Chemical Energetics Report: Endothermic and Exothermic Reactions
VerifiedAdded on 2020/06/04
|20
|3698
|384
Report
AI Summary
This report delves into the core concepts of chemical energetics, exploring enthalpy changes in various chemical reactions. It begins with an experimental analysis of a displacement reaction between zinc and copper sulfate, detailing the methodology, results, and calculations used to determine enthalpy changes, identifying it as exothermic. The report then covers key terms such as exothermic and endothermic reactions, and standard enthalpy changes. It further examines Hess's Law and its application in enthalpy cycles, including combustion reactions and the calculation of enthalpies for different reactions. Bond enthalpy data is used to develop enthalpy cycles and calculate bond dissociation enthalpies. The report includes examples such as the combustion of ethane, ethene, and hydrogen and also covers the combustion of ethanol. Finally, the report touches upon concepts like lattice enthalpy, enthalpy of ion hydration, and the relationship between enthalpy changes and solubility. The report provides comprehensive calculations and explanations, making it a valuable resource for understanding chemical energetics.

Chemical Energetics
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
Measuring Enthalpy Changes that take place in Endothermic and exothermic reactions through
experimentation................................................................................................................................1
Abstract:.................................................................................................................................1
Key terms:..............................................................................................................................1
Background Information:.......................................................................................................1
Hypothesis:.............................................................................................................................2
Apparatus Required:...............................................................................................................2
Assessment of Risk:...............................................................................................................2
Methods:.................................................................................................................................2
Results:...................................................................................................................................2
Calculations:...........................................................................................................................3
Conclusions:...........................................................................................................................3
Evaluation:..............................................................................................................................4
TASK 1............................................................................................................................................5
1.1 Exothermic and endothermic reactions ...........................................................................5
1.2, 1.3 Standard enthalpy changes .......................................................................................5
TASK 2............................................................................................................................................6
2.1 Conservation of Energy and Hess's Law (1)....................................................................6
2.2 Enthalpy cycles and use Hess's Law ...............................................................................6
2 a) Combustion reactions......................................................................................................6
2 b) Enthalpy of combustion..................................................................................................6
3) Enthalpy for given reaction................................................................................................7
4) Enthalpy of formation for NH3..........................................................................................7
5) Enthalpy of combustion.....................................................................................................8
6) Combustion of ethanol.......................................................................................................9
2.3 Developing enthalpy cycles using bond enthalpy data ....................................................9
7) Data ...................................................................................................................................9
8) Enthalpy for N-H bond.....................................................................................................10
9) Average bond dissociation enthalpy for C-Cl bond.........................................................11
Measuring Enthalpy Changes that take place in Endothermic and exothermic reactions through
experimentation................................................................................................................................1
Abstract:.................................................................................................................................1
Key terms:..............................................................................................................................1
Background Information:.......................................................................................................1
Hypothesis:.............................................................................................................................2
Apparatus Required:...............................................................................................................2
Assessment of Risk:...............................................................................................................2
Methods:.................................................................................................................................2
Results:...................................................................................................................................2
Calculations:...........................................................................................................................3
Conclusions:...........................................................................................................................3
Evaluation:..............................................................................................................................4
TASK 1............................................................................................................................................5
1.1 Exothermic and endothermic reactions ...........................................................................5
1.2, 1.3 Standard enthalpy changes .......................................................................................5
TASK 2............................................................................................................................................6
2.1 Conservation of Energy and Hess's Law (1)....................................................................6
2.2 Enthalpy cycles and use Hess's Law ...............................................................................6
2 a) Combustion reactions......................................................................................................6
2 b) Enthalpy of combustion..................................................................................................6
3) Enthalpy for given reaction................................................................................................7
4) Enthalpy of formation for NH3..........................................................................................7
5) Enthalpy of combustion.....................................................................................................8
6) Combustion of ethanol.......................................................................................................9
2.3 Developing enthalpy cycles using bond enthalpy data ....................................................9
7) Data ...................................................................................................................................9
8) Enthalpy for N-H bond.....................................................................................................10
9) Average bond dissociation enthalpy for C-Cl bond.........................................................11

10) Bond dissociation enthalpy............................................................................................11
11) Bond dissociation enthalpy ...........................................................................................12
12) Energy level diagram.....................................................................................................12
3.1 Energy level and reaction pathway diagrams for the combustion of ethane (13)..........14
4.1 Lattice enthalpy (a & b)..................................................................................................15
4.2 Enthalpy of ion hydration (c)........................................................................................15
4.3 Solutions (d)...................................................................................................................15
4.4 Relations between enthalpy changes and solubility of a substance...............................16
REFERENCES..............................................................................................................................18
11) Bond dissociation enthalpy ...........................................................................................12
12) Energy level diagram.....................................................................................................12
3.1 Energy level and reaction pathway diagrams for the combustion of ethane (13)..........14
4.1 Lattice enthalpy (a & b)..................................................................................................15
4.2 Enthalpy of ion hydration (c)........................................................................................15
4.3 Solutions (d)...................................................................................................................15
4.4 Relations between enthalpy changes and solubility of a substance...............................16
REFERENCES..............................................................................................................................18
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Measuring Enthalpy Changes that take place in Endothermic and exothermic
reactions through experimentation
Abstract:
The experiment which takes place between copper sulphate solution and zinc is
conducted for gaining insight regarding the enthalpy changes which take place in a displacement
reaction. The changes will be tracked using a calorimeter so that necessary enthalpy moderations
can be obtained.
