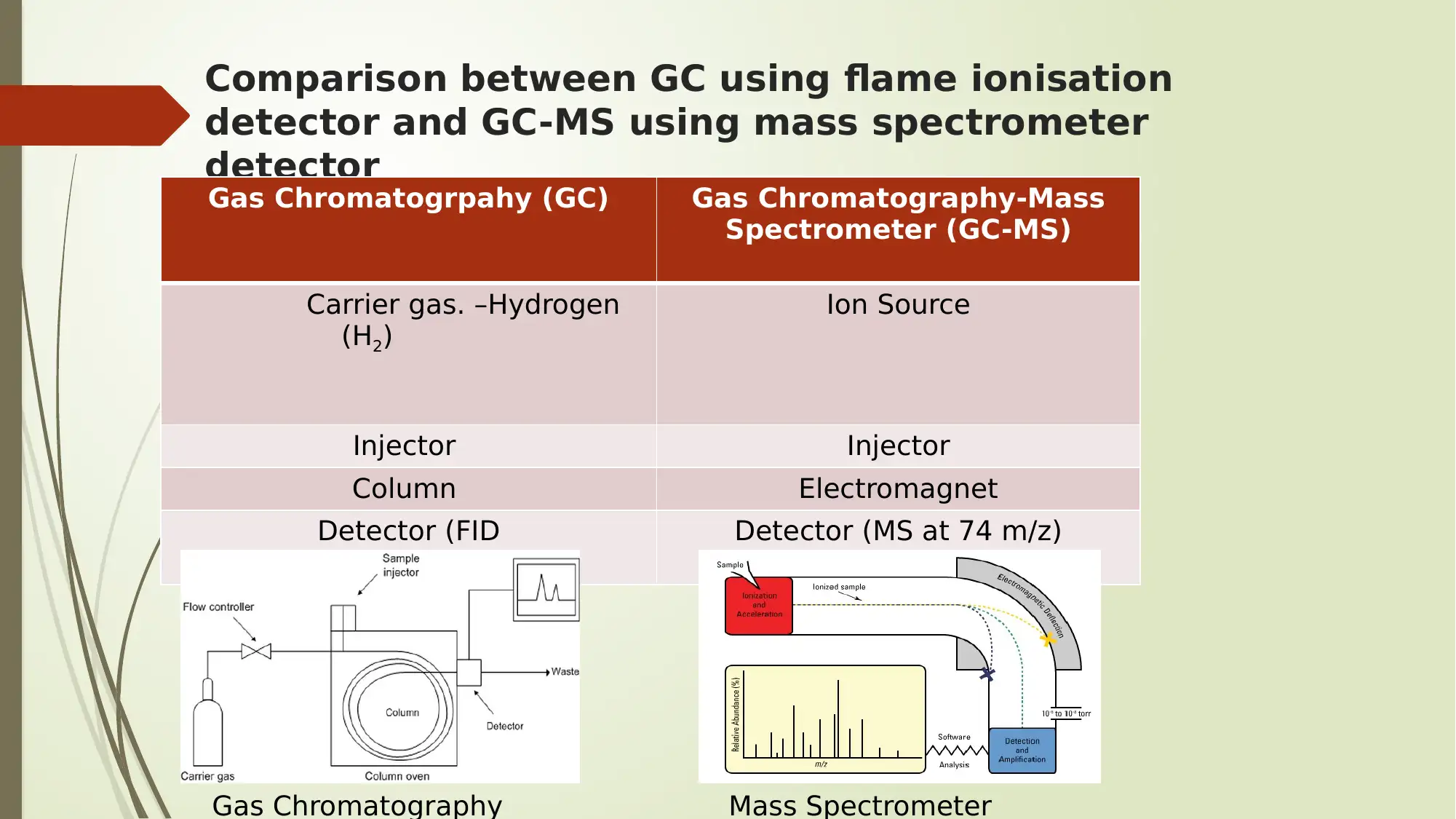
Comparison between GC using flame ionisation
detector and GC-MS using mass spectrometer
detector
Gas Chromatogrpahy (GC) Gas Chromatography-Mass
Spectrometer (GC-MS)
Carrier gas. –Hydrogen
(H2)
Ion Source
Injector Injector
Column Electromagnet
Detector (FID Detector (MS at 74 m/z)
Gas Chromatography Mass Spectrometer
detector and GC-MS using mass spectrometer
detector
