Cell Biology and Biochemistry: Enzymes, Activity, and Cell Transport
VerifiedAdded on 2023/03/24
|10
|1992
|84
Report
AI Summary
This report delves into the intricacies of cell biology and biochemistry, focusing on enzyme function, cell transport mechanisms, and the relationship between these processes and cell membrane structure. It begins by examining various methods of material exchange across cell membranes, including diffusion, osmosis, active transport, and phagocytosis, detailing how these processes are connected to the selective permeability of the cell membrane. The report further explores the structure and function of enzymes, explaining how their primary, secondary, and tertiary structures contribute to their catalytic activity. It evaluates the lock and key and induced fit models of enzyme action, highlighting their strengths and limitations in explaining substrate binding. Finally, the report discusses external factors such as temperature, pH, and substrate concentration that affect enzyme activity, providing a comprehensive overview of the principles governing cellular processes. Desklib offers a wealth of solved assignments and study resources for students.

Cell Biology and Bio chemistry
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TABLE OF CONTENTS
TASK 15..........................................................................................................................................1
TASK 16..........................................................................................................................................1
Exchange of materials across cell membranes.......................................................................1
Relation of these exchanges with the structure of cell membrane ........................................2
TASK 17..........................................................................................................................................2
Enzymes and the way they work............................................................................................2
Activation energy...................................................................................................................4
Evaluation of lock and key and induced fit models of enzyme action...................................5
Factors affecting enzyme activity...........................................................................................6
REFERENCES................................................................................................................................7
TASK 15..........................................................................................................................................1
TASK 16..........................................................................................................................................1
Exchange of materials across cell membranes.......................................................................1
Relation of these exchanges with the structure of cell membrane ........................................2
TASK 17..........................................................................................................................................2
Enzymes and the way they work............................................................................................2
Activation energy...................................................................................................................4
Evaluation of lock and key and induced fit models of enzyme action...................................5
Factors affecting enzyme activity...........................................................................................6
REFERENCES................................................................................................................................7

TASK 15
Completed in workbook
TASK 16
Exchange of materials across cell membranes
The movement of any substance across the cell membrane can be analysed by various
methods such as osmosis, diffusion, phagocytosis, active transport etc. Passive transport is
considered as the development of molecules covering the cell membranes and energy is not
required for it. Diffusion and osmosis is considered as a part of passive transport. Description of
these are discussed as under:
By diffusion
The process of diffusion is considered as a process of transfer of molecules from an area
of higher concentration to an area of lower concentration. Ions are moving here and there in
diffusion. The molecules inside have kinetic energy, so there has an availability of them colliding
with each other. Collisions occur in both the high concentration and the low concentration region
but more probability is of high concentration area (Cornish-Bowden and Cornish-Bowden,
2012). If there would be larger surface area, the rate of the reaction would be higher. A very
common type of it is called as facilitated diffusion. The size of the molecules is smaller and is
indirectly proportional to the rate of the reaction. In this method, ATP is not required but cell
membranes proteins are necessary. These are called the carrier molecules. These transfer the
molecules across the cell membranes from a region of higher concentration to that of lower
concentration
Osmosis
Osmosis is considered as a movement of water molecules covering a semi permeable
membrane. Only the molecules of water can move across the membrane. It does not even include
any solid or substance dissolved in the water. The blockage is being done by the semi permeable
membrane. It does not allow the restricted molecules to pass through the membrane.
Active Transport
Another method for the movement of molecules through the cell membrane can be
considered as the active transport. At the time of the active transport taking place, a particular
1
Completed in workbook
TASK 16
Exchange of materials across cell membranes
The movement of any substance across the cell membrane can be analysed by various
methods such as osmosis, diffusion, phagocytosis, active transport etc. Passive transport is
considered as the development of molecules covering the cell membranes and energy is not
required for it. Diffusion and osmosis is considered as a part of passive transport. Description of
these are discussed as under:
By diffusion
The process of diffusion is considered as a process of transfer of molecules from an area
of higher concentration to an area of lower concentration. Ions are moving here and there in
diffusion. The molecules inside have kinetic energy, so there has an availability of them colliding
with each other. Collisions occur in both the high concentration and the low concentration region
but more probability is of high concentration area (Cornish-Bowden and Cornish-Bowden,
2012). If there would be larger surface area, the rate of the reaction would be higher. A very
common type of it is called as facilitated diffusion. The size of the molecules is smaller and is
indirectly proportional to the rate of the reaction. In this method, ATP is not required but cell
membranes proteins are necessary. These are called the carrier molecules. These transfer the
molecules across the cell membranes from a region of higher concentration to that of lower
concentration
Osmosis
Osmosis is considered as a movement of water molecules covering a semi permeable
membrane. Only the molecules of water can move across the membrane. It does not even include
any solid or substance dissolved in the water. The blockage is being done by the semi permeable
membrane. It does not allow the restricted molecules to pass through the membrane.