Key terms:
The major terms related with this experimentation are enthalpy changes, displacement
reaction, calorimeter and exothermic/endothermic.
Background Information:
There are certain changes which take place when reactions take place. These changes
occur in the bond structure especially in case of displacement reactions. As per the Universal law
on Conservation of Energy, the energy cannot be created or destroyed. It can only be transferred
from one form to another. Hence, in every chemical reaction, the heat transfer takes place either
in the form of absorption or release. Copper (II) sulphate is a blue colour solution in aqueous
form. When standard conditions prevail i.e. pressure is 1 atm and temperature is 298K, then
enthalpy changes are considered to be standard.
The exothermic reactions are one in which energy is released while endothermic
reactions are based on absorption of energy. In terms of products and reactants, when energy is
quite less when reaction completes as compared to the initial state, then it is said to have
provided the energy to surroundings. However, in case of endothermic reactions the energy is
high in completion of products as compared to the initial state. This depicts that reaction is
endothermic in nature.
Bond dissociation enthalpy is derived from the energy which is required for breaking a
bond between two components. In case of this experiment, the ionic bond shared between
Copper and sulphate ions. When Zinc is introduced in this solution, then displacement takes
place and new product formed is zinc sulphate in a colourless solution.
1
reactions through experimentation
Abstract:
The experiment which takes place between copper sulphate solution and zinc is
conducted for gaining insight regarding the enthalpy changes which take place in a displacement
reaction. The changes will be tracked using a calorimeter so that necessary enthalpy moderations
can be obtained.
Key terms:
The major terms related with this experimentation are enthalpy changes, displacement
reaction, calorimeter and exothermic/endothermic.
Background Information:
There are certain changes which take place when reactions take place. These changes
occur in the bond structure especially in case of displacement reactions. As per the Universal law
on Conservation of Energy, the energy cannot be created or destroyed. It can only be transferred
from one form to another. Hence, in every chemical reaction, the heat transfer takes place either
in the form of absorption or release. Copper (II) sulphate is a blue colour solution in aqueous
form. When standard conditions prevail i.e. pressure is 1 atm and temperature is 298K, then
enthalpy changes are considered to be standard.
The exothermic reactions are one in which energy is released while endothermic
reactions are based on absorption of energy. In terms of products and reactants, when energy is
quite less when reaction completes as compared to the initial state, then it is said to have
provided the energy to surroundings. However, in case of endothermic reactions the energy is
high in completion of products as compared to the initial state. This depicts that reaction is
endothermic in nature.
Bond dissociation enthalpy is derived from the energy which is required for breaking a
bond between two components. In case of this experiment, the ionic bond shared between
Copper and sulphate ions. When Zinc is introduced in this solution, then displacement takes
place and new product formed is zinc sulphate in a colourless solution.
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hypothesis:
The estimated changes in enthalpy indicate prevalence of exothermic reaction.
Apparatus Required:
The apparatus or equipment which are required for this experiment include Zn powder
and Copper (II) Sulphate solution. A calorimeter with thermometer is also required for
conducting this experiment. Stop clock and weighing scale is required for making appropriate
measures.
Assessment of Risk:
Risks involved in this reaction include spilling of the solution when temperature shall
rise. Hence, use of lab coat is preferable for avoiding any sort of such accidents.
Methods:
After measuring 6g of zinc powder accurately over the weighing scale, the thermometer
is placed in the cup as a medium of stirring and measuring the temperature on a continuous basis.
