CHEG 472 - Water Treatment and Membrane Processes Homework Assignment
VerifiedAdded on 2022/09/12
|14
|3549
|8
Homework Assignment
AI Summary
This document presents a comprehensive solution to a water treatment assignment, addressing key concepts in the field. It begins by differentiating between conservative and non-conservative substances and provides the general material balance equations for each. The solution then defines the first and second laws of thermodynamics and distinguishes between batch, continuous, and plug flow reactors. It includes schematic diagrams of water treatment plants for both surface and groundwater sources and lists eight categories of raw water contaminants with examples. The document explores the biological, chemical, and physical characteristics of water, defines water hardness, and differentiates between carbonate and non-carbonate hardness, including relevant calculations. It covers water softening methods, specifically the lime-soda ash method, and outlines factors to consider when developing a water treatment process. The solution differentiates between coagulation and flocculation, explains floc formation, and describes the jar test for determining the optimum coagulant dose. It defines the sedimentation process and distinguishes between Type 1, Type 2, Type 3, and Type 4 sedimentation, including a discussion of rectangular sedimentation tanks. Finally, the document covers rapid sand filters, dual media filters, and slow sand filters. This assignment solution is designed to help students understand the core principles of water treatment and membrane processes.

Class Revision-Midterm 1
WATER TREATMENT AND MEMBRANE PROCESSES
By Name
Course
Instructor
Institution
Location
Date
WATER TREATMENT AND MEMBRANE PROCESSES
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Class Revision-Midterm 2
1. Conservative and Non-Conservative Substances
A conservative substance is defined as any substance that is not a subject of any reaction with no
decay or production. Non-conservative substances are defined as any substance that decays or is
generated in the system.
The general materials balance equations for conservative substances are:
Rate of accumulation = Input Rate – Output Rate+ Reaction Rate
; dM
dt = d( Min)
dt − d ( Mout )
dt +rV
Where; r is the rate of reaction and is negative (-) when the substance decays and positive (+)
when the substance is generated.
V is the system’s volume
M out and Min is the mass flowing out and in respectively
Mass is the accumulated mass within the boundary [1]
2. Second and First Law of Thermodynamics
1st Law of Thermodynamics states energy can neither be created or destroyed, but can only be
changed or transferred from one form to another or from one body to another.
The 2nd Law of Thermodynamics states that in a natural process of thermodynamics, the
summation of the entropies of interacting systems of thermodynamics increases.
3. Plug Flow Reactors, Continuous Reactors, and Batch Reactors
A Plug Flow Reactor, abbreviated as PFR and sometimes is referred to as the continuous tubular
reactor is where a single or various fluid reagents are pumped through a tube or pipe and the
chemical reactions continue traveling through the PFR.
1. Conservative and Non-Conservative Substances
A conservative substance is defined as any substance that is not a subject of any reaction with no
decay or production. Non-conservative substances are defined as any substance that decays or is
generated in the system.
The general materials balance equations for conservative substances are:
Rate of accumulation = Input Rate – Output Rate+ Reaction Rate
; dM
dt = d( Min)
dt − d ( Mout )
dt +rV
Where; r is the rate of reaction and is negative (-) when the substance decays and positive (+)
when the substance is generated.
V is the system’s volume
M out and Min is the mass flowing out and in respectively
Mass is the accumulated mass within the boundary [1]
2. Second and First Law of Thermodynamics
1st Law of Thermodynamics states energy can neither be created or destroyed, but can only be
changed or transferred from one form to another or from one body to another.
The 2nd Law of Thermodynamics states that in a natural process of thermodynamics, the
summation of the entropies of interacting systems of thermodynamics increases.
3. Plug Flow Reactors, Continuous Reactors, and Batch Reactors
A Plug Flow Reactor, abbreviated as PFR and sometimes is referred to as the continuous tubular
reactor is where a single or various fluid reagents are pumped through a tube or pipe and the
chemical reactions continue traveling through the PFR.
Class Revision-Midterm 3
In Batch Reactor, the material is loaded into a batch reactor which is a constant volume vessel in
the chemical process and the process of reaction continues with time [2].
In Continuous Reactors, a single or many fluid reagents are introduced into the reactor tank
which is normally stirred using impeller to promote proper reagent mixing which removing the
reactor effluent [3].