Gas Chromatogrpahy (GC) Gas Chromatography-Mass
Spectrometer (GC-MS)
Carrier gas. –Hydrogen
(H2)
Ion Source
Injector Injector
Column Electromagnet
Detector (FID Detector (MS at 74 m/z)
Gas Chromatography Mass Spectrometer
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
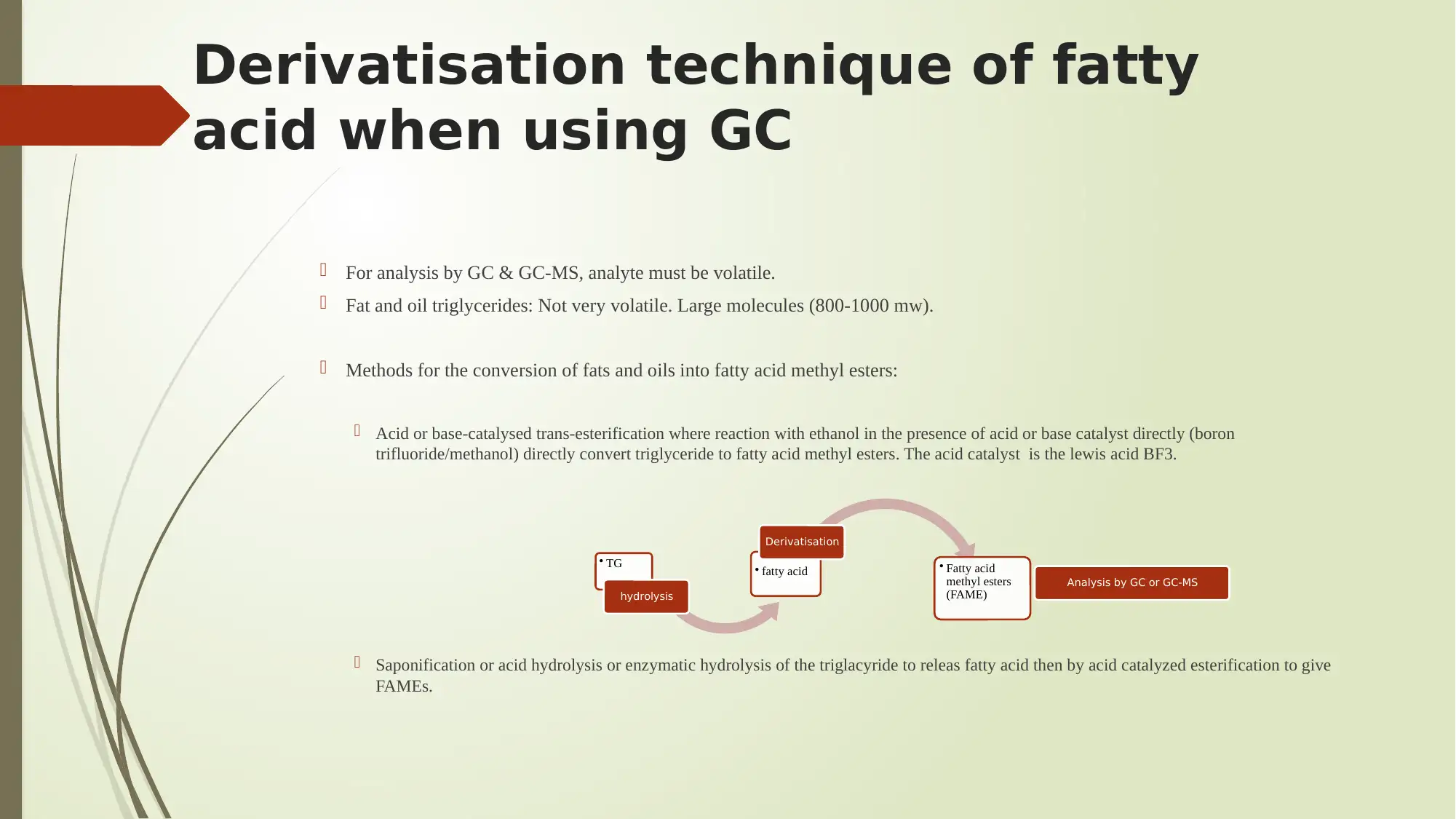
Derivatisation technique of fatty
acid when using GC
For analysis by GC & GC-MS, analyte must be volatile.