Solution present in the cup is aqueous copper sulphate in which 2.5g of CuSO4 was mixed with
water in limited amount through measuring cylinder. Soon after introducing zinc powder to the
solution, the cup is sealed with a lid and stirred occasionally for 9 minutes. This time is tracked
on a stop clock which helps in recording the changes that take place in the chemical reaction.
Results:
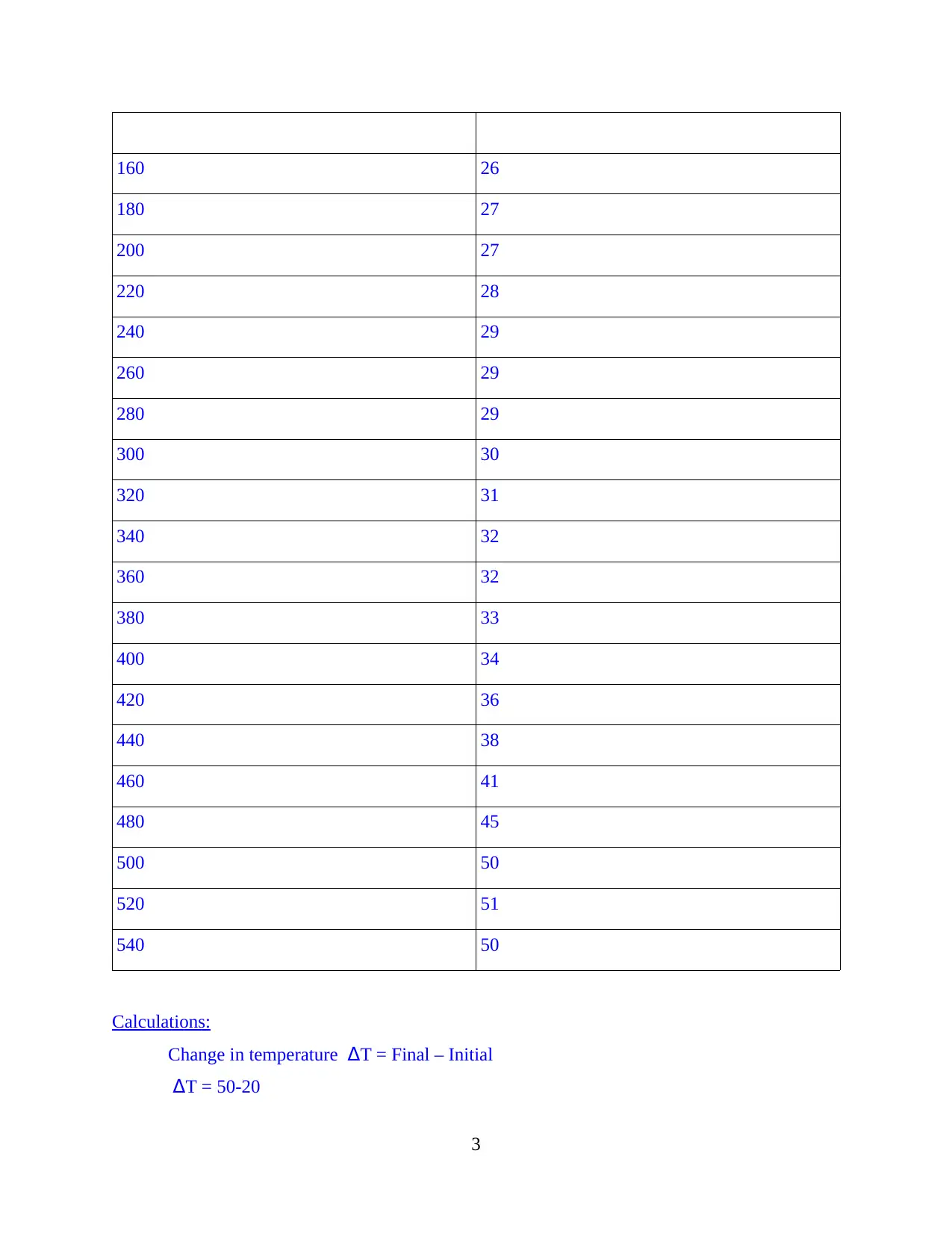
Following are the results obtained through this experimentation:
Time elapsing (s) Temperature recordings
0 20
20 21
40 23
60 25
80 25
100 26
120 26
140 26
2
The estimated changes in enthalpy indicate prevalence of exothermic reaction.
