Chemical Process Design Solution: Chemical Engineering Assignment
VerifiedAdded on 2020/05/04
|9
|1432
|34
Homework Assignment
AI Summary
This document presents a detailed solution to a chemical process design assignment, covering various aspects of chemical engineering. The solution begins with an overview of the chemical process design hierarchy, discussing the five levels of process design as defined by James Douglas, including mass balance, equipment selection, and economic analysis. The solution then delves into specific problems, such as calculating selectivity in reactions, designing a heat exchanger including LMTD and area calculations, and analyzing the block flow diagram for MTBE production, including material balance calculations around the reactor. Finally, the document provides a solution involving the calculation of mass balance in a chemical reaction. The document includes references to relevant literature and provides a comprehensive understanding of chemical process design principles and their application in solving practical problems.
Chemical Process Design
1 | P a g e
Chemical Engineering
1 | P a g e
Chemical Engineering
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemical Process Design
Contents
Solution 1.........................................................................................................................................3
Solution 1a)..................................................................................................................................3
Solution 1bi)................................................................................................................................4
Solution 1bii)...............................................................................................................................4
Solution 2i)......................................................................................................................................5
Solution 2ii).....................................................................................................................................6
Solution 3).......................................................................................................................................7
Solution 3a)..................................................................................................................................7
Solution 3b).................................................................................................................................7
Solution 4).......................................................................................................................................9
References......................................................................................................................................10
2 | P a g e
Contents
Solution 1.........................................................................................................................................3
Solution 1a)..................................................................................................................................3
Solution 1bi)................................................................................................................................4
Solution 1bii)...............................................................................................................................4
Solution 2i)......................................................................................................................................5
Solution 2ii).....................................................................................................................................6
Solution 3).......................................................................................................................................7
Solution 3a)..................................................................................................................................7
Solution 3b).................................................................................................................................7
Solution 4).......................................................................................................................................9
References......................................................................................................................................10
2 | P a g e

Chemical Process Design
Solution 1
Solution 1a)
For the purpose of chemical process most of the time we are following design and hierarchy
given by James Douglas, which divides their process into five steps. These five steps also called
level, which can be described as follows.
Level 1-
It has very basic process information; the need of the knowledge for this level is on very basic
level especially about main reaction that will occur in the process. The side reactions are not
considered in this process. Only mass balance is necessary to perform in input and output of the
reaction. The economic analysis can also be performed on the basis of simple reaction and
market value of these basic chemicals.
Level 2-
In this level user sets the basic input and output process of the flow of reaction. The end result is
assumed as perfect separation system, Recycling of reactant is also can be performed as desired.
The alternative of the process is considered as reversible by-product. The equipment selection is
based on the concept of oversizing of reaction, due to this reason additional cost and utility is
required in this step.
Level 3-
This is the level where structured and strict flow sheet decision are being finalised. The user has
to decide number and amount of reactor of reactor that can be feasible for particular reaction.
The separation of component can also be decided at this level. The operating condition of reactor
is also being considered, weather it will adiabatic or any other process. The potential of
economic feasibility is clearly visible because all components are visible in this stage.
Level 4-
This level basically involves the design of separation system as per phase and states of process
streams available. The design of vapour or liquid recovery also considered as condensation,
absorption, and membrane process are commonly used. The sequencing of column is a problem
in this level. The remove the product and recycle streams as distillates. A detailed economic
analysis is possible in this level; we can take the various equipment cost into capital equipment
costs and projected maintenance and replacement cost.
Level 5-
This level basically include the design of heat exchanger, temperature enthalpy curve can be
calculated at this level for each process stream. The use of information system and computer is
3 | P a g e
Solution 1
Solution 1a)
For the purpose of chemical process most of the time we are following design and hierarchy
given by James Douglas, which divides their process into five steps. These five steps also called
level, which can be described as follows.
Level 1-
It has very basic process information; the need of the knowledge for this level is on very basic
level especially about main reaction that will occur in the process. The side reactions are not
considered in this process. Only mass balance is necessary to perform in input and output of the
reaction. The economic analysis can also be performed on the basis of simple reaction and
market value of these basic chemicals.