Fat and oil triglycerides: Not very volatile. Large molecules (800-1000 mw).
Methods for the conversion of fats and oils into fatty acid methyl esters:
Acid or base-catalysed trans-esterification where reaction with ethanol in the presence of acid or base catalyst directly (boron
trifluoride/methanol) directly convert triglyceride to fatty acid methyl esters. The acid catalyst is the lewis acid BF3.
Saponification or acid hydrolysis or enzymatic hydrolysis of the triglacyride to releas fatty acid then by acid catalyzed esterification to give
FAMEs.
• TG
hydrolysis
• fatty acid
Derivatisation
• Fatty acid
methyl esters
(FAME)
Analysis by GC or GC-MS
acid when using GC
For analysis by GC & GC-MS, analyte must be volatile.
Fat and oil triglycerides: Not very volatile. Large molecules (800-1000 mw).
Methods for the conversion of fats and oils into fatty acid methyl esters:
Acid or base-catalysed trans-esterification where reaction with ethanol in the presence of acid or base catalyst directly (boron
trifluoride/methanol) directly convert triglyceride to fatty acid methyl esters. The acid catalyst is the lewis acid BF3.
Saponification or acid hydrolysis or enzymatic hydrolysis of the triglacyride to releas fatty acid then by acid catalyzed esterification to give
FAMEs.
• TG
hydrolysis
• fatty acid
Derivatisation
• Fatty acid
methyl esters
(FAME)
Analysis by GC or GC-MS

Procedure
Preparation of FAMEs from fats in snack food
Two snack foods were selected to be analyzed using GC and GC/MS. Twisties (contains 23.3 g/ 100g total fat) and
Pretzels (contains 3.6 g / 100 g total fat).
Approximately 5 g were crushed using clean mortar and pestle.
The nutritional information on the packing was examined for both snack foods and the aliquot mass was calculated
using the formula
Aliquotmass = (15000 / fat in products g/100 g) mg
Amount of 643.78 mg of Twisties and 4167.7mg of Pretzels were taken after crushing the sample and aliquoting them
Internal standard (tridodecanoin - C12:0 triglyceride) was added.
Aa volume of 20 mL of 1:1 (v/v) chloroform / methanol was added and the mixture was ultrasonicate for 5 minutes.
The snack foods were Filtered from the1:1 (v/v) chloroform / methanol solution using a Buchner funnel and the beaker
was washed with 5 mL of 1:1 (v/v) chloroform / methanol.
Remove 1 mL of the filtered and place in clean scintillation vial.
The sample was evaporated to dryness (oil form) by adding 2 mL of BF3/ methanol derivatisation reagent to the vial ,
the vial was caped and the mixture was shook vigorously.
The mixture was heated to 60 °C in the oven for 10 minutes and shaking of the mixture was maitained every 2 minutes.
The vial was removed from the oven and left to cool down. Then, 5 mL of hexanes and 5 mL of saturated NaCl were
added to the vial.
The vial was caped and the mixture was shook vigorously for a minute.
After separation of the two phases, 1 mL of the top layer was removed using pasture pipette and transfer into clean
vial. This is the sample to be analyzed contains methyl ester derivatives.