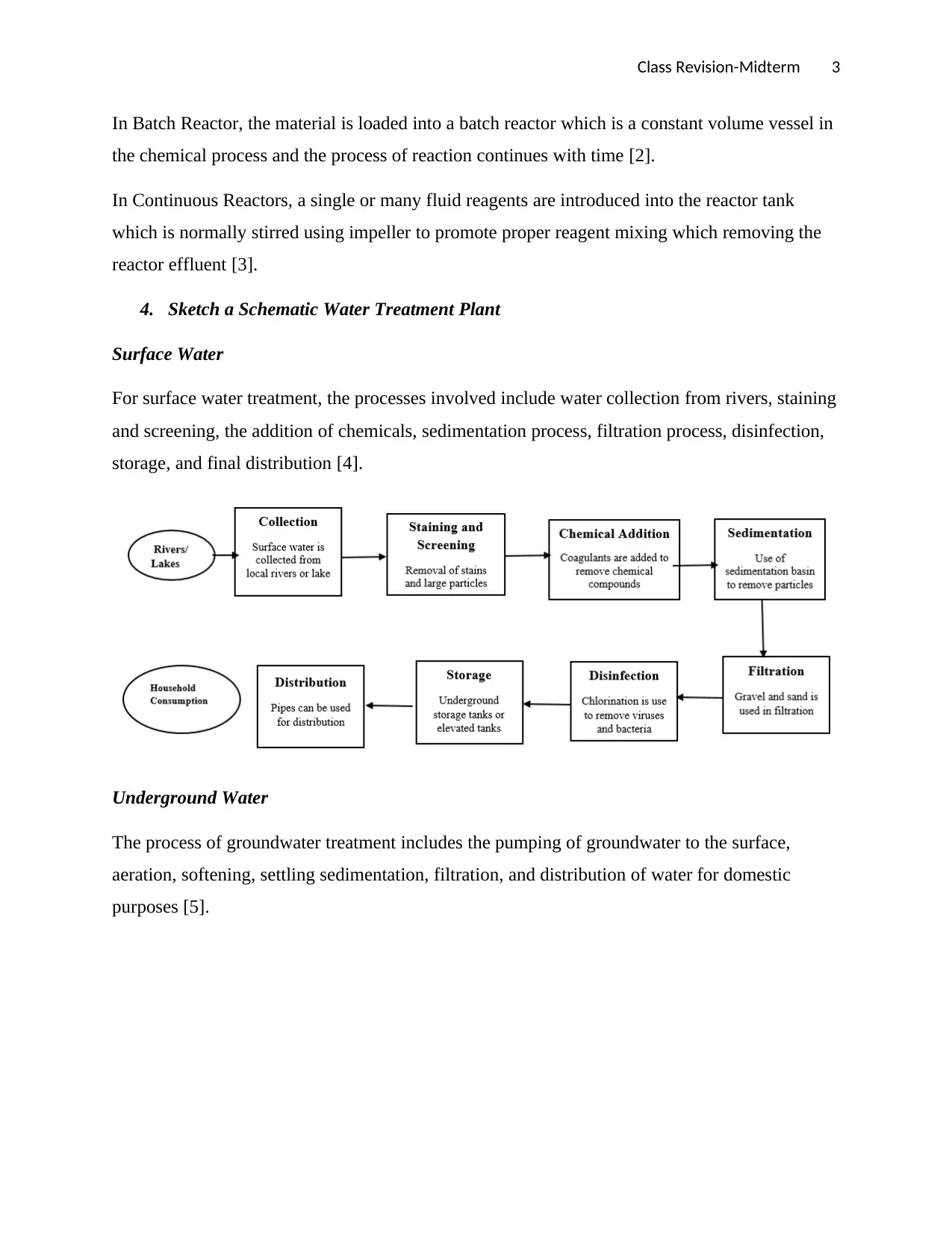
4. Sketch a Schematic Water Treatment Plant
Surface Water
For surface water treatment, the processes involved include water collection from rivers, staining
and screening, the addition of chemicals, sedimentation process, filtration process, disinfection,
storage, and final distribution [4].
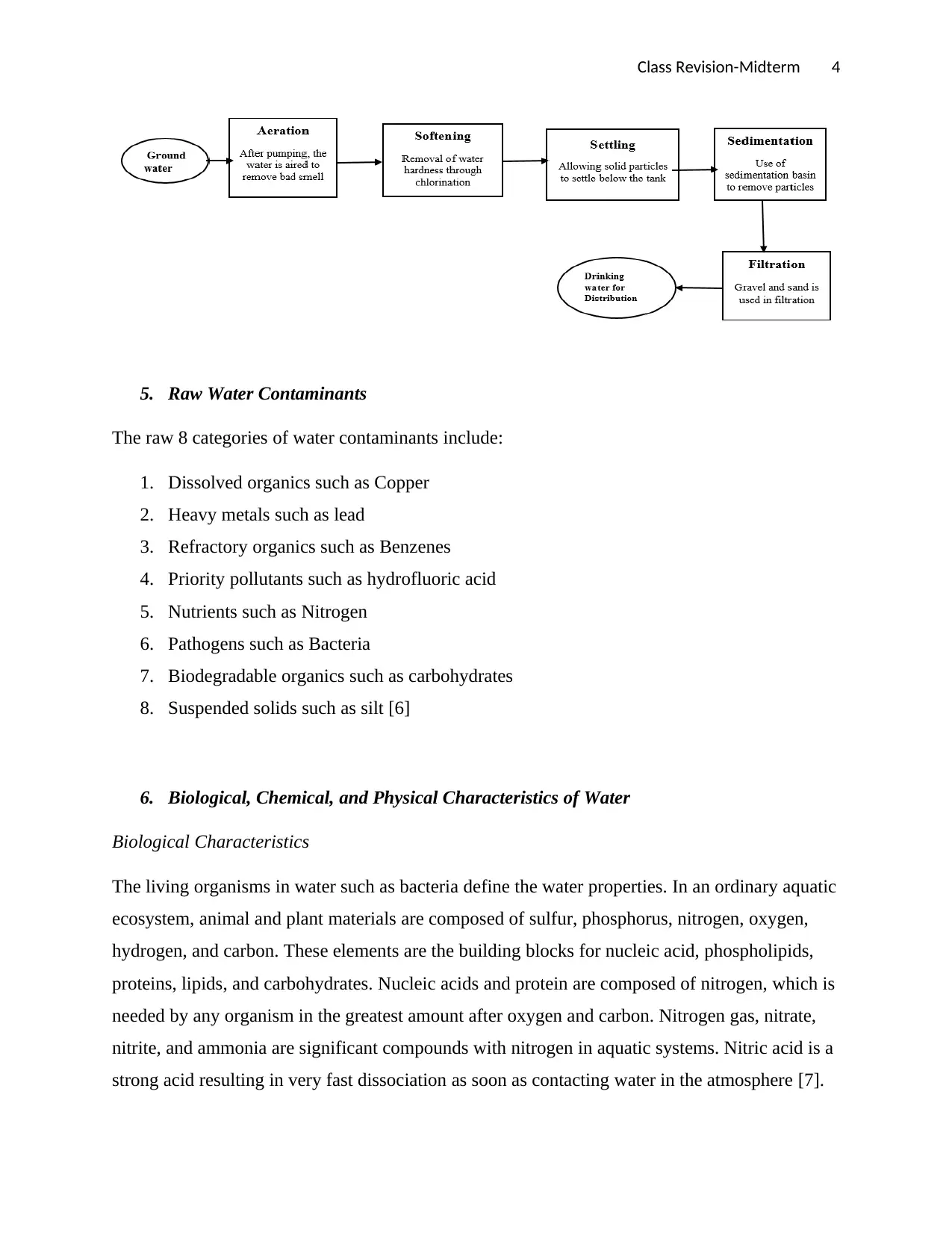
Underground Water
The process of groundwater treatment includes the pumping of groundwater to the surface,
aeration, softening, settling sedimentation, filtration, and distribution of water for domestic
purposes [5].
