University Chemistry: Chemical Spectroscopy NMR Assignment, Year 1
VerifiedAdded on 2022/08/13
|5
|1124
|51
Homework Assignment
AI Summary
This document provides a comprehensive solution to a chemistry assignment focusing on NMR (Nuclear Magnetic Resonance) spectroscopy, particularly proton NMR (1H-NMR). It begins with an explanation of the fundamental principles of NMR spectroscopy, including the magnetic properties of hydrogen nuclei and their behavior in an external magnetic field. The assignment then delves into interpreting NMR spectra, explaining how the number of peaks, peak splitting, and integration factors provide information about the structure of a compound. It includes detailed answers to questions about chemical shifts, the role of tetramethylsilane (TMS) as a reference, and how to analyze the NMR spectra of specific molecules like ethanol. The solutions also explain how to assign chemical shifts, splitting patterns, and integration factors to different fragments of molecules. References to key texts on molecular spectroscopy are also included.

Answers to LO3
Ans 1: The spectroscopy which deals with the spin of the hydrogen nucleus leading to the
absorption of the electromagnetic radiation by the hydrogen nucleus within the frequency
range of 4 to 900 MHz is termed as proton nuclear magnetic resonance (NMR) spectroscopy.
The principles of the NMR spectroscopy are as follows (Banwell & McCash, 1995):
a. The spinning of the hydrogen nucleus produces a magnetic field.
b. The spinning of the nuclei takes place randomly in all directions in absence of a
magnetic field.
c. The spinning of the nuclei becomes systematic in presence of an external magnetic
field.
d. The hydrogen nuclei align themselves to the external magnetic field in two ways: the
spin of the nuclei may be parallel () to the external magnetic field or antiparallel ()
to the external magnetic field (Gunther, 2013).
e. Thus there occurs a difference of energy level between the spin energy state and
spin energy state due to absorption of energy.
f. The absorbed energy in the form of radiofrequency is released when the spin reverts
back to the ground state (Keeler, 2010).
g. The emitted radiofrequency energy () is depicted as the signal of the NMR spectrum
which is directly proportional to the applied field strength as shown by the following
equation:
ν= γ Bo
2 π
Where Bo = magnetic field experienced by the proton due to external magnetic field
Ans 1: The spectroscopy which deals with the spin of the hydrogen nucleus leading to the
absorption of the electromagnetic radiation by the hydrogen nucleus within the frequency
range of 4 to 900 MHz is termed as proton nuclear magnetic resonance (NMR) spectroscopy.
The principles of the NMR spectroscopy are as follows (Banwell & McCash, 1995):
a. The spinning of the hydrogen nucleus produces a magnetic field.
b. The spinning of the nuclei takes place randomly in all directions in absence of a
magnetic field.
c. The spinning of the nuclei becomes systematic in presence of an external magnetic
field.
d. The hydrogen nuclei align themselves to the external magnetic field in two ways: the
spin of the nuclei may be parallel () to the external magnetic field or antiparallel ()
to the external magnetic field (Gunther, 2013).
e. Thus there occurs a difference of energy level between the spin energy state and
spin energy state due to absorption of energy.
f. The absorbed energy in the form of radiofrequency is released when the spin reverts
back to the ground state (Keeler, 2010).
g. The emitted radiofrequency energy () is depicted as the signal of the NMR spectrum
which is directly proportional to the applied field strength as shown by the following
equation:
ν= γ Bo
2 π
Where Bo = magnetic field experienced by the proton due to external magnetic field
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

= magnetogyric ratio
Ans 2a: The number of peaks exhibited by a compound in 1H NMR spectrum shows that the
hydrogen attached to the carbon are in different environments. The peaks gets further split
into doublet, triplet and so on due to the influence of the protons on the neighbouring carbon
atom.
Ans 2b: The area beneath the peak in 1H NMR spectrum represents the number of hydrogens
attached to the particular carbon atom. The ratio of the area is measured to find the number of
hydrogen atoms bonded to a particular carbon atom. The ratio under the peaks is termed as
the integration factors.
Ans 2c: The molecule used as a reference is tetramethylsilane which is also known as TMS in
NMR (Morisson et al, 2011). The TMS signal is displayed at a value of 0 ppm. This
molecule is taken as the standard and all other NMR values are measured in reference to this
molecule. There are 12 hydrogen atoms in the TMS molecule which are all in equal
environment. Thus, the TMS molecule displays a strong signal at = 0 ppm. This signal
appears on the extreme right of the scale and all other NMR signals appear on the left side
to this signal.
