The Changing Role of Academia in Drug Discovery - MP979
VerifiedAdded on 2022/10/09
|16
|3216
|29
Essay
AI Summary
This essay provides a comprehensive overview of the drug discovery process, from target identification and validation to clinical evaluation, approval, and marketing. It explores the traditional roles of academia and pharmaceutical companies in this process, highlighting how these roles have changed over the past 15-20 years. The essay also examines the emergence of new academic centers in the UK and Europe that are taking on roles traditionally held by big pharma, discussing their achievements. Finally, the essay offers a critical assessment of the future of academic-big pharma collaborations in drug discovery, considering the sustainability of these collaborations and their potential impact on the development of new therapeutics. The essay emphasizes the importance of collaboration, transparency, and translational research in accelerating the drug discovery process and reducing associated costs.

Running head: DRUG DISCOVERY
The Changing role of Academia in Drug Discovery
Name of the Student
Name of the University
Author Note
The Changing role of Academia in Drug Discovery
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1
DRUG DISCOVERY
Introduction
According to the Food and Drug Administration US (2019), drug is defined as a
substance that is used for diagnosis, mitigation, cure, treatment and effective prevention of
the disease. Alternatively it can be said that drug is a chemical substance that is intended to
affect the the pathological mechanism of the body. Analysis of the different therapeutic areas,
highlighted that the development of new medication starts with target identification followed
by approval for marketing. The overall process takes at least 12 years for successful
completion. Food and Drug Administration US (2019) stated that there is no simple step for
producing viable clinical compound. However, effective collaboration with the biology,
chemistry and toxicology and pharmacokinetic is important for the promotion of successful
drug discovery. The following essay aims to highlight the process of drug discovery along
with a brief overview of the clinical evaluation of the drug, approval and marketing of the
newly discovered drugs. This will be followed by traditional roles of the academic and the
pharmaceuticals in the process of drug discoveries and their role change during the course of
last 15 to 20 years. The new academic in the UK, Europe Scotland, that are now taking over
big pharmas will also be discussed in this essay. At the end, the paper will tend to ascertain
the future of this academic and bin-pharma collaborations towards the process of drug
discovery.
Process of Drug Discovery
The development or designing of drug is initiated when scientists identify a biological
target (either a protein receptor or a protein molecule, enzyme of gene) that is involved in a
specific biological process that is thought to be dysfunctional among the patients with any
particular disease for example, Alzheimer’s disease (Khachaturian 2015). The main goal of
the preclinical drug discovery program is to design one of more clinical target molecule. Each
DRUG DISCOVERY
Introduction
According to the Food and Drug Administration US (2019), drug is defined as a
substance that is used for diagnosis, mitigation, cure, treatment and effective prevention of
the disease. Alternatively it can be said that drug is a chemical substance that is intended to
affect the the pathological mechanism of the body. Analysis of the different therapeutic areas,
highlighted that the development of new medication starts with target identification followed
by approval for marketing. The overall process takes at least 12 years for successful
completion. Food and Drug Administration US (2019) stated that there is no simple step for
producing viable clinical compound. However, effective collaboration with the biology,
chemistry and toxicology and pharmacokinetic is important for the promotion of successful
drug discovery. The following essay aims to highlight the process of drug discovery along
with a brief overview of the clinical evaluation of the drug, approval and marketing of the
newly discovered drugs. This will be followed by traditional roles of the academic and the
pharmaceuticals in the process of drug discoveries and their role change during the course of
last 15 to 20 years. The new academic in the UK, Europe Scotland, that are now taking over
big pharmas will also be discussed in this essay. At the end, the paper will tend to ascertain
the future of this academic and bin-pharma collaborations towards the process of drug
discovery.