Apparatus Required:
The apparatus or equipment which are required for this experiment include Zn powder
and Copper (II) Sulphate solution. A calorimeter with thermometer is also required for
conducting this experiment. Stop clock and weighing scale is required for making appropriate
measures.
Assessment of Risk:
Risks involved in this reaction include spilling of the solution when temperature shall
rise. Hence, use of lab coat is preferable for avoiding any sort of such accidents.
Methods:
After measuring 6g of zinc powder accurately over the weighing scale, the thermometer
is placed in the cup as a medium of stirring and measuring the temperature on a continuous basis.
Solution present in the cup is aqueous copper sulphate in which 2.5g of CuSO4 was mixed with
water in limited amount through measuring cylinder. Soon after introducing zinc powder to the
solution, the cup is sealed with a lid and stirred occasionally for 9 minutes. This time is tracked
on a stop clock which helps in recording the changes that take place in the chemical reaction.
Results:
Following are the results obtained through this experimentation:
Time elapsing (s) Temperature recordings
0 20
20 21
40 23
60 25
80 25
100 26
120 26
140 26
2
160 26
180 27
200 27
220 28
240 29
260 29
280 29
300 30
320 31
340 32
360 32
380 33
400 34
420 36
440 38
460 41
480 45
500 50
520 51
540 50
Calculations:
Change in temperature ΔT = Final – Initial
ΔT = 50-20
3
180 27
200 27
220 28
240 29
260 29
280 29
300 30
320 31
340 32
360 32
380 33
400 34
420 36
440 38
460 41
480 45
500 50
520 51
540 50
Calculations:
Change in temperature ΔT = Final – Initial
ΔT = 50-20
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ΔT = 30°C
H = Heat generated = m.c. ΔT
Here, m = mass of the material = 25g
c = specific heat capacity = 4.18
H = 25* 4.18 * 30
H = 3135 kJ/mol
Conclusions:
From the above experimentation, it is inferred that when zinc reacts with copper(II)
sulphate solution, then displacement takes place followed by release of energy and completion of
an exothermic reaction. The temperature difference between initial and final time is ΔT = 50-20
ΔT = 30°C.
And the heat generated is H = 3135 kJ/mol
Evaluation:
There are possibilities of occurrence of flaws or defects in the calorimeter which
indicates that temperature changes might get affected. However, when using a bomb calorimeter
there is an automatic stirrer involved which results in greater changes in the temperature. The
heat loss which occurs on a minute scale also affects the readings and results on a minor scale.
On the other hand, the material insulation of the cup is also a major factor which regulates heat
flow. But polystyrene cups are more favourable as compared to other material equipment.
4
H = Heat generated = m.c. ΔT
Here, m = mass of the material = 25g
c = specific heat capacity = 4.18
H = 25* 4.18 * 30
H = 3135 kJ/mol
Conclusions:
From the above experimentation, it is inferred that when zinc reacts with copper(II)
sulphate solution, then displacement takes place followed by release of energy and completion of
an exothermic reaction. The temperature difference between initial and final time is ΔT = 50-20
ΔT = 30°C.
And the heat generated is H = 3135 kJ/mol
Evaluation:
There are possibilities of occurrence of flaws or defects in the calorimeter which
indicates that temperature changes might get affected. However, when using a bomb calorimeter
there is an automatic stirrer involved which results in greater changes in the temperature. The
heat loss which occurs on a minute scale also affects the readings and results on a minor scale.
On the other hand, the material insulation of the cup is also a major factor which regulates heat
flow. But polystyrene cups are more favourable as compared to other material equipment.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TASK 1
1.1 Exothermic and endothermic reactions
Every chemical reaction involves certain transformation of energy. The release or
absorption of energy is categorised as exothermic and endothermic reactions. These can be
understood in a better form with following examples:
Example 1: Reaction between zinc and copper sulphate
Zn(s) + Cu2+(aq) + SO42-(aq) → Cu(s) + Zn2+(aq) + SO42- (aq)
Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The above equation is an ionic equation when zinc and copper(II) sulphate react. The
oxidation of Zn ions and reduction of Cu ions depicts that this is a redox reaction. The reactivity
of Zinc is more than Copper. In this reaction, the aqueous solution of copper sulphate has less
reactive metal Cu which is displaced by Zn with release of energy (Fernández and Bickelhaupt,
2014). The overall temperature of this solution increases which indicates it is a exothermic
reaction.