Active Transport
Another method for the movement of molecules through the cell membrane can be
considered as the active transport. At the time of the active transport taking place, a particular
1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

substance is being moved by a protein across the cell membrane and it is being done from a
region of lower concentration to the one with higher concentration.
Phagocytosis
The process in which a small part of plasma membrane surrounds the molecules of fluid
near the surface of the cell is termed as endocytosis. Presence of solid vesicle matter in the
process makes it phagocytosis from endocytosis.
Relation of these exchanges with the structure of cell membrane
The connection between the transfer of materials within the cell members and its
structure is termed as selective permeability. In this process, even very smaller molecules can go
through the lipid bilayer of the cell membrane. If there is any substance that has to pass over the
cell membrane, it first has to pass through the transport proteins. The cell membrane helps the
cell to stay protected from its surroundings. It acts as a physical barrier.
TASK 17
Enzymes and the way they work
The enzymes are composed of the amino acids which are connected to each other by
peptide bonds in a linear chain. These are also considered as the proteins which contribute to the
participate in various processes of cellular metabolism. It is also capable of increasing the rate of
reaction of bio molecules ( Mandelkow and Mandelkow, 2012). Till this stage, it is called as the
primary structure. A protein or a polypeptide comes as an outcome of the whole amino acid
chain. The amino acids are being compressed through the DNA sequences of the particular gene.
Usually, the enzymes are proteins, so they act in accordance for their structure. As proteins, they
have a well-defined three-dimensional structure and as catalysts, they can act as a catalyst for
any biochemical or chemical reaction. They minimize the activation energy, thus maximising the
speed of the reaction. Its structure is being categorised in three parts which are discussed as
under:
Primary structure
In its primary structure, the enzymes, that are made up of amino acids connect with the
help of peptides in the form of a linear chain. The outcome is considered and the protein or a
polypeptide.
2
region of lower concentration to the one with higher concentration.
Phagocytosis
The process in which a small part of plasma membrane surrounds the molecules of fluid
near the surface of the cell is termed as endocytosis. Presence of solid vesicle matter in the
process makes it phagocytosis from endocytosis.
Relation of these exchanges with the structure of cell membrane
The connection between the transfer of materials within the cell members and its
structure is termed as selective permeability. In this process, even very smaller molecules can go
through the lipid bilayer of the cell membrane. If there is any substance that has to pass over the
cell membrane, it first has to pass through the transport proteins. The cell membrane helps the
cell to stay protected from its surroundings. It acts as a physical barrier.
TASK 17
Enzymes and the way they work
The enzymes are composed of the amino acids which are connected to each other by
peptide bonds in a linear chain. These are also considered as the proteins which contribute to the
participate in various processes of cellular metabolism. It is also capable of increasing the rate of
reaction of bio molecules ( Mandelkow and Mandelkow, 2012). Till this stage, it is called as the
primary structure. A protein or a polypeptide comes as an outcome of the whole amino acid
chain. The amino acids are being compressed through the DNA sequences of the particular gene.
Usually, the enzymes are proteins, so they act in accordance for their structure. As proteins, they
have a well-defined three-dimensional structure and as catalysts, they can act as a catalyst for
any biochemical or chemical reaction. They minimize the activation energy, thus maximising the
speed of the reaction. Its structure is being categorised in three parts which are discussed as
under:
Primary structure
In its primary structure, the enzymes, that are made up of amino acids connect with the
help of peptides in the form of a linear chain. The outcome is considered and the protein or a
polypeptide.