Process of Drug Discovery
The development or designing of drug is initiated when scientists identify a biological
target (either a protein receptor or a protein molecule, enzyme of gene) that is involved in a
specific biological process that is thought to be dysfunctional among the patients with any
particular disease for example, Alzheimer’s disease (Khachaturian 2015). The main goal of
the preclinical drug discovery program is to design one of more clinical target molecule. Each
2
DRUG DISCOVERY
of these target molecules has significant evidences backing their biological activity over the
target that is relevant to the diseases and also has drug-like properties so that it becomes
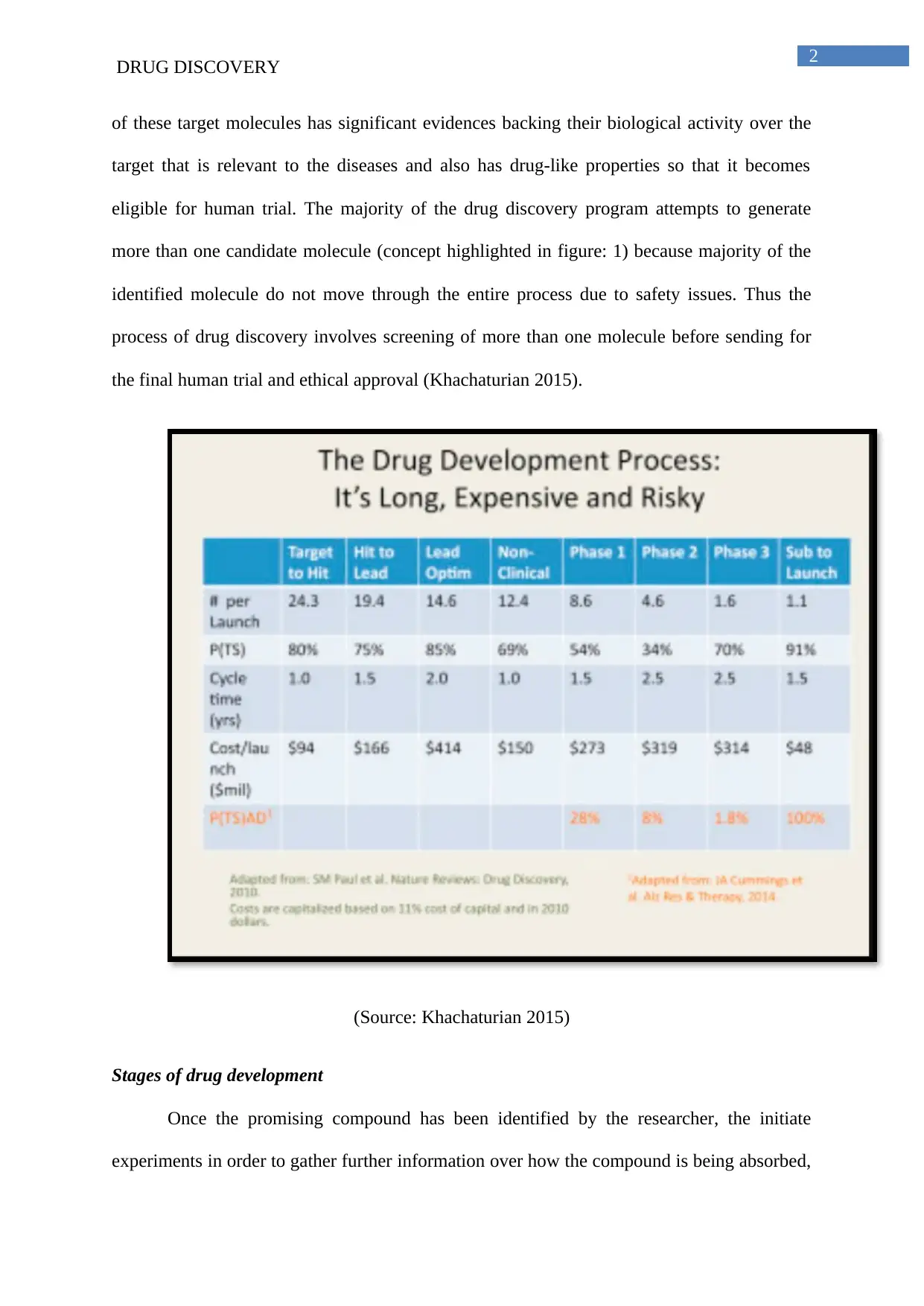
eligible for human trial. The majority of the drug discovery program attempts to generate
more than one candidate molecule (concept highlighted in figure: 1) because majority of the
identified molecule do not move through the entire process due to safety issues. Thus the
process of drug discovery involves screening of more than one molecule before sending for
the final human trial and ethical approval (Khachaturian 2015).
(Source: Khachaturian 2015)
Stages of drug development
Once the promising compound has been identified by the researcher, the initiate
experiments in order to gather further information over how the compound is being absorbed,
DRUG DISCOVERY
of these target molecules has significant evidences backing their biological activity over the
target that is relevant to the diseases and also has drug-like properties so that it becomes
eligible for human trial. The majority of the drug discovery program attempts to generate
more than one candidate molecule (concept highlighted in figure: 1) because majority of the
identified molecule do not move through the entire process due to safety issues. Thus the
process of drug discovery involves screening of more than one molecule before sending for
the final human trial and ethical approval (Khachaturian 2015).