Example 2: Reaction of ethanoic acid and calcium carbonate
The following equation depicts the respective reaction :
2CH3COOH + CaCO3 → Ca(CH3COO)2 + CO2 + H2O
The carbonate ions that are present in calcium carbonate react with acid (ethanoic acid)
which is followed by production of carbon dioxide. The proton ions released from acid form
water and the calcium ions combine with remaining ions of acid. Since, acetic or ethanoic acid is
quite weak, there is absorption of energy. The overall reaction is endothermic.
1.2, 1.3 Standard enthalpy changes
The change in temperature during the reaction of zinc and copper sulphate takes place
from 20°C to 50°C. The aqueous solution turns out to be colourless and the colour of zinc
powder changes to orange. On the other hand, a fizzing sound is generated when ethanoic acid
and calcium carbonate react. This is because of generation of carbon dioxide.
5
1.1 Exothermic and endothermic reactions
Every chemical reaction involves certain transformation of energy. The release or
absorption of energy is categorised as exothermic and endothermic reactions. These can be
understood in a better form with following examples:
Example 1: Reaction between zinc and copper sulphate
Zn(s) + Cu2+(aq) + SO42-(aq) → Cu(s) + Zn2+(aq) + SO42- (aq)
Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The above equation is an ionic equation when zinc and copper(II) sulphate react. The
oxidation of Zn ions and reduction of Cu ions depicts that this is a redox reaction. The reactivity
of Zinc is more than Copper. In this reaction, the aqueous solution of copper sulphate has less
reactive metal Cu which is displaced by Zn with release of energy (Fernández and Bickelhaupt,
2014). The overall temperature of this solution increases which indicates it is a exothermic
reaction.
Example 2: Reaction of ethanoic acid and calcium carbonate
The following equation depicts the respective reaction :
2CH3COOH + CaCO3 → Ca(CH3COO)2 + CO2 + H2O
The carbonate ions that are present in calcium carbonate react with acid (ethanoic acid)
which is followed by production of carbon dioxide. The proton ions released from acid form
water and the calcium ions combine with remaining ions of acid. Since, acetic or ethanoic acid is
quite weak, there is absorption of energy. The overall reaction is endothermic.
1.2, 1.3 Standard enthalpy changes
The change in temperature during the reaction of zinc and copper sulphate takes place
from 20°C to 50°C. The aqueous solution turns out to be colourless and the colour of zinc
powder changes to orange. On the other hand, a fizzing sound is generated when ethanoic acid
and calcium carbonate react. This is because of generation of carbon dioxide.
5

TASK 2
2.1 Conservation of Energy and Hess's Law (1)
According to the Hess's Law, irrespective of the number of steps involved in a chemical
reaction or process, the heat evolved or absorbed is absolutely the same. This is supportive for
the rule of conservation of energy that is universally applicable (Göstl, Senf and Hecht, 2014). It
determines that energy is neither created or destroyed during a particular process. It is only
conserved.
The Hess's Law is important in developing an understanding for the chemical process
because it helps in developing calculations for enthalpies regarding each reaction. The control
mechanisms can be activated for those reactions which have imbalanced flow of energy.
2.2 Enthalpy cycles and use Hess's Law
2 a) Combustion reactions
(I) Combustion of ethane
ethane + oxygen → water + carbon dioxide
C2H6 + 7/2O2 → 3H2O + 2CO2
(II) Combustion of ethene
C2H4 (g) + 3O2 (g)→ 2CO2 (g) + 2H2O (g)
(III) Combustion of hydrogen
2H2 (g)+ O2 (g)→ 2H2O (l)
2 b) Enthalpy of combustion
The enthalpy cycles and enthalpy of combustion for each case produced above is given as
follows:
Combustion of ethane
ΔcH° = 2ΔfH° (CO2) + 3ΔfH°(H2O) – ΔfH°(C2H6)
The value of ΔfH° (O2) is zero
ΔcH° = 2 * (-393) + 3 * (-285.5) - (-83.6)
ΔcH° = -786 – 856.5 + 83.6
ΔcH° = 83.6 – 1642.5
6
2.1 Conservation of Energy and Hess's Law (1)
According to the Hess's Law, irrespective of the number of steps involved in a chemical
reaction or process, the heat evolved or absorbed is absolutely the same. This is supportive for
the rule of conservation of energy that is universally applicable (Göstl, Senf and Hecht, 2014). It
determines that energy is neither created or destroyed during a particular process. It is only
conserved.
The Hess's Law is important in developing an understanding for the chemical process
because it helps in developing calculations for enthalpies regarding each reaction. The control
mechanisms can be activated for those reactions which have imbalanced flow of energy.