The computer was prepared to collect data and 0.5 mL of the sample was injected into GC and GC/MS.
Preparation of FAMEs from fats in snack food
Two snack foods were selected to be analyzed using GC and GC/MS. Twisties (contains 23.3 g/ 100g total fat) and
Pretzels (contains 3.6 g / 100 g total fat).
Approximately 5 g were crushed using clean mortar and pestle.
The nutritional information on the packing was examined for both snack foods and the aliquot mass was calculated
using the formula
Aliquotmass = (15000 / fat in products g/100 g) mg
Amount of 643.78 mg of Twisties and 4167.7mg of Pretzels were taken after crushing the sample and aliquoting them
Internal standard (tridodecanoin - C12:0 triglyceride) was added.
Aa volume of 20 mL of 1:1 (v/v) chloroform / methanol was added and the mixture was ultrasonicate for 5 minutes.
The snack foods were Filtered from the1:1 (v/v) chloroform / methanol solution using a Buchner funnel and the beaker
was washed with 5 mL of 1:1 (v/v) chloroform / methanol.
Remove 1 mL of the filtered and place in clean scintillation vial.
The sample was evaporated to dryness (oil form) by adding 2 mL of BF3/ methanol derivatisation reagent to the vial ,
the vial was caped and the mixture was shook vigorously.
The mixture was heated to 60 °C in the oven for 10 minutes and shaking of the mixture was maitained every 2 minutes.
The vial was removed from the oven and left to cool down. Then, 5 mL of hexanes and 5 mL of saturated NaCl were
added to the vial.
The vial was caped and the mixture was shook vigorously for a minute.
After separation of the two phases, 1 mL of the top layer was removed using pasture pipette and transfer into clean
vial. This is the sample to be analyzed contains methyl ester derivatives.
The computer was prepared to collect data and 0.5 mL of the sample was injected into GC and GC/MS.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Measurement of Fatty Acid Response Factor
The chromatographic conditions of GC-17A (SHIMADZU) and GCMS-QP5000
(SHIMADZU) were confirmed and the computer was prepared to collect data by
follow-up the steps mentioned in experiment 4 manual.
After the READY light on GC turns green and click sound on GC-MS was noticed,
0.5 μL of FAME standard was injected using 1μL syringe followed by pressing START
button on front of GC.
FAME standard 20 ug/mL contains C12:0, C14:0, C14:1, C16:0, C16:1, C18:0, C18:1, C18:2, C20:0 and C20:1
Check to see the run starts, wait for approximately 20 minutes to finished.
When ready light turn green again, inject the sample again (duplicate).
The chromatographic conditions of GC-17A (SHIMADZU) and GCMS-QP5000
(SHIMADZU) were confirmed and the computer was prepared to collect data by
follow-up the steps mentioned in experiment 4 manual.
After the READY light on GC turns green and click sound on GC-MS was noticed,
0.5 μL of FAME standard was injected using 1μL syringe followed by pressing START
button on front of GC.
FAME standard 20 ug/mL contains C12:0, C14:0, C14:1, C16:0, C16:1, C18:0, C18:1, C18:2, C20:0 and C20:1
Check to see the run starts, wait for approximately 20 minutes to finished.