(Source: Khachaturian 2015)
Stages of drug development
Once the promising compound has been identified by the researcher, the initiate
experiments in order to gather further information over how the compound is being absorbed,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3
DRUG DISCOVERY
metabolized, distributed and is excreted from the body. The potential benefits and mechanism
of action of selected compound, the optimal dosage and the medium of drug delivery. The
developmental process also includes side-effects of the selected drugs based on toxicology,
the affect of drugs on different age, gender, race and ethnicity and interaction of the drugs
with other potential drugs (Cabrera-Pérez et al. 2016).
(Source: Khachaturian 2015)
Target identification
The identification of drug target depends on few factors like pathophysiology of the
disease, the target expression uniformity in the body and the 3D structure of the target and
feasibility of obtaining that target. Other factors that are taken into consideration include
accessibility of the target under high-throughput screening, toxicity profile of the target,
DRUG DISCOVERY
metabolized, distributed and is excreted from the body. The potential benefits and mechanism
of action of selected compound, the optimal dosage and the medium of drug delivery. The
developmental process also includes side-effects of the selected drugs based on toxicology,
the affect of drugs on different age, gender, race and ethnicity and interaction of the drugs
with other potential drugs (Cabrera-Pérez et al. 2016).
(Source: Khachaturian 2015)
Target identification
The identification of drug target depends on few factors like pathophysiology of the
disease, the target expression uniformity in the body and the 3D structure of the target and