In Batch Reactor, the material is loaded into a batch reactor which is a constant volume vessel in
the chemical process and the process of reaction continues with time [2].
In Continuous Reactors, a single or many fluid reagents are introduced into the reactor tank
which is normally stirred using impeller to promote proper reagent mixing which removing the
reactor effluent [3].
4. Sketch a Schematic Water Treatment Plant
Surface Water
For surface water treatment, the processes involved include water collection from rivers, staining
and screening, the addition of chemicals, sedimentation process, filtration process, disinfection,
storage, and final distribution [4].
Underground Water
The process of groundwater treatment includes the pumping of groundwater to the surface,
aeration, softening, settling sedimentation, filtration, and distribution of water for domestic
purposes [5].
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Class Revision-Midterm 4
5. Raw Water Contaminants
The raw 8 categories of water contaminants include:
1. Dissolved organics such as Copper
2. Heavy metals such as lead
3. Refractory organics such as Benzenes
4. Priority pollutants such as hydrofluoric acid
5. Nutrients such as Nitrogen
6. Pathogens such as Bacteria
7. Biodegradable organics such as carbohydrates
8. Suspended solids such as silt [6]
6. Biological, Chemical, and Physical Characteristics of Water
Biological Characteristics
The living organisms in water such as bacteria define the water properties. In an ordinary aquatic
ecosystem, animal and plant materials are composed of sulfur, phosphorus, nitrogen, oxygen,
hydrogen, and carbon. These elements are the building blocks for nucleic acid, phospholipids,
proteins, lipids, and carbohydrates. Nucleic acids and protein are composed of nitrogen, which is
needed by any organism in the greatest amount after oxygen and carbon. Nitrogen gas, nitrate,
nitrite, and ammonia are significant compounds with nitrogen in aquatic systems. Nitric acid is a
strong acid resulting in very fast dissociation as soon as contacting water in the atmosphere [7].
5. Raw Water Contaminants
The raw 8 categories of water contaminants include:
1. Dissolved organics such as Copper
2. Heavy metals such as lead
3. Refractory organics such as Benzenes
4. Priority pollutants such as hydrofluoric acid
5. Nutrients such as Nitrogen
6. Pathogens such as Bacteria
7. Biodegradable organics such as carbohydrates
8. Suspended solids such as silt [6]
6. Biological, Chemical, and Physical Characteristics of Water
Biological Characteristics
The living organisms in water such as bacteria define the water properties. In an ordinary aquatic
ecosystem, animal and plant materials are composed of sulfur, phosphorus, nitrogen, oxygen,
hydrogen, and carbon. These elements are the building blocks for nucleic acid, phospholipids,
proteins, lipids, and carbohydrates. Nucleic acids and protein are composed of nitrogen, which is
needed by any organism in the greatest amount after oxygen and carbon. Nitrogen gas, nitrate,
nitrite, and ammonia are significant compounds with nitrogen in aquatic systems. Nitric acid is a
strong acid resulting in very fast dissociation as soon as contacting water in the atmosphere [7].
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Class Revision-Midterm 5
Sulfate is present in natural water as sulfate, hydrogen sulfide, element sulfur, and organic sulfur.
Hydrogen sulfide is toxic to various microorganisms and also causes water odour.
Physical Characteristics
The physical features of water such as odour, taste, color, and temperature are determined by the
senses of taste, smell, sight, and touch. For instance, odour by smell, suspended solids, turbidity,
floating debris, and color by tough. The water temperature influences majority of the significant
physical water characteristics and properties such as dissolved gases solubility, salinity, specific
conductivity, surface tension, viscosity, specific weight, density, and thermal capacity [7]. The
color of the water is majorly concerned with the aesthetic quality of water as it determines if the
water is fit for drinking. The odour and taste of water are affected by caffeine (bitter), sour
(hydrochloric acid), salty (sodium chloride), and sweet (sucrose).
Chemical Characteristics
The chemical properties of water are determined by the rocks and solid that is in contact with
water. Consequently, industrial treated wastewater, urban and agricultural runoff affect the
quality of water. The chemical and microbial changes also impact the chemical properties of
water. The total dissolved solid concertation is associated with specific conductance or electrical
conductivity. The conductivity determines the ability of water to convey electricity.