2
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
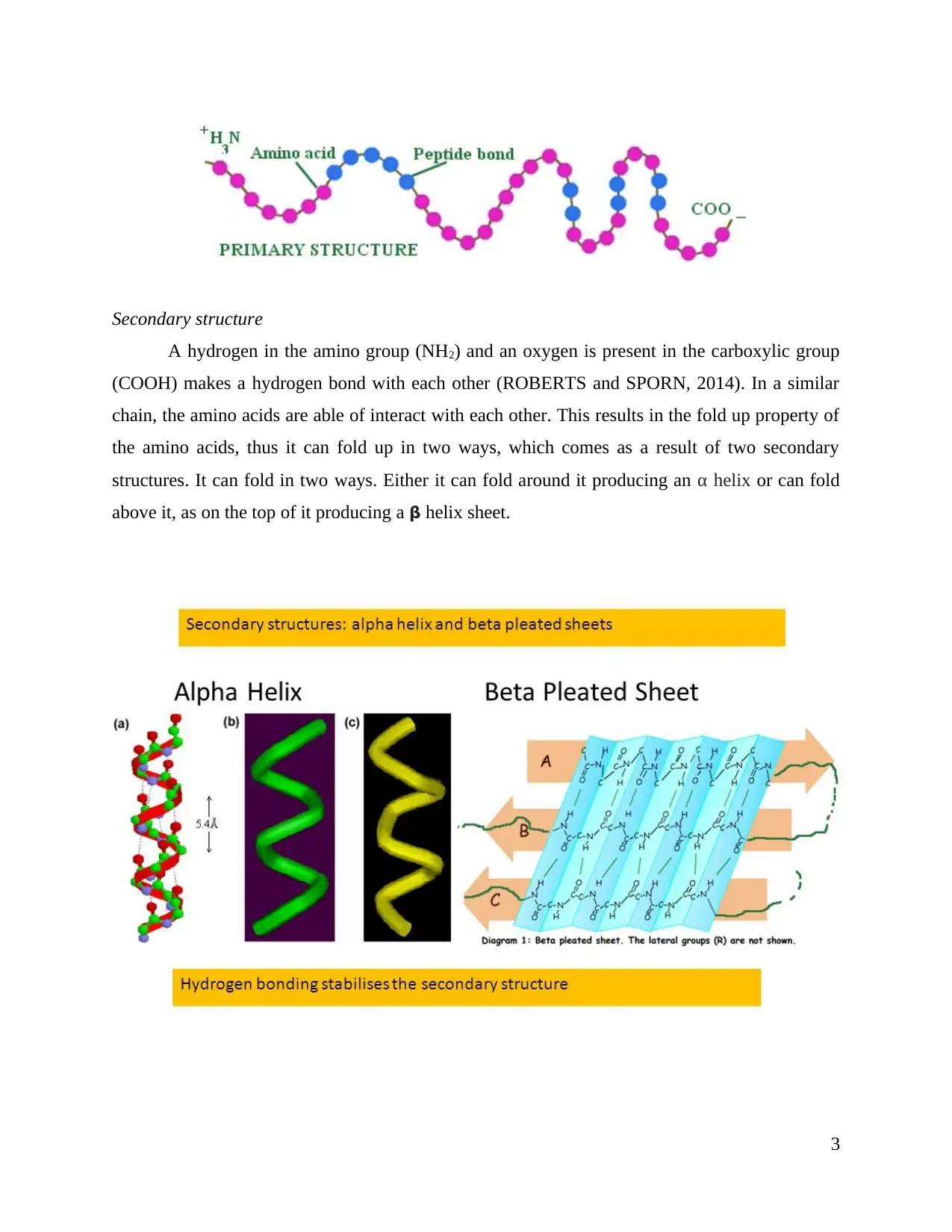
Secondary structure
A hydrogen in the amino group (NH2) and an oxygen is present in the carboxylic group
(COOH) makes a hydrogen bond with each other (ROBERTS and SPORN, 2014). In a similar
chain, the amino acids are able of interact with each other. This results in the fold up property of
the amino acids, thus it can fold up in two ways, which comes as a result of two secondary
structures. It can fold in two ways. Either it can fold around it producing an α helix or can fold
above it, as on the top of it producing a β helix sheet.
3
A hydrogen in the amino group (NH2) and an oxygen is present in the carboxylic group
(COOH) makes a hydrogen bond with each other (ROBERTS and SPORN, 2014). In a similar
chain, the amino acids are able of interact with each other. This results in the fold up property of
the amino acids, thus it can fold up in two ways, which comes as a result of two secondary
structures. It can fold in two ways. Either it can fold around it producing an α helix or can fold
above it, as on the top of it producing a β helix sheet.
3

Tertiary Structure
As a result, the enzymes get up to two dimensional folds in the secondary structure, the
proteins can expand further as well and are capable of achieving a three dimensional structure. It
is considered as the rounded structure.