feasibility of obtaining that target. Other factors that are taken into consideration include
accessibility of the target under high-throughput screening, toxicity profile of the target,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
4
DRUG DISCOVERY
potential adverse affects of the target and its phenotypic data (Wright and Sieber 2016). The
intellectual property status of the targets is also important for the target identification and this
factor is relevant for the pharma-companies that apply for the patent rights for the discovered
drugs. The drug targets are identified by taking help form the public databases creating a
competition between the drug develops (Wright and Sieber 2016).
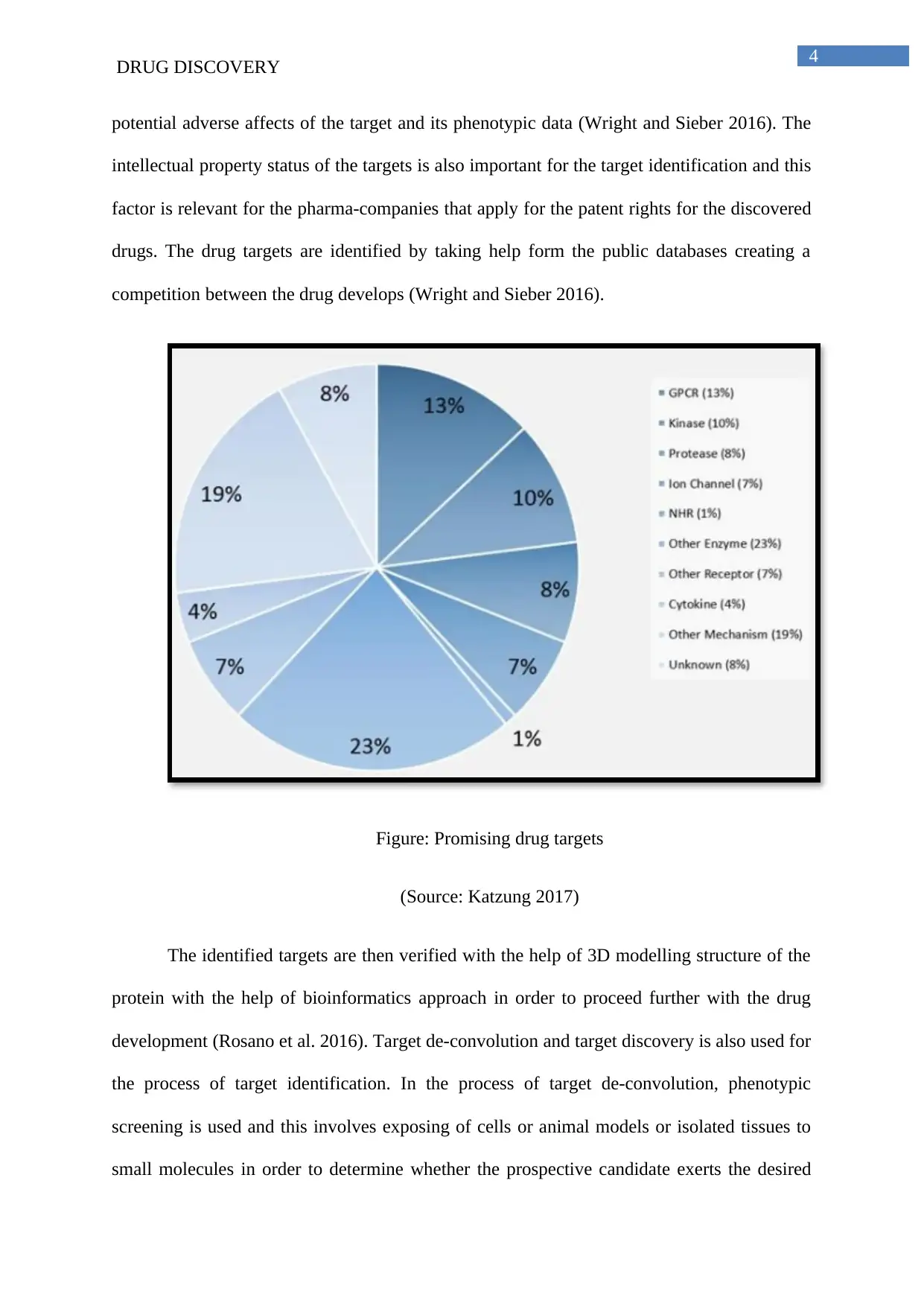
Figure: Promising drug targets
(Source: Katzung 2017)
The identified targets are then verified with the help of 3D modelling structure of the
protein with the help of bioinformatics approach in order to proceed further with the drug
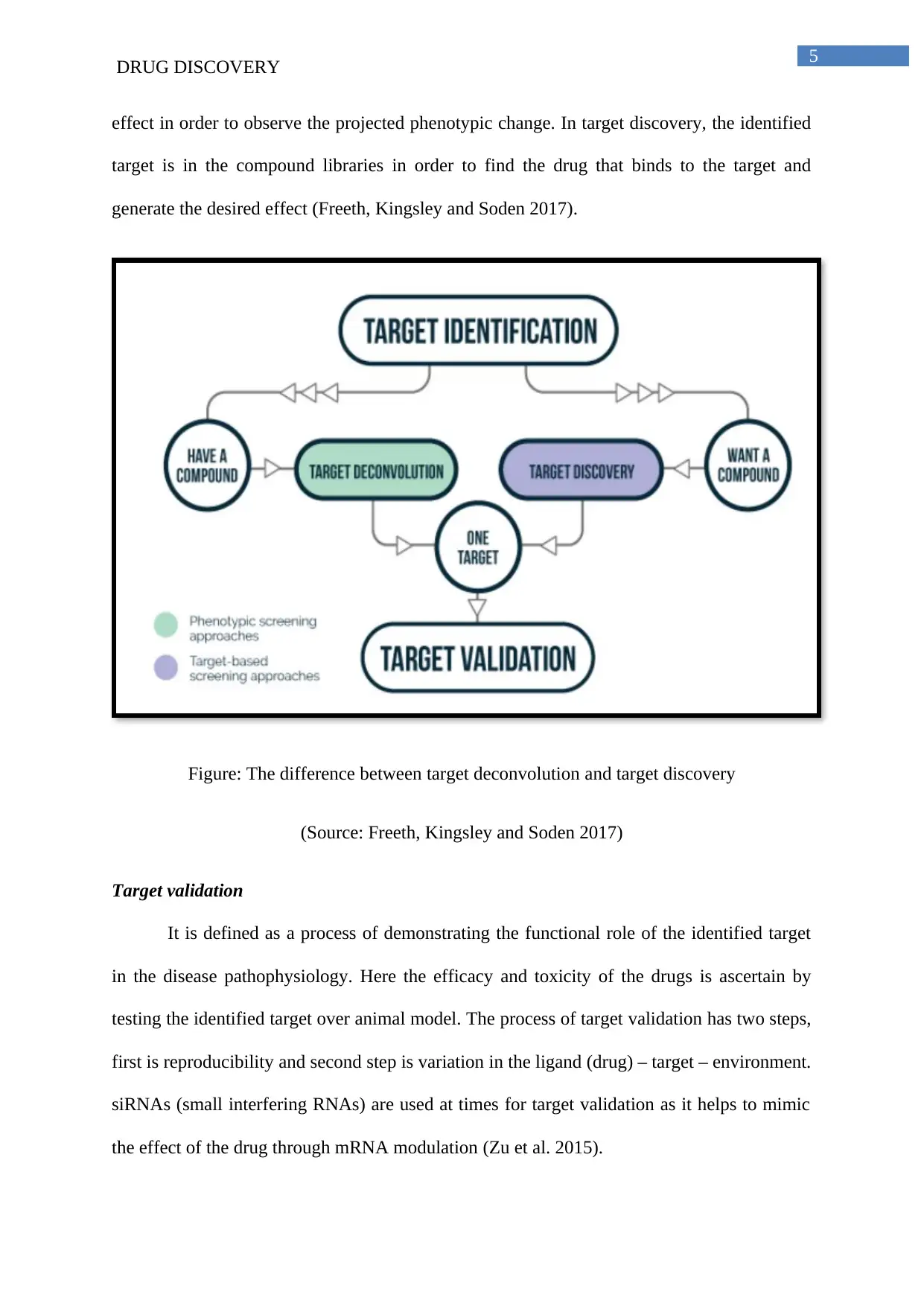
development (Rosano et al. 2016). Target de-convolution and target discovery is also used for
the process of target identification. In the process of target de-convolution, phenotypic
screening is used and this involves exposing of cells or animal models or isolated tissues to
small molecules in order to determine whether the prospective candidate exerts the desired
DRUG DISCOVERY
potential adverse affects of the target and its phenotypic data (Wright and Sieber 2016). The
intellectual property status of the targets is also important for the target identification and this
factor is relevant for the pharma-companies that apply for the patent rights for the discovered
drugs. The drug targets are identified by taking help form the public databases creating a
competition between the drug develops (Wright and Sieber 2016).