2.2 Enthalpy cycles and use Hess's Law
2 a) Combustion reactions
(I) Combustion of ethane
ethane + oxygen → water + carbon dioxide
C2H6 + 7/2O2 → 3H2O + 2CO2
(II) Combustion of ethene
C2H4 (g) + 3O2 (g)→ 2CO2 (g) + 2H2O (g)
(III) Combustion of hydrogen
2H2 (g)+ O2 (g)→ 2H2O (l)
2 b) Enthalpy of combustion
The enthalpy cycles and enthalpy of combustion for each case produced above is given as
follows:
Combustion of ethane
ΔcH° = 2ΔfH° (CO2) + 3ΔfH°(H2O) – ΔfH°(C2H6)
The value of ΔfH° (O2) is zero
ΔcH° = 2 * (-393) + 3 * (-285.5) - (-83.6)
ΔcH° = -786 – 856.5 + 83.6
ΔcH° = 83.6 – 1642.5
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
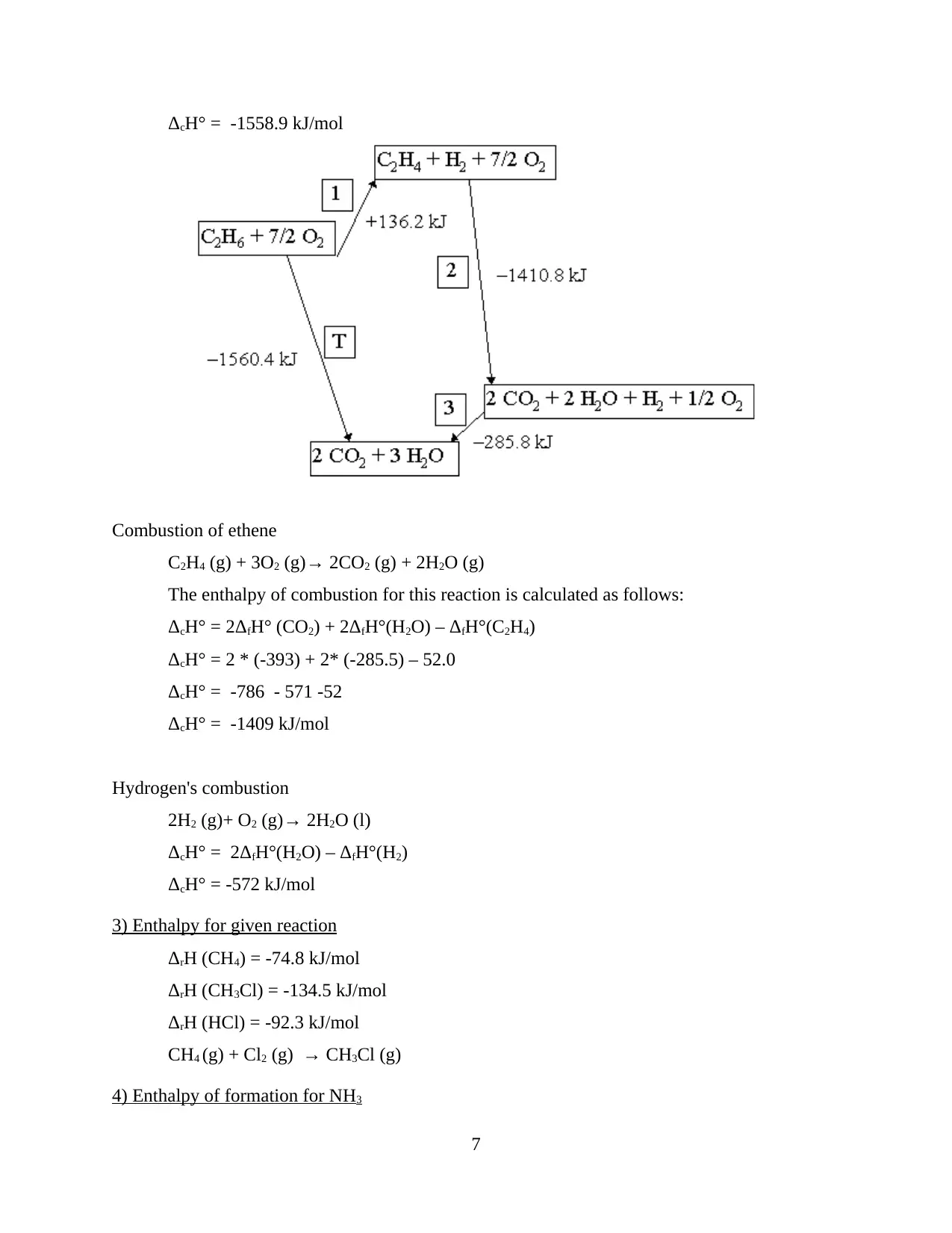
ΔcH° = -1558.9 kJ/mol
Combustion of ethene
C2H4 (g) + 3O2 (g)→ 2CO2 (g) + 2H2O (g)
The enthalpy of combustion for this reaction is calculated as follows:
ΔcH° = 2ΔfH° (CO2) + 2ΔfH°(H2O) – ΔfH°(C2H4)
ΔcH° = 2 * (-393) + 2* (-285.5) – 52.0
ΔcH° = -786 - 571 -52
ΔcH° = -1409 kJ/mol
Hydrogen's combustion
2H2 (g)+ O2 (g)→ 2H2O (l)
ΔcH° = 2ΔfH°(H2O) – ΔfH°(H2)
ΔcH° = -572 kJ/mol
3) Enthalpy for given reaction
ΔrH (CH4) = -74.8 kJ/mol
ΔrH (CH3Cl) = -134.5 kJ/mol
ΔrH (HCl) = -92.3 kJ/mol
CH4 (g) + Cl2 (g) → CH3Cl (g)
4) Enthalpy of formation for NH3
7
Combustion of ethene
C2H4 (g) + 3O2 (g)→ 2CO2 (g) + 2H2O (g)
The enthalpy of combustion for this reaction is calculated as follows:
ΔcH° = 2ΔfH° (CO2) + 2ΔfH°(H2O) – ΔfH°(C2H4)