7. Water Hardness
This is water that has a high content of minerals and is developed during percolation of water
through gypsum, chalk or limestone deposits. Permanent water hardness is as a result of the
presence of magnesium chloride or magnesium sulfate and/or calcium chloride or calcium
sulfate. The temporary water hardness is as a result of the bicarbonate mineral presence such as
calcium bicarbonate and magnesium bicarbonate that have dissolved in water [8].
The following equilibrium reaction illustrates the formation and dissolving of calcium
bicarbonate and calcium carbonate:
Sulfate is present in natural water as sulfate, hydrogen sulfide, element sulfur, and organic sulfur.
Hydrogen sulfide is toxic to various microorganisms and also causes water odour.
Physical Characteristics
The physical features of water such as odour, taste, color, and temperature are determined by the
senses of taste, smell, sight, and touch. For instance, odour by smell, suspended solids, turbidity,
floating debris, and color by tough. The water temperature influences majority of the significant
physical water characteristics and properties such as dissolved gases solubility, salinity, specific
conductivity, surface tension, viscosity, specific weight, density, and thermal capacity [7]. The
color of the water is majorly concerned with the aesthetic quality of water as it determines if the
water is fit for drinking. The odour and taste of water are affected by caffeine (bitter), sour
(hydrochloric acid), salty (sodium chloride), and sweet (sucrose).
Chemical Characteristics
The chemical properties of water are determined by the rocks and solid that is in contact with
water. Consequently, industrial treated wastewater, urban and agricultural runoff affect the
quality of water. The chemical and microbial changes also impact the chemical properties of
water. The total dissolved solid concertation is associated with specific conductance or electrical
conductivity. The conductivity determines the ability of water to convey electricity.
7. Water Hardness
This is water that has a high content of minerals and is developed during percolation of water
through gypsum, chalk or limestone deposits. Permanent water hardness is as a result of the
presence of magnesium chloride or magnesium sulfate and/or calcium chloride or calcium
sulfate. The temporary water hardness is as a result of the bicarbonate mineral presence such as
calcium bicarbonate and magnesium bicarbonate that have dissolved in water [8].
The following equilibrium reaction illustrates the formation and dissolving of calcium
bicarbonate and calcium carbonate:

Class Revision-Midterm 6
Non-Carbonate Hardness and Carbonate Hardness
Non-carbonate hardness is a type of hardness caused by sulfates and chloride of magnesium and
calcium. It is also referred to as permanent hardness. Non-carbonate hardness is as a result of
calcium chloride, magnesium sulphate, and calcium sulphate [8]. Carbonate hardness is a type of
hardness produced by bicarbonates of magnesium and calcium.
8. Calculation of Carbonate Hardness and Non-Carbonate Hardness for the total
Hardness and Alkalinity
Carbonate Hardness, CH
Given that Non-carbonate hardness, NCH, and Carbonate hardness, CH
Total Hardness, TH = NCH + CH
Carbon hardness is related directly to alkalinity;
CH in meq/L = (Alk in meq/L)
CH in o dH = 2.809 x [Alk in meq/L]
Therefore, Carbonate Hardness, CH = Alkalinity
Non-Carbonate Hardness, NCH
From the Equation: Total Hardness, TH = NCH + CH
Therefore, Non-Carbonate Hardness, NCH = Total Hardness – Carbonate Hardness
NCH = TH – CH [9]
9. Water Softening Method
The water softening method is defined as the process of removing dissolved calcium salts,
magnesium salts, and certain other metallic cations in hard water. The resultant soft water needs
less soap to form lather hence reducing the quantity of soap used in the cleaning process.
Non-Carbonate Hardness and Carbonate Hardness
Non-carbonate hardness is a type of hardness caused by sulfates and chloride of magnesium and
calcium. It is also referred to as permanent hardness. Non-carbonate hardness is as a result of
calcium chloride, magnesium sulphate, and calcium sulphate [8]. Carbonate hardness is a type of
hardness produced by bicarbonates of magnesium and calcium.
8. Calculation of Carbonate Hardness and Non-Carbonate Hardness for the total
Hardness and Alkalinity
Carbonate Hardness, CH
Given that Non-carbonate hardness, NCH, and Carbonate hardness, CH
Total Hardness, TH = NCH + CH
Carbon hardness is related directly to alkalinity;
CH in meq/L = (Alk in meq/L)
CH in o dH = 2.809 x [Alk in meq/L]
Therefore, Carbonate Hardness, CH = Alkalinity
Non-Carbonate Hardness, NCH
From the Equation: Total Hardness, TH = NCH + CH
Therefore, Non-Carbonate Hardness, NCH = Total Hardness – Carbonate Hardness
NCH = TH – CH [9]
9. Water Softening Method
The water softening method is defined as the process of removing dissolved calcium salts,
magnesium salts, and certain other metallic cations in hard water. The resultant soft water needs
less soap to form lather hence reducing the quantity of soap used in the cleaning process.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Class Revision-Midterm 7
Lime-Soda Ash Method
In the Lime-soda ash method, lime and soda ash are added resulting into formation of
magnesium hydroxide from magnesium hardness precipitation and calcium carbonate from
calcium hardness precipitation [10]The chemical reactions appearing during the method include:
10. Factors Considered When Developing Water Treatment Process
Discharge management: There is a need for determining the rules regarding the disposal of
discharge after-treatment process.
Desired treated water quality: The water quality expected after the treatment process depends on
the treatment processes. Drinking water should be thoroughly treated but not as water treated for
construction purposes.
Source of water to be treated: The source of water determines the treatment process. Surface
water treatment is different from underground water treatment due to the difference in water
quality [11].