Figure: Promising drug targets
(Source: Katzung 2017)
The identified targets are then verified with the help of 3D modelling structure of the
protein with the help of bioinformatics approach in order to proceed further with the drug
development (Rosano et al. 2016). Target de-convolution and target discovery is also used for
the process of target identification. In the process of target de-convolution, phenotypic
screening is used and this involves exposing of cells or animal models or isolated tissues to
small molecules in order to determine whether the prospective candidate exerts the desired
5
DRUG DISCOVERY
effect in order to observe the projected phenotypic change. In target discovery, the identified
target is in the compound libraries in order to find the drug that binds to the target and
generate the desired effect (Freeth, Kingsley and Soden 2017).
Figure: The difference between target deconvolution and target discovery
(Source: Freeth, Kingsley and Soden 2017)
Target validation
It is defined as a process of demonstrating the functional role of the identified target
in the disease pathophysiology. Here the efficacy and toxicity of the drugs is ascertain by
testing the identified target over animal model. The process of target validation has two steps,
first is reproducibility and second step is variation in the ligand (drug) – target – environment.
siRNAs (small interfering RNAs) are used at times for target validation as it helps to mimic
the effect of the drug through mRNA modulation (Zu et al. 2015).
DRUG DISCOVERY
effect in order to observe the projected phenotypic change. In target discovery, the identified
target is in the compound libraries in order to find the drug that binds to the target and
generate the desired effect (Freeth, Kingsley and Soden 2017).
Figure: The difference between target deconvolution and target discovery
(Source: Freeth, Kingsley and Soden 2017)
Target validation
It is defined as a process of demonstrating the functional role of the identified target
in the disease pathophysiology. Here the efficacy and toxicity of the drugs is ascertain by
testing the identified target over animal model. The process of target validation has two steps,
first is reproducibility and second step is variation in the ligand (drug) – target – environment.
siRNAs (small interfering RNAs) are used at times for target validation as it helps to mimic
the effect of the drug through mRNA modulation (Zu et al. 2015).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6
DRUG DISCOVERY
Drug approval and drug marketing
FDA does the drug approval and the process takes place within a structured
framework that has three basic steps. The first step involves analysis of the target disease and
the available treatments. FDA reviewers studied the condition or the process of illness for
which the drug is being discovered and then evaluate the present treatment landscape that
provides the context for weighing the risk and the benefits of drugs. The second step involves
the assessment of the benefits and risks of the clinical data (FDA 2017). Here the FDA
reviews the clinical benefits and risk information that is submitted by the drug discovered
while taking into account of the uncertainties and might evolve from incomplete data. The
third step involves strategies for managing risks. This step includes an FDA-approved drug
label that describes the benefits and risks of the drug and how that risk or benefits can be
managed effectively. For the mitigation of risk, the drug discoverer is required to process
Risk Management and Mitigation Strategy (REMS) (FDA 2018).
WHO has specific ethical criteria for promotion of medical drug. The aim of the drug
promotion program is used improving health for the rational use of the drug by the use of
proper ethical foundation of the truthfulness and righteousness. The intended audience
include industry, dispensers, prescribes, governments and teachers (Parker Williams and Bero
2018).