Ans 2d: Chemical shift denotes that the hydrogens attached to the carbon atoms are at
different environments. The value of the chemical shift depends on the atoms attached to the
carbon containing the hydrogen atom which exhibits the NMR peak. When the hydrogen
atoms are in different environment, they require different magnitude of the external magnetic
field to resonate with the specific radio frequency. Thus the hydrogens display peaks at
different values. The carbon attached to an electronegative atom will show high chemical
shift as compared to another carbon bonded to non polar atoms.
Ans 2a: The number of peaks exhibited by a compound in 1H NMR spectrum shows that the
hydrogen attached to the carbon are in different environments. The peaks gets further split
into doublet, triplet and so on due to the influence of the protons on the neighbouring carbon
atom.
Ans 2b: The area beneath the peak in 1H NMR spectrum represents the number of hydrogens
attached to the particular carbon atom. The ratio of the area is measured to find the number of
hydrogen atoms bonded to a particular carbon atom. The ratio under the peaks is termed as
the integration factors.
Ans 2c: The molecule used as a reference is tetramethylsilane which is also known as TMS in
NMR (Morisson et al, 2011). The TMS signal is displayed at a value of 0 ppm. This
molecule is taken as the standard and all other NMR values are measured in reference to this
molecule. There are 12 hydrogen atoms in the TMS molecule which are all in equal
environment. Thus, the TMS molecule displays a strong signal at = 0 ppm. This signal
appears on the extreme right of the scale and all other NMR signals appear on the left side
to this signal.
Ans 2d: Chemical shift denotes that the hydrogens attached to the carbon atoms are at
different environments. The value of the chemical shift depends on the atoms attached to the
carbon containing the hydrogen atom which exhibits the NMR peak. When the hydrogen
atoms are in different environment, they require different magnitude of the external magnetic