Environmental factors: Environmental factors such as temperature, residual waste management,
and energy requirements should be considered in selecting treatment processes.
Cost: The financial status is important is selecting the treatment process since some processes are
very capital and labor-intensive [12].
Lime-Soda Ash Method
In the Lime-soda ash method, lime and soda ash are added resulting into formation of
magnesium hydroxide from magnesium hardness precipitation and calcium carbonate from
calcium hardness precipitation [10]The chemical reactions appearing during the method include:
10. Factors Considered When Developing Water Treatment Process
Discharge management: There is a need for determining the rules regarding the disposal of
discharge after-treatment process.
Desired treated water quality: The water quality expected after the treatment process depends on
the treatment processes. Drinking water should be thoroughly treated but not as water treated for
construction purposes.
Source of water to be treated: The source of water determines the treatment process. Surface
water treatment is different from underground water treatment due to the difference in water
quality [11].
Environmental factors: Environmental factors such as temperature, residual waste management,
and energy requirements should be considered in selecting treatment processes.
Cost: The financial status is important is selecting the treatment process since some processes are
very capital and labor-intensive [12].
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Class Revision-Midterm 8
Location: The location situated for treatment plants may affect the water quality due to cross-
contamination hence resulting in advanced treatment processes.
11. Coagulation and Flocculation
Flocculation is the process by which colloids being exposed from suspension in a form of flake
or floc by adding clarifying agents or spontaneously while coagulation is the process of
destabilizing the charges of the particles with opposite charges to those solids suspended to
neutralize the negative charges on the non-settable solids disperses such as organic substances
and clay [13].
The phenomenon of Floc Formation
Floc is a loosely, the small aggregated mass of flocculent materials precipitated from or
suspended in a liquid. Floc consists of suspended particles finely divided into a gelatinous
particle as a result of adhesion or attraction to a coagulant compound [13]. Floc formation
involves the combination of suspended materials in the raw water together with precipitated and
adsorbed solids acquired through the coagulation process.
12. Jar test to obtain an optimum coagulant dose
The coagulant dose to be used can be obtained by the jar test. The jar test entails exposing the
same sample volumes of water to be treated to different coagulant doses and then mixing the
samples simultaneously at a constant spontaneous time of mixing. The microfloc developed after
the process of coagulation undergoes flocculation further and is permitted to settle. Then the
turbidity of the samples is determined and the dose of the lowest turbidity can be said to be an
optimum coagulant dose [14].
13. Sedimentation Process
The sedimentation process is defined as the tendency of suspension particles to settle out of the
fluid suspension under the effects of gravity. The elements settling out from the fluid suspension
are the sediments while the water treatment is referred to as sludge. The process of sedimentation
Location: The location situated for treatment plants may affect the water quality due to cross-
contamination hence resulting in advanced treatment processes.
11. Coagulation and Flocculation
Flocculation is the process by which colloids being exposed from suspension in a form of flake
or floc by adding clarifying agents or spontaneously while coagulation is the process of
destabilizing the charges of the particles with opposite charges to those solids suspended to
neutralize the negative charges on the non-settable solids disperses such as organic substances
and clay [13].
The phenomenon of Floc Formation
Floc is a loosely, the small aggregated mass of flocculent materials precipitated from or
suspended in a liquid. Floc consists of suspended particles finely divided into a gelatinous
particle as a result of adhesion or attraction to a coagulant compound [13]. Floc formation
involves the combination of suspended materials in the raw water together with precipitated and
adsorbed solids acquired through the coagulation process.
12. Jar test to obtain an optimum coagulant dose
The coagulant dose to be used can be obtained by the jar test. The jar test entails exposing the
same sample volumes of water to be treated to different coagulant doses and then mixing the
samples simultaneously at a constant spontaneous time of mixing. The microfloc developed after
the process of coagulation undergoes flocculation further and is permitted to settle. Then the
turbidity of the samples is determined and the dose of the lowest turbidity can be said to be an
optimum coagulant dose [14].
13. Sedimentation Process
The sedimentation process is defined as the tendency of suspension particles to settle out of the
fluid suspension under the effects of gravity. The elements settling out from the fluid suspension
are the sediments while the water treatment is referred to as sludge. The process of sedimentation

Class Revision-Midterm 9
is because of their motion through the response of fluid to the forces acting on them, these forces
can be as a result if electromagnetism, centrifugal acceleration, or gravity. Sedimentation in
water treatment can be used to minimize the concentration of particles in suspension before the
coagulation application [15]. Settling is the falling of the elements of sedimentation through the
fluid while the sedimentation is the end of the process of settling.
Type 1, Type 2, Type 3 and Type 4 Sedimentation
Type 1: This type of sedimentation is defined by elements that discretely settle at settling
constant velocity or through the iron mineral deposition to streamlines down to the source point.
The elements settle as separable elements and do not stick or flocculate to each other during
settling.
Type 2: This type of sedimentation is defined by flocculated particles during the process of
sedimentation and due to this, their sizes are changing constantly and hence their velocity of
settling is also changing.
Type 3: This category of sedimentation is also referred to as zone sedimentation. During this
process, the particles are highly concentrated such that the particles have the tendency of settling
as a mass and a particular sludge zone and clear zone exist. Zone settling takes place in sludge
thickeners, active sludge sedimentation, sedimentation, and lime-softening [16].
Type 4: In this type of sedimentation, the suspensions are concentrated and the particles tend to
settle at a particular sludge zone resulting in sludge thickening.