Level 2-
In this level user sets the basic input and output process of the flow of reaction. The end result is
assumed as perfect separation system, Recycling of reactant is also can be performed as desired.
The alternative of the process is considered as reversible by-product. The equipment selection is
based on the concept of oversizing of reaction, due to this reason additional cost and utility is
required in this step.
Level 3-
This is the level where structured and strict flow sheet decision are being finalised. The user has
to decide number and amount of reactor of reactor that can be feasible for particular reaction.
The separation of component can also be decided at this level. The operating condition of reactor
is also being considered, weather it will adiabatic or any other process. The potential of
economic feasibility is clearly visible because all components are visible in this stage.
Level 4-
This level basically involves the design of separation system as per phase and states of process
streams available. The design of vapour or liquid recovery also considered as condensation,
absorption, and membrane process are commonly used. The sequencing of column is a problem
in this level. The remove the product and recycle streams as distillates. A detailed economic
analysis is possible in this level; we can take the various equipment cost into capital equipment
costs and projected maintenance and replacement cost.
Level 5-
This level basically include the design of heat exchanger, temperature enthalpy curve can be
calculated at this level for each process stream. The use of information system and computer is
3 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemical Process Design
also used in the form of various software packages, e.g., FLOWTRAN, which is very useful in
finding the heating and cooling requirement of the process (WESTERBERG, 2008).
Solution 1bi)
As given in question,
Feed -> Product with r1 = k1CFa1
Feed -> By-product with r2 = k2CFa2
The selectivity of the reaction = rate of formation of desired product / Rate of formation of
undesired product
= k1CFa1 / k2CFa2 ……………(i)
Suppose α is the solution of above equation,
The selectivity for the above reaction depends upon the concentration, if concentration of
product feed is increases the selectivity will increases,
Therefore to keep maximising the desirable product we have to increase the concentration of
product and decrease the concentration of by-product.
Solution 1bii)
For the case of parallel reaction as given equation, it depends upon multiple factors but as
given in equation, the increase and decrease in the selectivity will be referred as follows
If a1 > a2 Selectivity increases as conversion increases
If a1 < a2 Selectivity decreases as conversion increases
The initial setting for reactor, the conversion setting of the be in the order of 95% (Smith, 2016).
4 | P a g e
also used in the form of various software packages, e.g., FLOWTRAN, which is very useful in
finding the heating and cooling requirement of the process (WESTERBERG, 2008).
Solution 1bi)
As given in question,
Feed -> Product with r1 = k1CFa1
Feed -> By-product with r2 = k2CFa2
The selectivity of the reaction = rate of formation of desired product / Rate of formation of
undesired product
= k1CFa1 / k2CFa2 ……………(i)
Suppose α is the solution of above equation,
The selectivity for the above reaction depends upon the concentration, if concentration of
product feed is increases the selectivity will increases,
Therefore to keep maximising the desirable product we have to increase the concentration of
product and decrease the concentration of by-product.
Solution 1bii)
For the case of parallel reaction as given equation, it depends upon multiple factors but as
given in equation, the increase and decrease in the selectivity will be referred as follows
If a1 > a2 Selectivity increases as conversion increases
If a1 < a2 Selectivity decreases as conversion increases
The initial setting for reactor, the conversion setting of the be in the order of 95% (Smith, 2016).