Roles of academia and pharmaceutical companies in drug discovery (200)
The pharmaceutical industries continue to receive challenge by the skyrocketing costs
and by the plummeting productivity in R&D while numerous key products are experiencing
patent expiration. In other words, it can be said that pharmaceutical industries are
experiencing challenging times in the process of designing of new drugs. On the other hand,
the academic drug discovery sector has significant potential towards a contribution of
DRUG DISCOVERY
Drug approval and drug marketing
FDA does the drug approval and the process takes place within a structured
framework that has three basic steps. The first step involves analysis of the target disease and
the available treatments. FDA reviewers studied the condition or the process of illness for
which the drug is being discovered and then evaluate the present treatment landscape that
provides the context for weighing the risk and the benefits of drugs. The second step involves
the assessment of the benefits and risks of the clinical data (FDA 2017). Here the FDA
reviews the clinical benefits and risk information that is submitted by the drug discovered
while taking into account of the uncertainties and might evolve from incomplete data. The
third step involves strategies for managing risks. This step includes an FDA-approved drug
label that describes the benefits and risks of the drug and how that risk or benefits can be
managed effectively. For the mitigation of risk, the drug discoverer is required to process
Risk Management and Mitigation Strategy (REMS) (FDA 2018).
WHO has specific ethical criteria for promotion of medical drug. The aim of the drug
promotion program is used improving health for the rational use of the drug by the use of
proper ethical foundation of the truthfulness and righteousness. The intended audience
include industry, dispensers, prescribes, governments and teachers (Parker Williams and Bero
2018).
Roles of academia and pharmaceutical companies in drug discovery (200)
The pharmaceutical industries continue to receive challenge by the skyrocketing costs
and by the plummeting productivity in R&D while numerous key products are experiencing
patent expiration. In other words, it can be said that pharmaceutical industries are
experiencing challenging times in the process of designing of new drugs. On the other hand,
the academic drug discovery sector has significant potential towards a contribution of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7
DRUG DISCOVERY
meaningful discovery of novel drug targets. It also helped in the process of development of
new mode-of-action of therapeutics against a wide range of different diseases including both
neglected diseases and rare diseases (Loregian and Palù 2013). The pharmaceutical
companies have started global science hubs in the main academic centres in order to inspire
the biomedical innovation. Based on the requirement of the institution and collaborative
enterprises, an array of innovation model has been implemented under the tie-up of royalty
payments and intellectual property rights Palmer and Chaguturu 2017). The role of the
academic experts is to perform a traditional role in the process of target identification and
validation for probing of tool molecules against the identified disease targets in order to
explore therapeutic relevance (Palmer and Chaguturu 2017). Bickle (2019) stated that at
present academic centres or the academic experts are aiming towards the development of the
proposal of translational project in order to produce therapeutic molecules for rare diseases.
Roles change over time
According to the pharma industry, it takes more than decade to develop a drug
however; failure is associated with 95% of cases. At present a new discovery model has be
established in order to establish effective collaboration with the academic experts and the
pharmaceutical industry partners. This approach is helping to develop transformative
experience resulting in increased access to the novel disease targets while decreasing the
overall cost and the risk of developing therapeutics. The discovery of the high thought-put
sequencing and foundational translational research have paved the way for quick
identification of the disease targets and thereby helping to reduce cost. High-throughput
screening was generated by pharmaceutical companies until the end of 20th century, it is
carried out within its world.
DRUG DISCOVERY
meaningful discovery of novel drug targets. It also helped in the process of development of
new mode-of-action of therapeutics against a wide range of different diseases including both
neglected diseases and rare diseases (Loregian and Palù 2013). The pharmaceutical
companies have started global science hubs in the main academic centres in order to inspire
the biomedical innovation. Based on the requirement of the institution and collaborative
enterprises, an array of innovation model has been implemented under the tie-up of royalty
payments and intellectual property rights Palmer and Chaguturu 2017). The role of the
academic experts is to perform a traditional role in the process of target identification and
validation for probing of tool molecules against the identified disease targets in order to
explore therapeutic relevance (Palmer and Chaguturu 2017). Bickle (2019) stated that at
present academic centres or the academic experts are aiming towards the development of the
proposal of translational project in order to produce therapeutic molecules for rare diseases.
Roles change over time
According to the pharma industry, it takes more than decade to develop a drug
however; failure is associated with 95% of cases. At present a new discovery model has be
established in order to establish effective collaboration with the academic experts and the
pharmaceutical industry partners. This approach is helping to develop transformative
experience resulting in increased access to the novel disease targets while decreasing the
overall cost and the risk of developing therapeutics. The discovery of the high thought-put
sequencing and foundational translational research have paved the way for quick
identification of the disease targets and thereby helping to reduce cost. High-throughput
screening was generated by pharmaceutical companies until the end of 20th century, it is
carried out within its world.