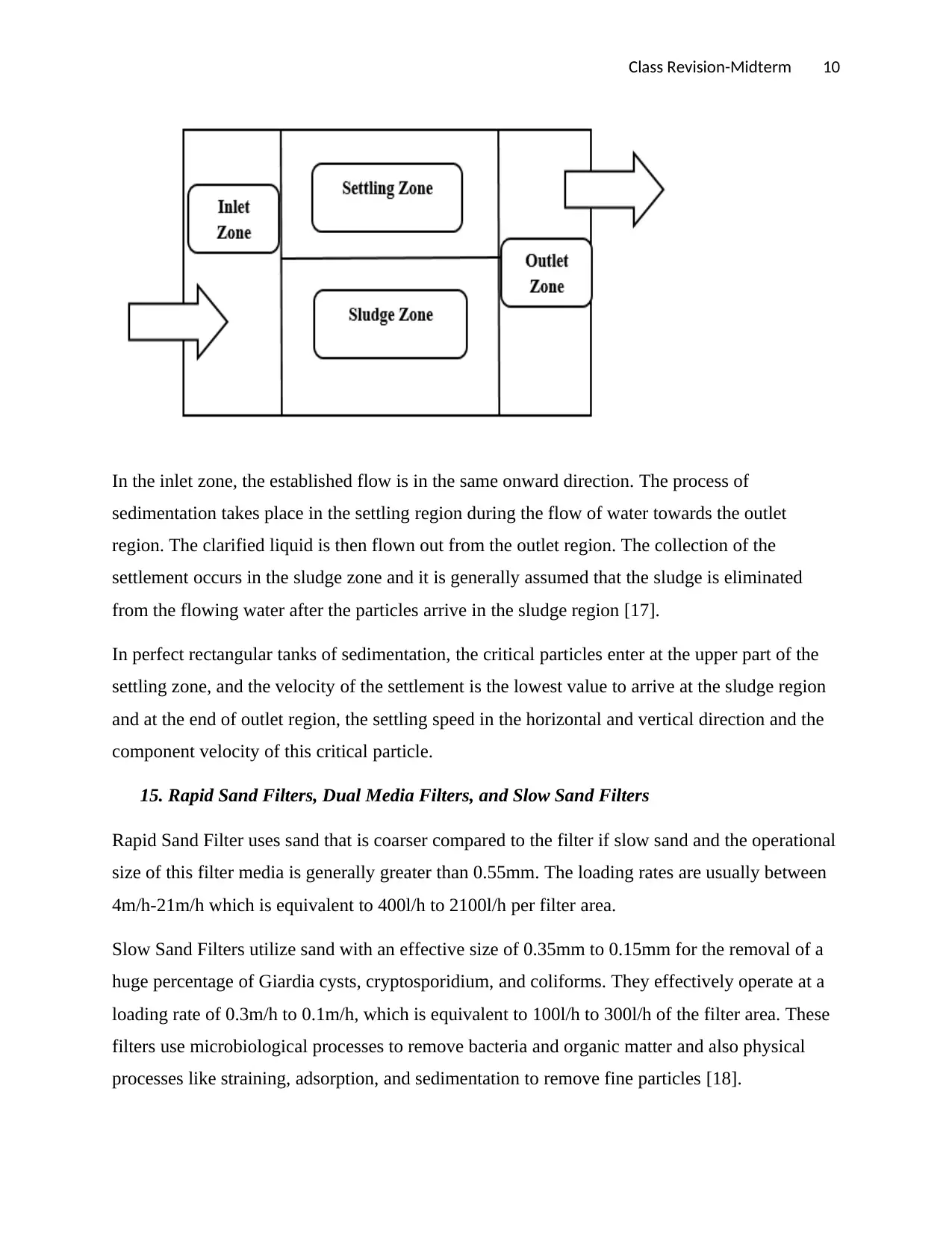
14. Rectangular Sedimentation Tank
The surface area of the rectangular sedimentation tank is the major factor in the rate of
sedimentation. All the continuous flow settling rectangular tank are categorized into four
sections, namely outlet, sludge, settling, and inlet zones as shown in the figure below:
is because of their motion through the response of fluid to the forces acting on them, these forces
can be as a result if electromagnetism, centrifugal acceleration, or gravity. Sedimentation in
water treatment can be used to minimize the concentration of particles in suspension before the
coagulation application [15]. Settling is the falling of the elements of sedimentation through the
fluid while the sedimentation is the end of the process of settling.
Type 1, Type 2, Type 3 and Type 4 Sedimentation
Type 1: This type of sedimentation is defined by elements that discretely settle at settling
constant velocity or through the iron mineral deposition to streamlines down to the source point.
The elements settle as separable elements and do not stick or flocculate to each other during
settling.
Type 2: This type of sedimentation is defined by flocculated particles during the process of
sedimentation and due to this, their sizes are changing constantly and hence their velocity of
settling is also changing.
Type 3: This category of sedimentation is also referred to as zone sedimentation. During this
process, the particles are highly concentrated such that the particles have the tendency of settling
as a mass and a particular sludge zone and clear zone exist. Zone settling takes place in sludge
thickeners, active sludge sedimentation, sedimentation, and lime-softening [16].
Type 4: In this type of sedimentation, the suspensions are concentrated and the particles tend to
settle at a particular sludge zone resulting in sludge thickening.
14. Rectangular Sedimentation Tank
The surface area of the rectangular sedimentation tank is the major factor in the rate of
sedimentation. All the continuous flow settling rectangular tank are categorized into four
sections, namely outlet, sludge, settling, and inlet zones as shown in the figure below:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Class Revision-Midterm 10
In the inlet zone, the established flow is in the same onward direction. The process of
sedimentation takes place in the settling region during the flow of water towards the outlet
region. The clarified liquid is then flown out from the outlet region. The collection of the
settlement occurs in the sludge zone and it is generally assumed that the sludge is eliminated
from the flowing water after the particles arrive in the sludge region [17].