ΔcH° = 2 * (-393) + 2* (-285.5) – 52.0
ΔcH° = -786 - 571 -52
ΔcH° = -1409 kJ/mol
Hydrogen's combustion
2H2 (g)+ O2 (g)→ 2H2O (l)
ΔcH° = 2ΔfH°(H2O) – ΔfH°(H2)
ΔcH° = -572 kJ/mol
3) Enthalpy for given reaction
ΔrH (CH4) = -74.8 kJ/mol
ΔrH (CH3Cl) = -134.5 kJ/mol
ΔrH (HCl) = -92.3 kJ/mol
CH4 (g) + Cl2 (g) → CH3Cl (g)
4) Enthalpy of formation for NH3
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4NH3(g) + 3O2(g) → 2N2(g) + 6H2O(l), H = -1530kJmol-1 .. (i)
H2(g) + 1/2O2(g) →H2O(l), H = -288 kJmol-1 … (ii)
Flip and divide the equation (I) by 4,
New Reaction will be
NH3(g) + 3/4O2(g)→ ½ N2(g) + 3/2 H2O(l) H = +382.5 kJmol-1 … (iii)
Multiply equation (ii) by 2
2H2(g) + O2(g) →2H2O(l), H = -564 kJmol-1 … (iv)
Now we will comment on the oxygen part of the given equation
For equation (i), put 3/4O2 on the right side
For equation (ii), put O2 on the right side
the new equations will become
NH3(g) + 3/4O2(g) → ½ N2(g) + 3/2 H2O(l) + 3/4O2(g H = +382.5 kJmol-1
2H2(g) + O2(g)→ 2H2O(l),+ O2(g) H = -564 kJmol-1
By adding both the equations and cancel; out the oxygen present, the new equation will be;
NH3(g) + 2H2(g)→ ½ N2(g) + 3/2 H2O(l) + 2H2O(l),
NH3(g) + 2H2(g) → ½ N2(g) + 4H2O
The enthalpy will become
H = - 184.5 kJmol-1
5) Enthalpy of combustion
The combustion of gaseous diborane has following formula:
B2H6 (g) + 3O2 → B2O3 (s) + 3H2O
ΔcH° is the enthalpy of combustion for this combustion process.