8
DRUG DISCOVERY
Now the pharma companies and the academic experts work in unison with the help of
the bio-informatics approach in order to develop the potential drug target. This change in role
is initiated by the establishment of the National Institutes of Health Molecular Libraries
Program. The level of transparency in the model helped to increase the level of trust between
the academic experts and the pharma-companies and thereby helping to reduce the overall
time of drug discovery via effective collaboration. The focus of the funding of the pharma-
companies has also shifted during the recent times and academic has reciprocated with
process of translational research (Palmer and Chaguturu 2017). However, Birnbaum (2016)
highlighted a completely different perspective about the academia and the pharma-
companies. Birnbaum (2016) stated that there is an ongoing cross-talk with the academic and
pharmaceutical industry. Under this cross talk there are obstacles in development of
optimally reproductive relationships. Birnbaum (2016) stated that failure in communication
among the academia and the pharma-companies leading to legal complications in the process
of discovery of the new drug.
New academic groups
New academic centres that aim towards developing small molecule drugs are now
replacing the roles that are being traditionally performed by big pharma. For example,
Edinburgh Cancer Discovery Unit, an academic research group that provides
multidisciplinary core skills embracing advanced technological platforms and other disease
models. This non-profit organization helps to drive innovations in the domain of oncology-
based drug discovery and development. At present this organization has succeeded in
developing 2D and 3D cell and tissue based assay in order to conduct drug trial. They also
provide full-cost recovery model for projects conducted with external partners and also
provides open-access model to the students of the University of Edinburgh and external
DRUG DISCOVERY
Now the pharma companies and the academic experts work in unison with the help of
the bio-informatics approach in order to develop the potential drug target. This change in role
is initiated by the establishment of the National Institutes of Health Molecular Libraries
Program. The level of transparency in the model helped to increase the level of trust between
the academic experts and the pharma-companies and thereby helping to reduce the overall
time of drug discovery via effective collaboration. The focus of the funding of the pharma-
companies has also shifted during the recent times and academic has reciprocated with
process of translational research (Palmer and Chaguturu 2017). However, Birnbaum (2016)
highlighted a completely different perspective about the academia and the pharma-
companies. Birnbaum (2016) stated that there is an ongoing cross-talk with the academic and
pharmaceutical industry. Under this cross talk there are obstacles in development of
optimally reproductive relationships. Birnbaum (2016) stated that failure in communication
among the academia and the pharma-companies leading to legal complications in the process
of discovery of the new drug.
New academic groups
New academic centres that aim towards developing small molecule drugs are now
replacing the roles that are being traditionally performed by big pharma. For example,
Edinburgh Cancer Discovery Unit, an academic research group that provides
multidisciplinary core skills embracing advanced technological platforms and other disease
models. This non-profit organization helps to drive innovations in the domain of oncology-
based drug discovery and development. At present this organization has succeeded in
developing 2D and 3D cell and tissue based assay in order to conduct drug trial. They also
provide full-cost recovery model for projects conducted with external partners and also
provides open-access model to the students of the University of Edinburgh and external
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9
DRUG DISCOVERY
academic or other industrial organization (Bickle 2019). Technology Development Studio is
an academic screening facility established in 2004 at the Max Planck Institute of Molecular
Cell Biology and Genetics in Germany. Theu provide state-of-art cellular screening services
and develop novel technologies for drug designing. They have facilities of both genomic and
chemical screen run along with genome wide access of RNAi libraries. They have also
developed protocols for handling 3D non-adherent object (Bickle 2019). Pharmacy
Chemical Biology Centre (PCBC) is located in the University of Pittsburgh in 2011. They
perform high-throughput screening (HTS). They help in the process of detection of the novel
drug molecules (Bickle 2019).
Shanks, Ketteler and Ebner (2015) are of the opinion that there are 24 different
dedicated drug discovery facilities present in the UK. These facilities have a wide range of
operational frameworks and other academic facilities with several sources of funding. The
common theme that links these drug discovery groups include the open-access to drug
discovery facilities that is accessible to other UK-wide academic groups and each of the
facilities employ personnel from academic and thus helping to increase the strength of the
UK academic community (Shanks, Ketteler and Ebner 2015).