In perfect rectangular tanks of sedimentation, the critical particles enter at the upper part of the
settling zone, and the velocity of the settlement is the lowest value to arrive at the sludge region
and at the end of outlet region, the settling speed in the horizontal and vertical direction and the
component velocity of this critical particle.
15. Rapid Sand Filters, Dual Media Filters, and Slow Sand Filters
Rapid Sand Filter uses sand that is coarser compared to the filter if slow sand and the operational
size of this filter media is generally greater than 0.55mm. The loading rates are usually between
4m/h-21m/h which is equivalent to 400l/h to 2100l/h per filter area.
Slow Sand Filters utilize sand with an effective size of 0.35mm to 0.15mm for the removal of a
huge percentage of Giardia cysts, cryptosporidium, and coliforms. They effectively operate at a
loading rate of 0.3m/h to 0.1m/h, which is equivalent to 100l/h to 300l/h of the filter area. These
filters use microbiological processes to remove bacteria and organic matter and also physical
processes like straining, adsorption, and sedimentation to remove fine particles [18].
In the inlet zone, the established flow is in the same onward direction. The process of
sedimentation takes place in the settling region during the flow of water towards the outlet
region. The clarified liquid is then flown out from the outlet region. The collection of the
settlement occurs in the sludge zone and it is generally assumed that the sludge is eliminated
from the flowing water after the particles arrive in the sludge region [17].
In perfect rectangular tanks of sedimentation, the critical particles enter at the upper part of the
settling zone, and the velocity of the settlement is the lowest value to arrive at the sludge region
and at the end of outlet region, the settling speed in the horizontal and vertical direction and the
component velocity of this critical particle.
15. Rapid Sand Filters, Dual Media Filters, and Slow Sand Filters
Rapid Sand Filter uses sand that is coarser compared to the filter if slow sand and the operational
size of this filter media is generally greater than 0.55mm. The loading rates are usually between
4m/h-21m/h which is equivalent to 400l/h to 2100l/h per filter area.
Slow Sand Filters utilize sand with an effective size of 0.35mm to 0.15mm for the removal of a
huge percentage of Giardia cysts, cryptosporidium, and coliforms. They effectively operate at a
loading rate of 0.3m/h to 0.1m/h, which is equivalent to 100l/h to 300l/h of the filter area. These
filters use microbiological processes to remove bacteria and organic matter and also physical
processes like straining, adsorption, and sedimentation to remove fine particles [18].
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Class Revision-Midterm 11
Dual Media Filters are majorly used for the removal of suspended solids and turbidity solids with
an effective of 0.01mm to 0.02mm. The dual media filter provides very effective removal of
particles under the conditions of a high rate of filtration. They effectively operate at a loading
rate of 0.0086m/h to 0.017m/h, which is equivalent to 0.86l/h to 1.7l/h of the filter area.
16. Disinfection Process
The disinfection process describes the process of killing, deactivating or removing pathogenic
microorganisms resulting in the termination of their reproduction and growth. When
microorganisms are not eliminated from drinking water, then the consumption of this water will
cause those drinking the water to fall in. Some of the chemicals used during the disinfection
process include alcohols, felons, bromine chloride, halogens, chlorine dioxide, and chlorine [19].
Dissolution (Hydrolysis) of Chlorine Gas in Water
The hydrolysis product formed from the reaction between water and chloride molecule is
hypochlorous acid (HOCL). The equation of the reaction is as shown below:
Cl2 + H2O ⇋ HOCl + H+ + Cl-
Hypochlorous acid can undergo the degree of ionization as follow depending on the temperature
and pH of water:
HOCl ⇋ H+ + OCl-
The hypochlorite ion and hypochlorous acid concentrations are about 25oC and pH 7.5.
[19]
17. Free Available Chlorine and Residual Chlorine
During the dissolution of sodium hypochlorite (NaOCl) in water, hypochlorite ion and
hypochlorous acid are produced. These hypochlorite ions are referred to as Free Chlorine while
the amount of chlorine that remains in water after the process of chlorination is referred as
Residual Chloride. Free Chlorine is a portion of total residual chlorine, the percentage of
dissolved chlorine gas that is not bonded to any other water reactants [20].
Dual Media Filters are majorly used for the removal of suspended solids and turbidity solids with
an effective of 0.01mm to 0.02mm. The dual media filter provides very effective removal of
particles under the conditions of a high rate of filtration. They effectively operate at a loading
rate of 0.0086m/h to 0.017m/h, which is equivalent to 0.86l/h to 1.7l/h of the filter area.
16. Disinfection Process
The disinfection process describes the process of killing, deactivating or removing pathogenic
microorganisms resulting in the termination of their reproduction and growth. When
microorganisms are not eliminated from drinking water, then the consumption of this water will
cause those drinking the water to fall in. Some of the chemicals used during the disinfection
process include alcohols, felons, bromine chloride, halogens, chlorine dioxide, and chlorine [19].
Dissolution (Hydrolysis) of Chlorine Gas in Water
The hydrolysis product formed from the reaction between water and chloride molecule is
hypochlorous acid (HOCL). The equation of the reaction is as shown below:
Cl2 + H2O ⇋ HOCl + H+ + Cl-
Hypochlorous acid can undergo the degree of ionization as follow depending on the temperature
and pH of water:
HOCl ⇋ H+ + OCl-
The hypochlorite ion and hypochlorous acid concentrations are about 25oC and pH 7.5.