When ready light turn green again, inject the sample again (duplicate).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
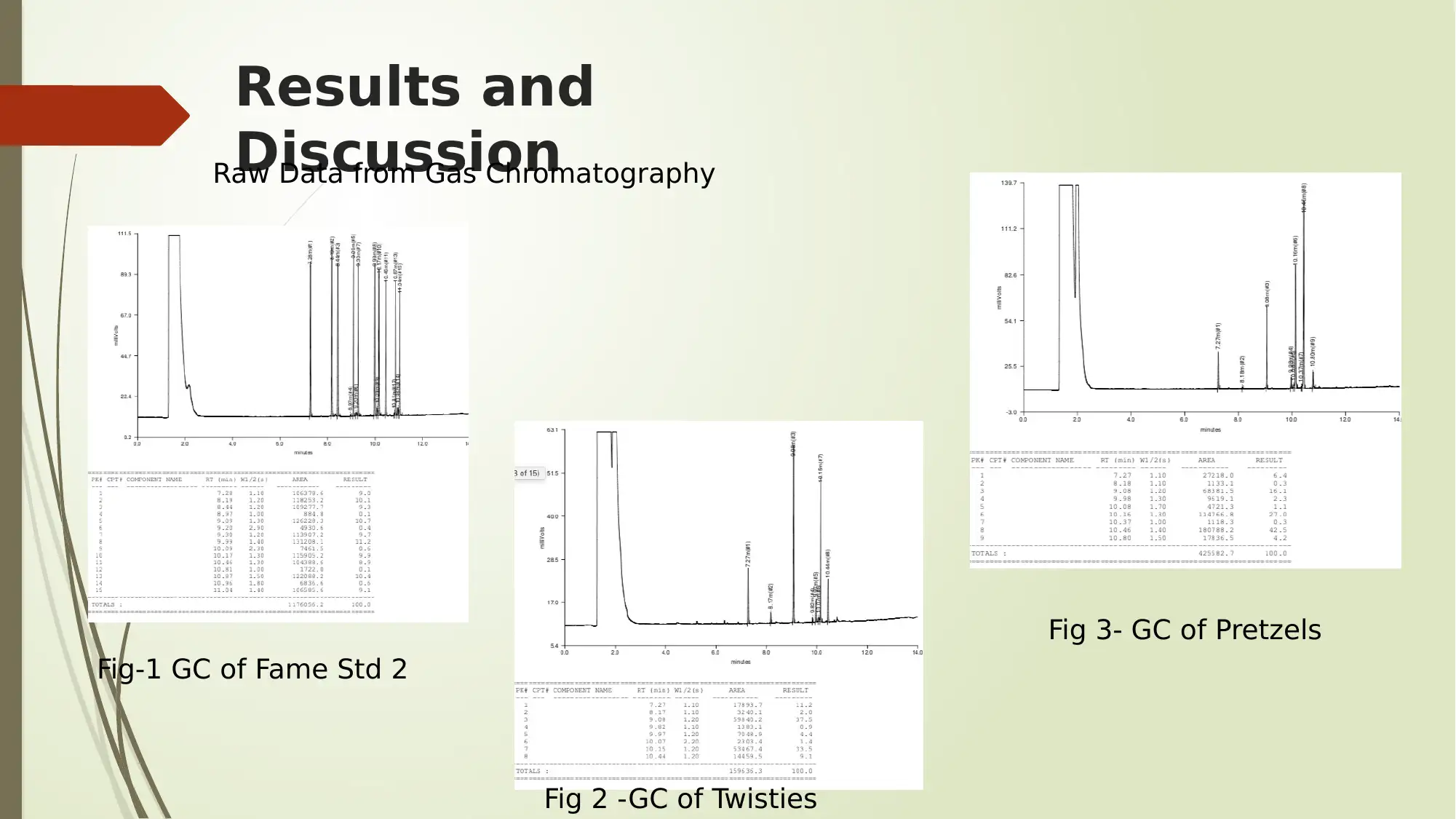
Results and
DiscussionRaw Data from Gas Chromatography
Fig-1 GC of Fame Std 2
Fig 2 -GC of Twisties
Fig 3- GC of Pretzels
DiscussionRaw Data from Gas Chromatography
Fig-1 GC of Fame Std 2
Fig 2 -GC of Twisties