field to resonate with the specific radio frequency. Thus the hydrogens display peaks at
different values. The carbon attached to an electronegative atom will show high chemical
shift as compared to another carbon bonded to non polar atoms.
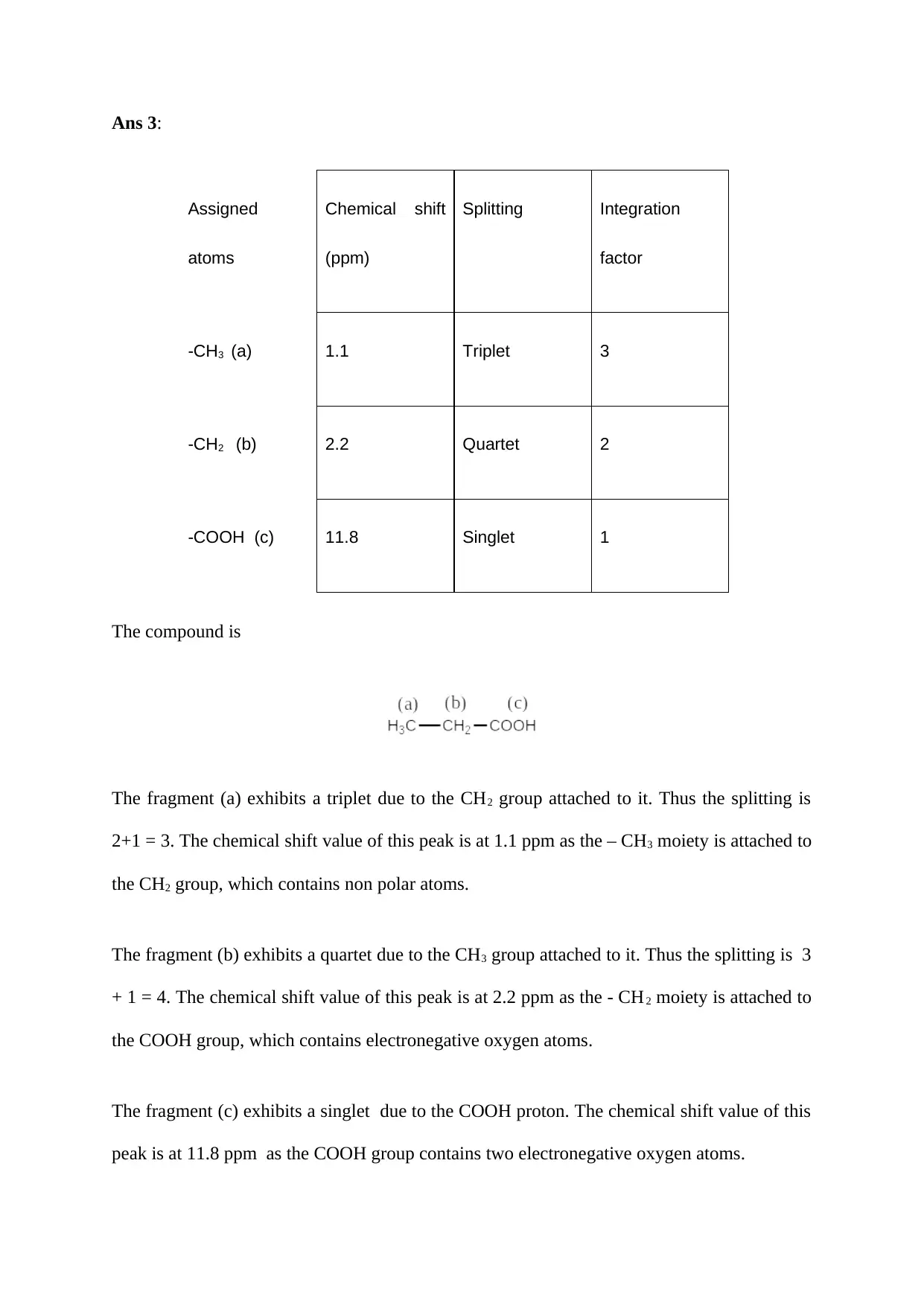
Ans 3:
Assigned
atoms
Chemical shift
(ppm)
Splitting Integration
factor
-CH3 (a) 1.1 Triplet 3
-CH2 (b) 2.2 Quartet 2
-COOH (c) 11.8 Singlet 1
The compound is
The fragment (a) exhibits a triplet due to the CH2 group attached to it. Thus the splitting is
2+1 = 3. The chemical shift value of this peak is at 1.1 ppm as the – CH3 moiety is attached to
the CH2 group, which contains non polar atoms.
The fragment (b) exhibits a quartet due to the CH3 group attached to it. Thus the splitting is 3
+ 1 = 4. The chemical shift value of this peak is at 2.2 ppm as the - CH2 moiety is attached to
the COOH group, which contains electronegative oxygen atoms.
The fragment (c) exhibits a singlet due to the COOH proton. The chemical shift value of this
peak is at 11.8 ppm as the COOH group contains two electronegative oxygen atoms.
Assigned
atoms
Chemical shift
(ppm)
Splitting Integration
factor
-CH3 (a) 1.1 Triplet 3
-CH2 (b) 2.2 Quartet 2
-COOH (c) 11.8 Singlet 1
The compound is
The fragment (a) exhibits a triplet due to the CH2 group attached to it. Thus the splitting is
2+1 = 3. The chemical shift value of this peak is at 1.1 ppm as the – CH3 moiety is attached to
the CH2 group, which contains non polar atoms.
The fragment (b) exhibits a quartet due to the CH3 group attached to it. Thus the splitting is 3
+ 1 = 4. The chemical shift value of this peak is at 2.2 ppm as the - CH2 moiety is attached to
the COOH group, which contains electronegative oxygen atoms.
The fragment (c) exhibits a singlet due to the COOH proton. The chemical shift value of this
peak is at 11.8 ppm as the COOH group contains two electronegative oxygen atoms.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