Activation energy
The activation energy is considered as the energy that is required for the reaction to
occur. Enzymes minimizes the rate of the activation energy. Although these enzymes do not get
produced and used in a reaction, but there they behave as catalysts that maximizes the overall
rate of the reaction. While increasing the rate of a reaction, enzymes lowers the activation energy
that is required for the process. Activation energy is necessary for starting of a reaction. It is also
measured as difference of the threshold energy and the average energy of the reactant. The
4
As a result, the enzymes get up to two dimensional folds in the secondary structure, the
proteins can expand further as well and are capable of achieving a three dimensional structure. It
is considered as the rounded structure.
Activation energy
The activation energy is considered as the energy that is required for the reaction to
occur. Enzymes minimizes the rate of the activation energy. Although these enzymes do not get
produced and used in a reaction, but there they behave as catalysts that maximizes the overall
rate of the reaction. While increasing the rate of a reaction, enzymes lowers the activation energy
that is required for the process. Activation energy is necessary for starting of a reaction. It is also
measured as difference of the threshold energy and the average energy of the reactant. The
4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
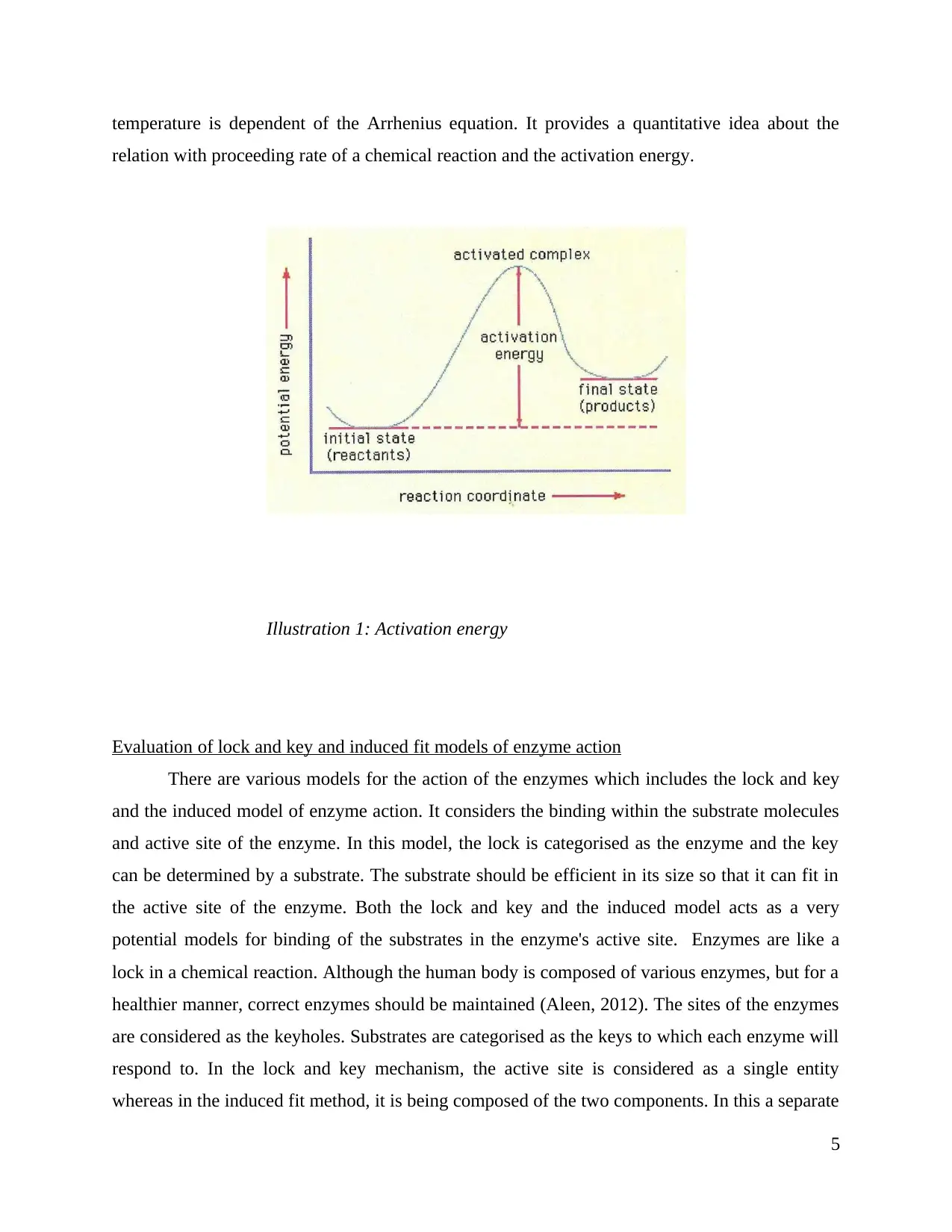
temperature is dependent of the Arrhenius equation. It provides a quantitative idea about the
relation with proceeding rate of a chemical reaction and the activation energy.