Putting the values from the given data
ΔcH° = -1270 -(3*242) -31.4
8
H2(g) + 1/2O2(g) →H2O(l), H = -288 kJmol-1 … (ii)
Flip and divide the equation (I) by 4,
New Reaction will be
NH3(g) + 3/4O2(g)→ ½ N2(g) + 3/2 H2O(l) H = +382.5 kJmol-1 … (iii)
Multiply equation (ii) by 2
2H2(g) + O2(g) →2H2O(l), H = -564 kJmol-1 … (iv)
Now we will comment on the oxygen part of the given equation
For equation (i), put 3/4O2 on the right side
For equation (ii), put O2 on the right side
the new equations will become
NH3(g) + 3/4O2(g) → ½ N2(g) + 3/2 H2O(l) + 3/4O2(g H = +382.5 kJmol-1
2H2(g) + O2(g)→ 2H2O(l),+ O2(g) H = -564 kJmol-1
By adding both the equations and cancel; out the oxygen present, the new equation will be;
NH3(g) + 2H2(g)→ ½ N2(g) + 3/2 H2O(l) + 2H2O(l),
NH3(g) + 2H2(g) → ½ N2(g) + 4H2O
The enthalpy will become
H = - 184.5 kJmol-1
5) Enthalpy of combustion
The combustion of gaseous diborane has following formula:
B2H6 (g) + 3O2 → B2O3 (s) + 3H2O
ΔcH° is the enthalpy of combustion for this combustion process.
Putting the values from the given data
ΔcH° = -1270 -(3*242) -31.4
8

ΔcH° = -1301.4 – 726
ΔcH° = -2027.4
The combustion of gaseous benzene is provided as follows:
C6H6 (g) + 7.5O2 (g) → 6CO2 (g) + 3H2O (g)
ΔcH° is the combustion enthalpy for the above formula is calculated as follows:
ΔcH° = 6 * (-393) + 3 (-242) - (+83.9)
ΔcH° = - 2358 -726 – 83.9
ΔcH° = -3167.9 kJ/mol
6) Combustion of ethanol
The combustion of ethanol in the presence of oxygen produces water and carbon-dioxide.
Furthermore, the energy produced = 1368 kJ/mol.
ΔfH° = Enthalpy of formation of ethanol
ΔfH° = -393.7 kJ/mol for CO2
ΔfH° = -285.9 kJ/mol for H2O
ΔcH° = 1368 kJ/mol
The chemical reaction is produced as follows:
C2H5OH (g) + 3O2 (g) → 2CO2 (g) + 3H2O (g)
ΔcH° = 1368 = 2 ΔfH°(CO2 ) + 3 ΔfH° (H2O) - ΔfH° (C2H5OH)
1368 = 2 (-393.7) + 3(-285.9) - ΔfH° ( C2H5OH)
ΔfH° ( C2H5OH) = 3013.1 kJ/mol
2.3 Developing enthalpy cycles using bond enthalpy data
7) Data
a) The H for the following reaction: CH4(g) + Br2(g) → CH3Br(g) + HBr(g)
H is the enthalpy which has been utilised for breaking the bonds between methane and
Br-Br
H = 193+ 413
H = 606 kJ/mol
b) The H for the following reaction: CH4(g) + F2(g)→ CH3F(g) + HF(g)
H = is the energy that has been required for breaking the bonds between C-H and F-F .
H = 413 + 158
9
ΔcH° = -2027.4
The combustion of gaseous benzene is provided as follows:
C6H6 (g) + 7.5O2 (g) → 6CO2 (g) + 3H2O (g)
ΔcH° is the combustion enthalpy for the above formula is calculated as follows:
ΔcH° = 6 * (-393) + 3 (-242) - (+83.9)
ΔcH° = - 2358 -726 – 83.9
ΔcH° = -3167.9 kJ/mol
6) Combustion of ethanol
The combustion of ethanol in the presence of oxygen produces water and carbon-dioxide.
Furthermore, the energy produced = 1368 kJ/mol.
ΔfH° = Enthalpy of formation of ethanol
ΔfH° = -393.7 kJ/mol for CO2
ΔfH° = -285.9 kJ/mol for H2O
ΔcH° = 1368 kJ/mol
The chemical reaction is produced as follows:
C2H5OH (g) + 3O2 (g) → 2CO2 (g) + 3H2O (g)
ΔcH° = 1368 = 2 ΔfH°(CO2 ) + 3 ΔfH° (H2O) - ΔfH° (C2H5OH)
1368 = 2 (-393.7) + 3(-285.9) - ΔfH° ( C2H5OH)
ΔfH° ( C2H5OH) = 3013.1 kJ/mol
2.3 Developing enthalpy cycles using bond enthalpy data
7) Data
a) The H for the following reaction: CH4(g) + Br2(g) → CH3Br(g) + HBr(g)
H is the enthalpy which has been utilised for breaking the bonds between methane and
Br-Br
H = 193+ 413
H = 606 kJ/mol
b) The H for the following reaction: CH4(g) + F2(g)→ CH3F(g) + HF(g)
H = is the energy that has been required for breaking the bonds between C-H and F-F .
H = 413 + 158
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 20
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