Fig 3- GC of Pretzels
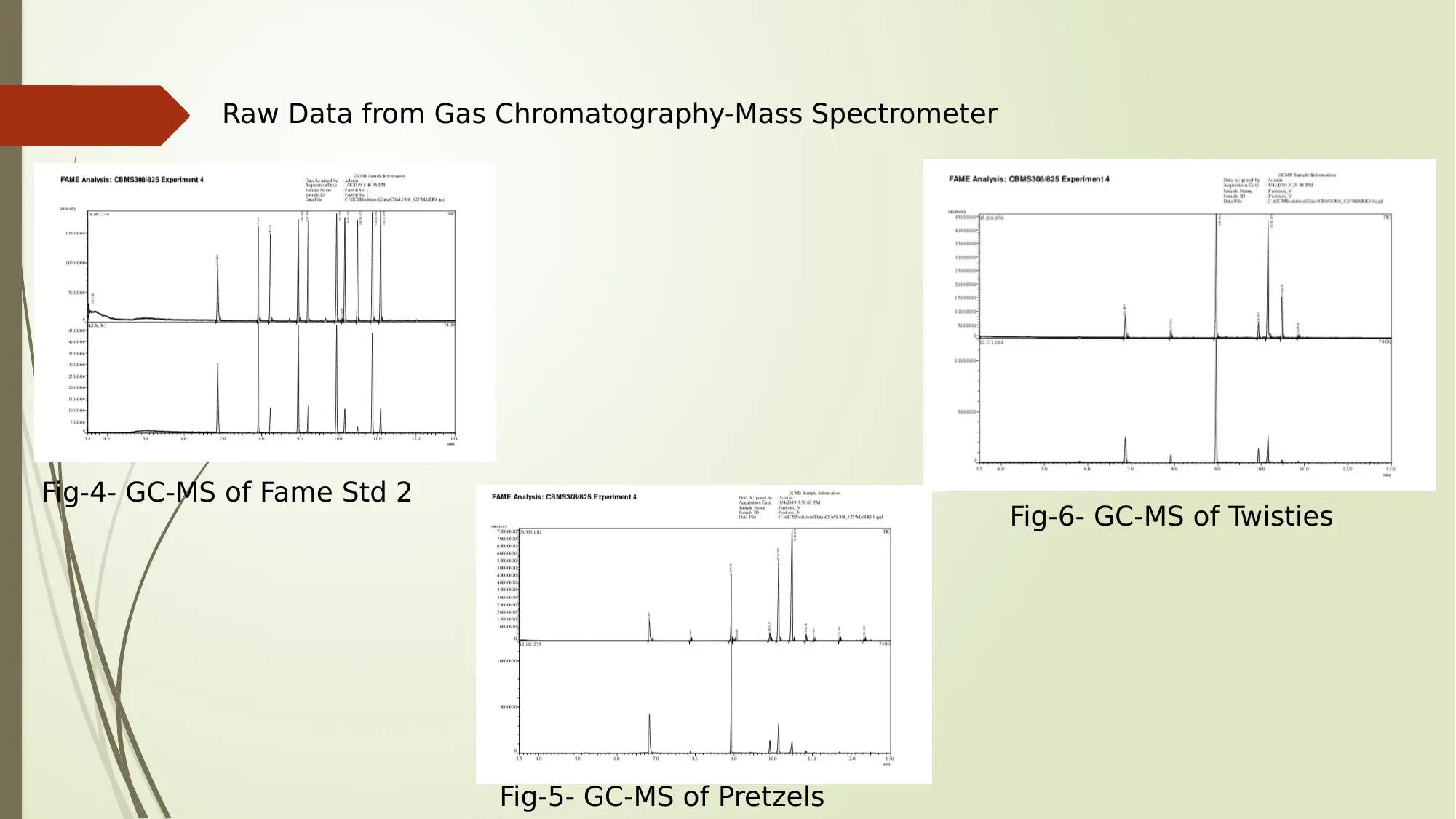
Raw Data from Gas Chromatography-Mass Spectrometer
Fig-4- GC-MS of Fame Std 2
Fig-5- GC-MS of Pretzels
Fig-6- GC-MS of Twisties
Fig-4- GC-MS of Fame Std 2
Fig-5- GC-MS of Pretzels
Fig-6- GC-MS of Twisties
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 26
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.