Evaluation of lock and key and induced fit models of enzyme action
There are various models for the action of the enzymes which includes the lock and key
and the induced model of enzyme action. It considers the binding within the substrate molecules
and active site of the enzyme. In this model, the lock is categorised as the enzyme and the key
can be determined by a substrate. The substrate should be efficient in its size so that it can fit in
the active site of the enzyme. Both the lock and key and the induced model acts as a very
potential models for binding of the substrates in the enzyme's active site. Enzymes are like a
lock in a chemical reaction. Although the human body is composed of various enzymes, but for a
healthier manner, correct enzymes should be maintained (Aleen, 2012). The sites of the enzymes
are considered as the keyholes. Substrates are categorised as the keys to which each enzyme will
respond to. In the lock and key mechanism, the active site is considered as a single entity
whereas in the induced fit method, it is being composed of the two components. In this a separate
5
Illustration 1: Activation energy
relation with proceeding rate of a chemical reaction and the activation energy.
Evaluation of lock and key and induced fit models of enzyme action
There are various models for the action of the enzymes which includes the lock and key
and the induced model of enzyme action. It considers the binding within the substrate molecules
and active site of the enzyme. In this model, the lock is categorised as the enzyme and the key
can be determined by a substrate. The substrate should be efficient in its size so that it can fit in
the active site of the enzyme. Both the lock and key and the induced model acts as a very
potential models for binding of the substrates in the enzyme's active site. Enzymes are like a
lock in a chemical reaction. Although the human body is composed of various enzymes, but for a
healthier manner, correct enzymes should be maintained (Aleen, 2012). The sites of the enzymes
are considered as the keyholes. Substrates are categorised as the keys to which each enzyme will
respond to. In the lock and key mechanism, the active site is considered as a single entity
whereas in the induced fit method, it is being composed of the two components. In this a separate
5
Illustration 1: Activation energy
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

group for the catalyst is also visible whereas in the lock and key, there are no separate groups. In
the lock and key, active site is determined as a single entity but in the induced fit model, it does
not act static.
Factors affecting enzyme activity
There are various factors which affect the activity of the enzymes along with the
proceeding rate of the reaction (Chatterjea and Shinde, 2011). These includes pH, concentration
of the substrate, temperature and availability of the activators. Describing its effect below:
Temperature
If the temperature increases, it maximises the rate of kinetic energy as well. It refers to
more collisions. It is directly proportional to the vibrational energy as well which means as there
is an increase in the temperature, the vibrational energy is also being maximised. The weaker
bonds at the time of maximisation of the temperature, breaks as an outcome of the strain. It
further increases the overall rate of the reaction. As more the bonds will decrease, the rate of the
reaction also starts decreasing because of the lesser and loosened bonds.
PH – Acidity or Basicity
It measures the basicity and the acidity of a solution. The acidic solutions are those
having a pH of below 7 whereas the solutions having a pH of above 7 is considered as a base
(Bar-Even and et.al, 2011). Any minor change in the pH does not mean a permanent change in
the enzymes or its structure, because the reformation of the bonds can be easily done.
Concentration
When the concentration level of an enzyme or a substrate is being modified, it affects the
rate of an enzyme catalysed reaction. The organism can increase its metabolism and enzyme
activity by various means as by controlling these factors. The concentration can be of enzyme as
well as of substrate. Maximization in the concentration of the substrate increases the rate of the
reaction and same is being followed by the enzymes.
6
the lock and key, active site is determined as a single entity but in the induced fit model, it does
not act static.