[19]
17. Free Available Chlorine and Residual Chlorine
During the dissolution of sodium hypochlorite (NaOCl) in water, hypochlorite ion and
hypochlorous acid are produced. These hypochlorite ions are referred to as Free Chlorine while
the amount of chlorine that remains in water after the process of chlorination is referred as
Residual Chloride. Free Chlorine is a portion of total residual chlorine, the percentage of
dissolved chlorine gas that is not bonded to any other water reactants [20].
Class Revision-Midterm 12
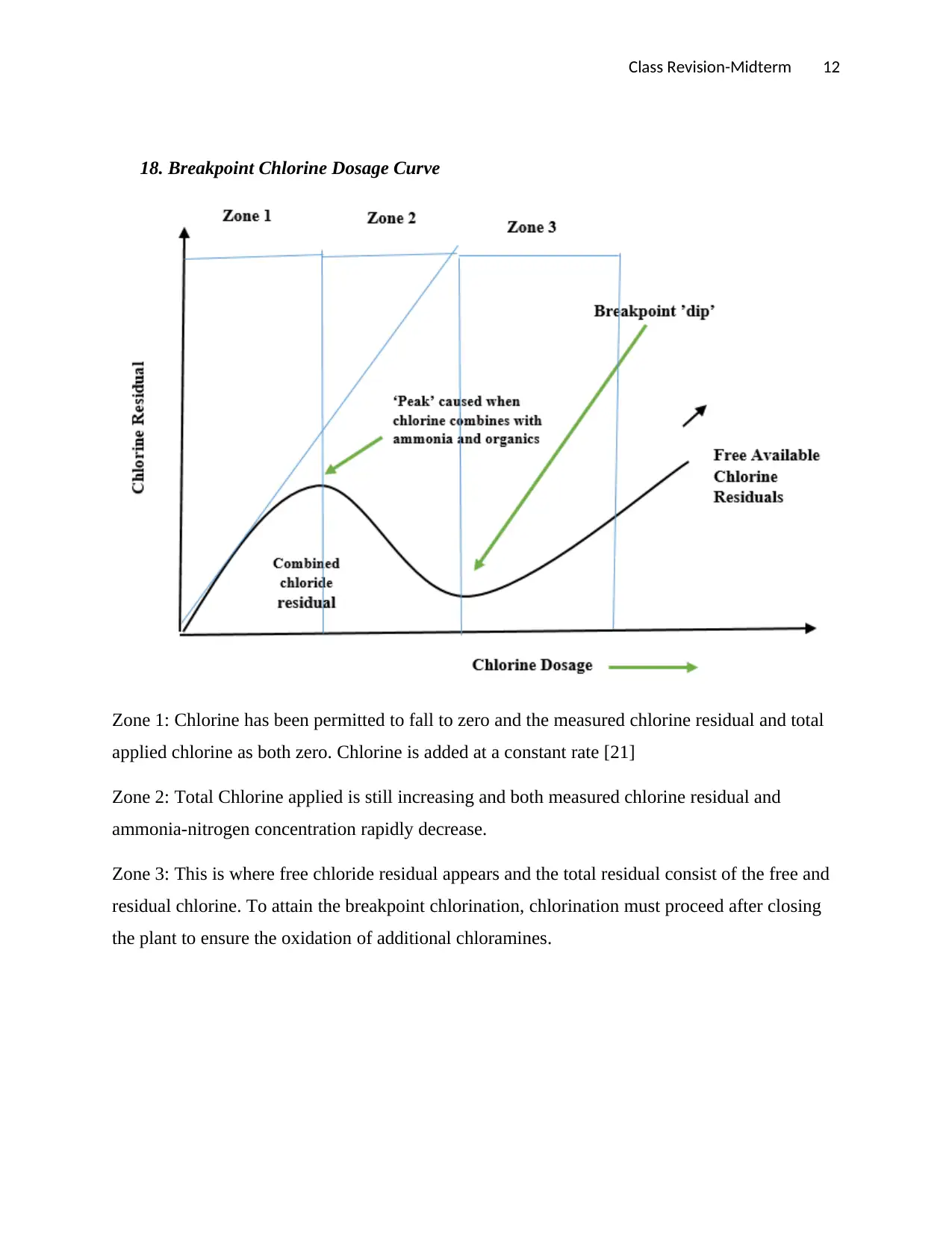
18. Breakpoint Chlorine Dosage Curve
Zone 1: Chlorine has been permitted to fall to zero and the measured chlorine residual and total
applied chlorine as both zero. Chlorine is added at a constant rate [21]
Zone 2: Total Chlorine applied is still increasing and both measured chlorine residual and
ammonia-nitrogen concentration rapidly decrease.
Zone 3: This is where free chloride residual appears and the total residual consist of the free and
residual chlorine. To attain the breakpoint chlorination, chlorination must proceed after closing
the plant to ensure the oxidation of additional chloramines.
18. Breakpoint Chlorine Dosage Curve
Zone 1: Chlorine has been permitted to fall to zero and the measured chlorine residual and total
applied chlorine as both zero. Chlorine is added at a constant rate [21]
Zone 2: Total Chlorine applied is still increasing and both measured chlorine residual and
ammonia-nitrogen concentration rapidly decrease.
Zone 3: This is where free chloride residual appears and the total residual consist of the free and
residual chlorine. To attain the breakpoint chlorination, chlorination must proceed after closing
the plant to ensure the oxidation of additional chloramines.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.