DRUG DISCOVERY
academic or other industrial organization (Bickle 2019). Technology Development Studio is
an academic screening facility established in 2004 at the Max Planck Institute of Molecular
Cell Biology and Genetics in Germany. Theu provide state-of-art cellular screening services
and develop novel technologies for drug designing. They have facilities of both genomic and
chemical screen run along with genome wide access of RNAi libraries. They have also
developed protocols for handling 3D non-adherent object (Bickle 2019). Pharmacy
Chemical Biology Centre (PCBC) is located in the University of Pittsburgh in 2011. They
perform high-throughput screening (HTS). They help in the process of detection of the novel
drug molecules (Bickle 2019).
Shanks, Ketteler and Ebner (2015) are of the opinion that there are 24 different
dedicated drug discovery facilities present in the UK. These facilities have a wide range of
operational frameworks and other academic facilities with several sources of funding. The
common theme that links these drug discovery groups include the open-access to drug
discovery facilities that is accessible to other UK-wide academic groups and each of the
facilities employ personnel from academic and thus helping to increase the strength of the
UK academic community (Shanks, Ketteler and Ebner 2015).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10
DRUG DISCOVERY
Figure: Common Drug Discovery Facilities in the UK, Scotland and in Europe
(Source: Shanks, Ketteler and Ebner 2015)
Conclusion
Thus from the above discussion, it can be concluded that a considerable wave of
achievement has flooded the process of selection of the new target molecule and the overall
process of drug discovery in the UK. During the course of time with the advent of the high
thoughput sequencing the overall time that is being invested in the process of drug discovery
have reduced considerably. Moreover, at present there are limited chances of failure in
comparison to the scenario that prevailed 15 to 20 years before. The new pharma and
academic experts are now planning to work in unison in order to refine the overall process of
drug delivery. However, at times both the groups are experiences conflict of interest,
resulting in a delay in the overall procedure and reduction in the efficacy of the drug
designing.
DRUG DISCOVERY
Figure: Common Drug Discovery Facilities in the UK, Scotland and in Europe
(Source: Shanks, Ketteler and Ebner 2015)
Conclusion
Thus from the above discussion, it can be concluded that a considerable wave of
achievement has flooded the process of selection of the new target molecule and the overall
process of drug discovery in the UK. During the course of time with the advent of the high
thoughput sequencing the overall time that is being invested in the process of drug discovery
have reduced considerably. Moreover, at present there are limited chances of failure in
comparison to the scenario that prevailed 15 to 20 years before. The new pharma and
academic experts are now planning to work in unison in order to refine the overall process of
drug delivery. However, at times both the groups are experiences conflict of interest,
resulting in a delay in the overall procedure and reduction in the efficacy of the drug
designing.

11
DRUG DISCOVERY
Shamas-Din and Schimmer (2015) are of the opinion that participation of academic
organization in the process of drug discovery, target identification and series of clinical trials
is increasing rapidly. However, the process of drug discovery by the academic centers
continues to stall in the domain of clinical probes. This cast a significant hurdle for academic
groups. However, with the help of the high-quality chemical probes and increase in the level
of projected clinical trials, it can be stated academic can be able to sustain their new role with
a special mention to the oncology based drug discovery. This oncology oriented drug
discovery approach is not expected to falter as it is being backed by blessings of the
technological advancements. However, increase in the drug discovery cost and lack of proper
funding can decrease the momentum of the overall process of drug delivery. There are
numerous potential drugs that are being identified by these academic centers that are
succesful in reaching the clinical for therapeutic use. Some of the common examples include
paclitaxel,, taxol and abraxane. All these medications are used in the domain of oncology and
is being discovered by Research Triangle Institute that is a joint partner with the Bristol-
Myers Squib.
Other list of drugs identified by academic centres reached the clinic are highlighted
in the table below
DRUG DISCOVERY
Shamas-Din and Schimmer (2015) are of the opinion that participation of academic
organization in the process of drug discovery, target identification and series of clinical trials
is increasing rapidly. However, the process of drug discovery by the academic centers
continues to stall in the domain of clinical probes. This cast a significant hurdle for academic
groups. However, with the help of the high-quality chemical probes and increase in the level
of projected clinical trials, it can be stated academic can be able to sustain their new role with
a special mention to the oncology based drug discovery. This oncology oriented drug
discovery approach is not expected to falter as it is being backed by blessings of the
technological advancements. However, increase in the drug discovery cost and lack of proper
funding can decrease the momentum of the overall process of drug delivery. There are
numerous potential drugs that are being identified by these academic centers that are
succesful in reaching the clinical for therapeutic use. Some of the common examples include
paclitaxel,, taxol and abraxane. All these medications are used in the domain of oncology and
is being discovered by Research Triangle Institute that is a joint partner with the Bristol-
Myers Squib.
Other list of drugs identified by academic centres reached the clinic are highlighted
in the table below
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