Factors affecting enzyme activity
There are various factors which affect the activity of the enzymes along with the
proceeding rate of the reaction (Chatterjea and Shinde, 2011). These includes pH, concentration
of the substrate, temperature and availability of the activators. Describing its effect below:
Temperature
If the temperature increases, it maximises the rate of kinetic energy as well. It refers to
more collisions. It is directly proportional to the vibrational energy as well which means as there
is an increase in the temperature, the vibrational energy is also being maximised. The weaker
bonds at the time of maximisation of the temperature, breaks as an outcome of the strain. It
further increases the overall rate of the reaction. As more the bonds will decrease, the rate of the
reaction also starts decreasing because of the lesser and loosened bonds.
PH – Acidity or Basicity
It measures the basicity and the acidity of a solution. The acidic solutions are those
having a pH of below 7 whereas the solutions having a pH of above 7 is considered as a base
(Bar-Even and et.al, 2011). Any minor change in the pH does not mean a permanent change in
the enzymes or its structure, because the reformation of the bonds can be easily done.
Concentration
When the concentration level of an enzyme or a substrate is being modified, it affects the
rate of an enzyme catalysed reaction. The organism can increase its metabolism and enzyme
activity by various means as by controlling these factors. The concentration can be of enzyme as
well as of substrate. Maximization in the concentration of the substrate increases the rate of the
reaction and same is being followed by the enzymes.
6

REFERENCES
Books and Journals
Mandelkow, E. M. and Mandelkow, E., 2012. Biochemistry and cell biology of tau protein in
neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2(7).
p.a006247.
Alberts, B. and et.al., 2013. Essential cell biology. Garland Science.
Aleen, R. ed., 2012. Primitive motile systems in cell biology. Elsevier.
Bar-Even, A. and et.al., 2011. The moderately efficient enzyme: evolutionary and
physicochemical trends shaping enzyme parameters. Biochemistry. 50(21). pp.4402-
4410.
Bernstein, B. W. and Bamburg, J. R., 2010. ADF/cofilin: a functional node in cell biology.
Trends in cell biology. 20(4). pp.187-195.
Chatterjee, M. N. and Shinde, R., 2011. Textbook of medical biochemistry. Wife Goes On.
Cornish-Bowden, A. and Cornish-Bowden, A., 2012. Fundamentals of enzyme kinetics.
Dugas, H. and Penney, C., 2013. Bioorganic chemistry: a chemical approach to enzyme action.
Springer Science & Business Media.
ROBERTS, A. B. and SPORN, M. B., 2014. Cellular Biology and biochemistry. The retinoids.
2. p.209.
Rothfield, L. I. ed., 2014. Structure and function of biological membranes. Academic Press.
Wingard, L. B., Katchalski-Katzir, E. and Goldstein, L. eds., 2014. Immobilized enzyme
principles: applied biochemistry and bioengineering (Vol. 1). Elsevier.
Online
Eukaryotic Cells, 2017. [Online]. Available through:
<https://courses.lumenlearning.com/boundless-biology/chapter/eukaryotic-cells/>.
7
Books and Journals
Mandelkow, E. M. and Mandelkow, E., 2012. Biochemistry and cell biology of tau protein in
neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2(7).
p.a006247.
Alberts, B. and et.al., 2013. Essential cell biology. Garland Science.
Aleen, R. ed., 2012. Primitive motile systems in cell biology. Elsevier.
Bar-Even, A. and et.al., 2011. The moderately efficient enzyme: evolutionary and
physicochemical trends shaping enzyme parameters. Biochemistry. 50(21). pp.4402-
4410.
Bernstein, B. W. and Bamburg, J. R., 2010. ADF/cofilin: a functional node in cell biology.
Trends in cell biology. 20(4). pp.187-195.
Chatterjee, M. N. and Shinde, R., 2011. Textbook of medical biochemistry. Wife Goes On.
Cornish-Bowden, A. and Cornish-Bowden, A., 2012. Fundamentals of enzyme kinetics.
Dugas, H. and Penney, C., 2013. Bioorganic chemistry: a chemical approach to enzyme action.
Springer Science & Business Media.
ROBERTS, A. B. and SPORN, M. B., 2014. Cellular Biology and biochemistry. The retinoids.
2. p.209.
Rothfield, L. I. ed., 2014. Structure and function of biological membranes. Academic Press.
Wingard, L. B., Katchalski-Katzir, E. and Goldstein, L. eds., 2014. Immobilized enzyme
principles: applied biochemistry and bioengineering (Vol. 1). Elsevier.
Online
Eukaryotic Cells, 2017. [Online]. Available through:
<https://courses.lumenlearning.com/boundless-biology/chapter/eukaryotic-cells/>.
7
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

8
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