4 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemical Process Design
Solution 2i)
Flow rate = 50000 kg /hr = 10.0 kg /s 13.88
Temperature difference = 70-10 = 60
Pressure drop = 0.7 bar
Pressure drop at nozzle = Pn = 0.25
ID = 15
Od = 19 Length = 4094 mm triangular
Average temperature for water = T = 283 + 343/2 = 313 oK
Specific heat of water at 313K = 4.179 kJ/Kg.K
Now total heat generated will be = 3480 KJ
Hence the total heat generated = 3480 kJ
Thermal conductivity of water 1000 w/m2 oC and
Temperature for heated steam = 2.7 bar the temperature of heated steam = 273+130 = 403 K
Now I have to calculate
LMTD= ( T −T 2 )−(T −T 1)
ln¿ ¿
Putting the value and solving
LMTD = 86 o C = 359 K
5 | P a g e
Solution 2i)
Flow rate = 50000 kg /hr = 10.0 kg /s 13.88
Temperature difference = 70-10 = 60
Pressure drop = 0.7 bar
Pressure drop at nozzle = Pn = 0.25
ID = 15
Od = 19 Length = 4094 mm triangular
Average temperature for water = T = 283 + 343/2 = 313 oK
Specific heat of water at 313K = 4.179 kJ/Kg.K
Now total heat generated will be = 3480 KJ
Hence the total heat generated = 3480 kJ
Thermal conductivity of water 1000 w/m2 oC and
Temperature for heated steam = 2.7 bar the temperature of heated steam = 273+130 = 403 K
Now I have to calculate
LMTD= ( T −T 2 )−(T −T 1)
ln¿ ¿
Putting the value and solving
LMTD = 86 o C = 359 K
5 | P a g e
Chemical Process Design
Solution 2ii)
Now I must calculate total area
A = 3480 x 1000 / 1000 x 86 = 40.04 m2
No of tube for heat exchanger = 40.04 / 3.14 * 19 x10-3 x 4.094 = 163
Since the given number of tube is 110, in this condition the heat exchanger is not suitable for
duty when water inside the tube
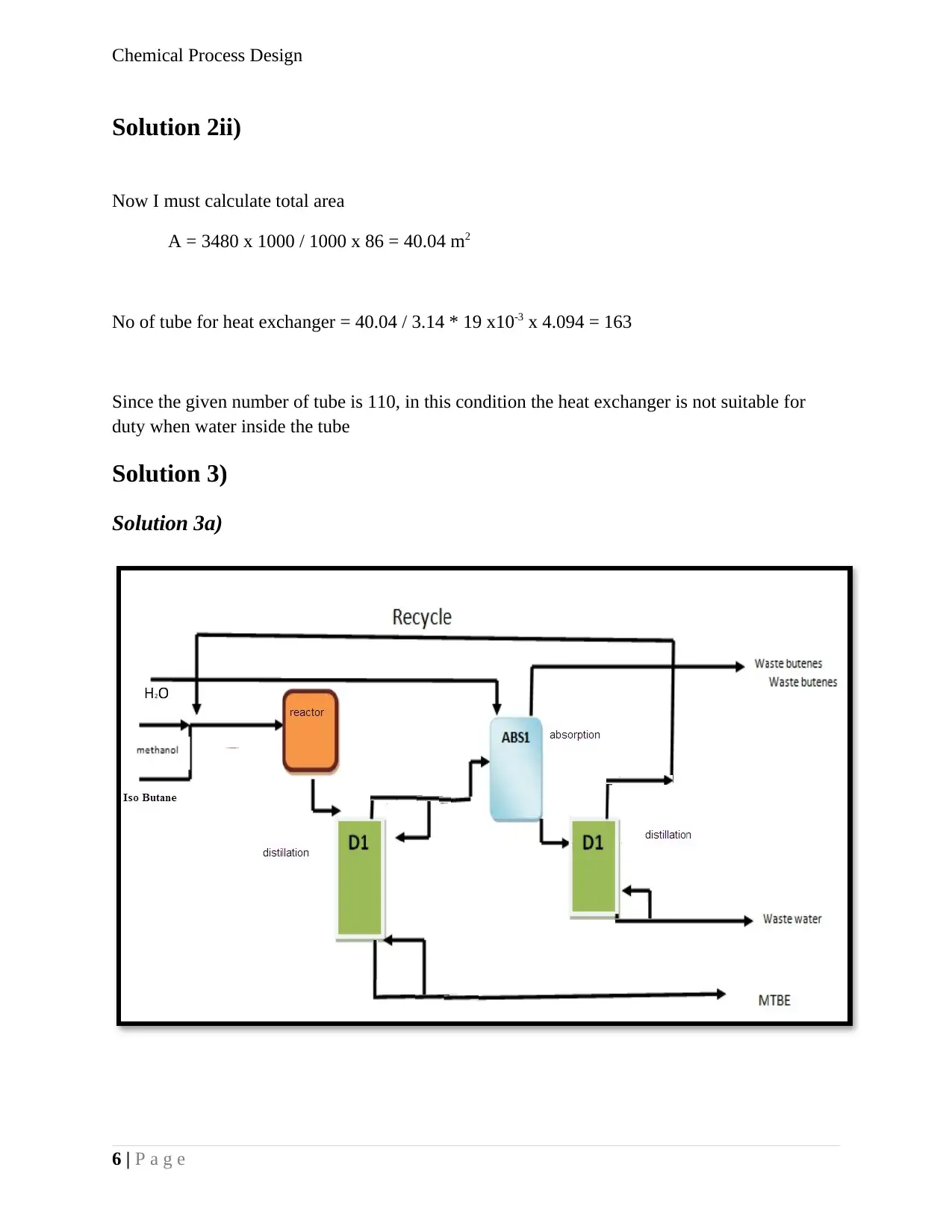
Solution 3)
Solution 3a)
6 | P a g e
Solution 2ii)
Now I must calculate total area
A = 3480 x 1000 / 1000 x 86 = 40.04 m2
No of tube for heat exchanger = 40.04 / 3.14 * 19 x10-3 x 4.094 = 163
Since the given number of tube is 110, in this condition the heat exchanger is not suitable for
duty when water inside the tube
Solution 3)
Solution 3a)
6 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemical Process Design
Solution 3b)
Since we know that,
Materialout = Materialin + Generation – Consumption - accumulation
As given in question,
The calculation around the reactor as follows
As given, 100 mole/h of isobutene feed to the reactor
In this condition,
Material balance around reactor
Input – reacted = output
Input = 100,
According to feed stock composition
n-butane, = 2, Butence-1 = 31, Butane-2 = 18 and Isobutene = 49%
Amount of Iso-butane = 100
Then Butane-2 = 100/49*18 36.73 moles/h
Butence-1 = 100/49*31 = 63.26 moles/h
N-butane = 100/49*2 = 4.1 moles/h
At entry level
Methanol / Iso = 2
Then methanol = 100*2 = 200 moles / hour
Then total input = 200+4.1+63.26+36.73+100 = 404.09
Since as per reaction, 2 moles of reactant produce one mole of MTBE
Then total MTBE = 404.09/2 = 101.045 moles of MTBE
7 | P a g e
Figure 1-Block Flow diagram of MTBE
Solution 3b)
Since we know that,
Materialout = Materialin + Generation – Consumption - accumulation
As given in question,
The calculation around the reactor as follows
As given, 100 mole/h of isobutene feed to the reactor
In this condition,
Material balance around reactor
Input – reacted = output
Input = 100,
According to feed stock composition
n-butane, = 2, Butence-1 = 31, Butane-2 = 18 and Isobutene = 49%
Amount of Iso-butane = 100
Then Butane-2 = 100/49*18 36.73 moles/h
Butence-1 = 100/49*31 = 63.26 moles/h
N-butane = 100/49*2 = 4.1 moles/h
At entry level
Methanol / Iso = 2
Then methanol = 100*2 = 200 moles / hour
Then total input = 200+4.1+63.26+36.73+100 = 404.09
Since as per reaction, 2 moles of reactant produce one mole of MTBE
Then total MTBE = 404.09/2 = 101.045 moles of MTBE
7 | P a g e
Figure 1-Block Flow diagram of MTBE
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemical Process Design
Then % of Pure MTBE = 101.045 * 0.97 = 98.013 moles
Unreacted methanol and C4 compound = 101.045 – 98.013 = 3.03102 moles / h Ans
Solution 4)
As given in question, Mole % of N2 = 33.4 % for 100 moles (33.4 moles)
Mole % of H2 = 66.4 % for 100 moles (66.4moles)
Argon = 0.2% = 0.2 moles in 100 moles of feed
Suppose x is the recycle of N2
Conversion pass in the reactor for ammonia = 100 – 11.8 (Which is going to recycle) = 88.2%
Purging of Argon = PAr = 0.05P
The amount of nitrogen reacting = .882 * 33.4 = 29.4588 moles
Amount of H2 reaction = 29.4588 * 3 = 88.3764
Reaction of N2 and H2 produces = 2 * 29.4588 = 58.92 moles of NH3.
11.8 moles of ammonia will contain = 11.8 x 33.4 = 3.9412 N2
11.8 moles of ammonia will contain = 11.8 x 66.4 = 7.8352 H2
11.8 moles of ammonia will contain = 11.8 x 0.2 = 0.0236 Ar
Now we must compute the mass balance
For Argon, 0.0236 = ARR + APP = ARR + 0.050P
For Nitrogen, 3.9412 = NRR + NPP ……….(i)
For Hydrogen, 7.8532 = HRR + HPP ………..(ii)
For recycled stream, AR + NR + HR = 1 replacing I with value of ZP
Replacing the all value with P value
3.9412 = (88.2 – P)-(58.56 – HPP)-(0.2 – 0.005P) + (1-0.005P – YP)P
After simplifying the equation
-0.005P2 + 0.005P = 0 then P = 1 mol
8 | P a g e
Then % of Pure MTBE = 101.045 * 0.97 = 98.013 moles
Unreacted methanol and C4 compound = 101.045 – 98.013 = 3.03102 moles / h Ans
Solution 4)
As given in question, Mole % of N2 = 33.4 % for 100 moles (33.4 moles)
Mole % of H2 = 66.4 % for 100 moles (66.4moles)
Argon = 0.2% = 0.2 moles in 100 moles of feed
Suppose x is the recycle of N2
Conversion pass in the reactor for ammonia = 100 – 11.8 (Which is going to recycle) = 88.2%
Purging of Argon = PAr = 0.05P
The amount of nitrogen reacting = .882 * 33.4 = 29.4588 moles
Amount of H2 reaction = 29.4588 * 3 = 88.3764
Reaction of N2 and H2 produces = 2 * 29.4588 = 58.92 moles of NH3.
11.8 moles of ammonia will contain = 11.8 x 33.4 = 3.9412 N2
11.8 moles of ammonia will contain = 11.8 x 66.4 = 7.8352 H2
11.8 moles of ammonia will contain = 11.8 x 0.2 = 0.0236 Ar
Now we must compute the mass balance
For Argon, 0.0236 = ARR + APP = ARR + 0.050P
For Nitrogen, 3.9412 = NRR + NPP ……….(i)
For Hydrogen, 7.8532 = HRR + HPP ………..(ii)
For recycled stream, AR + NR + HR = 1 replacing I with value of ZP
Replacing the all value with P value
3.9412 = (88.2 – P)-(58.56 – HPP)-(0.2 – 0.005P) + (1-0.005P – YP)P
After simplifying the equation
-0.005P2 + 0.005P = 0 then P = 1 mol
8 | P a g e

Chemical Process Design
In this condition the Purged flow rate = 11.2 +1 = 12.2 moles Ans
References
Kutz, M, 2007, Environmentally Conscious Materials and Chemicals Processing. 2nd ed.
Toronto: John WIley and SOns.
Smith, R, 2016. Chemical Process Design and Integration. 2nd ed. West sussex: John Wiley.
WESTERBERG, D, &., 2008. Cephda: Chemical engineering procee hierarchical design with
ascend. Department of Chemical Engineering and Engineering Design Research Center,
140(92), pp. 1-16.
9 | P a g e
In this condition the Purged flow rate = 11.2 +1 = 12.2 moles Ans
References
Kutz, M, 2007, Environmentally Conscious Materials and Chemicals Processing. 2nd ed.
Toronto: John WIley and SOns.
Smith, R, 2016. Chemical Process Design and Integration. 2nd ed. West sussex: John Wiley.
WESTERBERG, D, &., 2008. Cephda: Chemical engineering procee hierarchical design with
ascend. Department of Chemical Engineering and Engineering Design Research Center,
140(92), pp. 1-16.
9 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