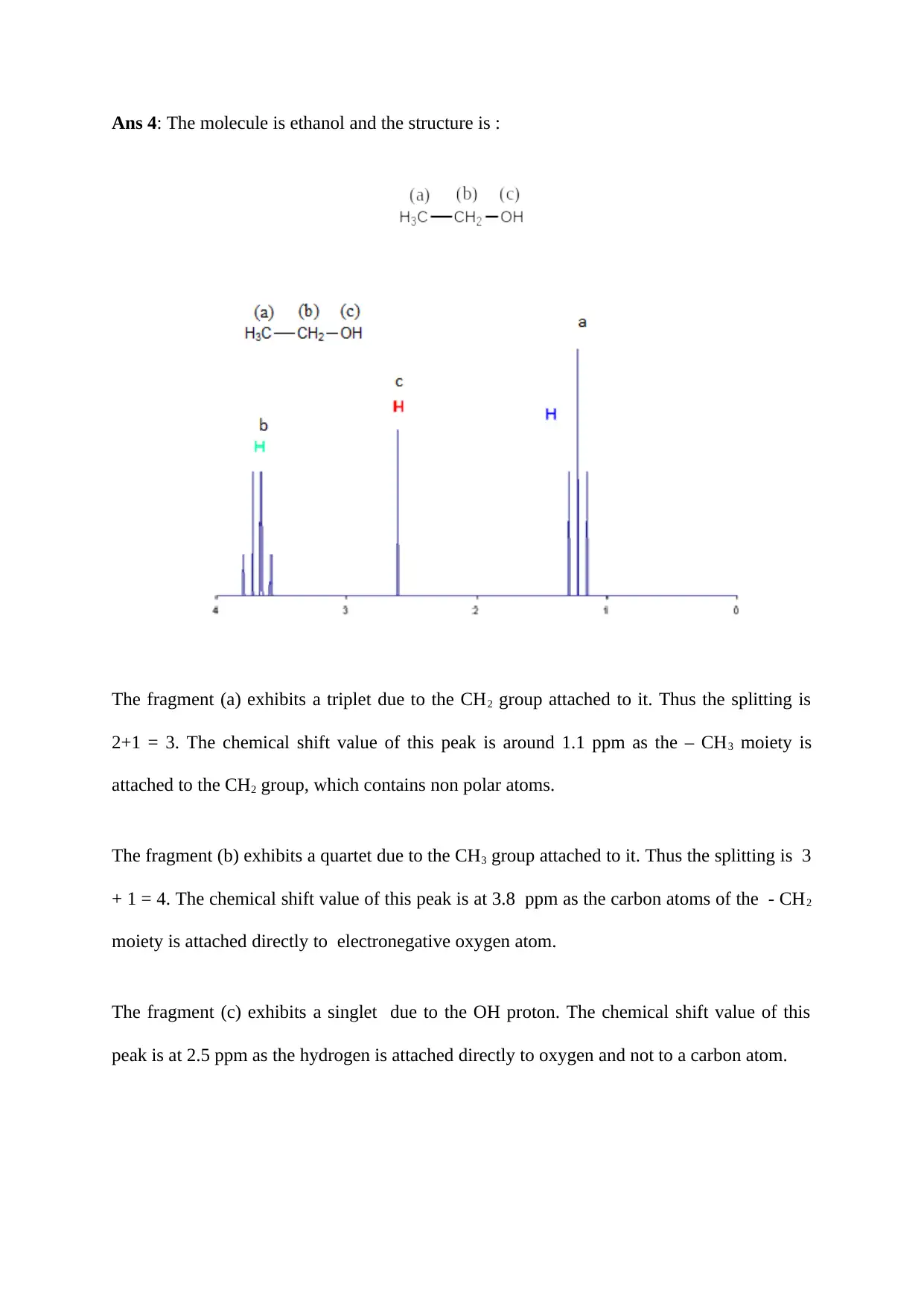
Ans 4: The molecule is ethanol and the structure is :
The fragment (a) exhibits a triplet due to the CH2 group attached to it. Thus the splitting is
2+1 = 3. The chemical shift value of this peak is around 1.1 ppm as the – CH3 moiety is
attached to the CH2 group, which contains non polar atoms.
The fragment (b) exhibits a quartet due to the CH3 group attached to it. Thus the splitting is 3
+ 1 = 4. The chemical shift value of this peak is at 3.8 ppm as the carbon atoms of the - CH2
moiety is attached directly to electronegative oxygen atom.
The fragment (c) exhibits a singlet due to the OH proton. The chemical shift value of this
peak is at 2.5 ppm as the hydrogen is attached directly to oxygen and not to a carbon atom.
The fragment (a) exhibits a triplet due to the CH2 group attached to it. Thus the splitting is
2+1 = 3. The chemical shift value of this peak is around 1.1 ppm as the – CH3 moiety is
attached to the CH2 group, which contains non polar atoms.
The fragment (b) exhibits a quartet due to the CH3 group attached to it. Thus the splitting is 3
+ 1 = 4. The chemical shift value of this peak is at 3.8 ppm as the carbon atoms of the - CH2
moiety is attached directly to electronegative oxygen atom.
The fragment (c) exhibits a singlet due to the OH proton. The chemical shift value of this
peak is at 2.5 ppm as the hydrogen is attached directly to oxygen and not to a carbon atom.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Reference
Banwell, C. N. & McCash, E. M. (1995) Fundamentals of molecular spectroscopy. 5th
edition ,Tata McGraw- Hill.
Keeler, J ( 2010 ) Understanding NMR spectroscopy. 2nd edition, John Wiley & Sons Ltd
publications.
Gunther, H (2013) NMR spectroscopy: Basic principles, concepts and applications in
chemistry, 3rd edition, Wiley VCH Verlag.
Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. (2011) Organic Chemistry, 7th edition,
Pearson press.
Book: print
Author/Editor (if it is an editor always put (ed.) after the name)
(Year of publication)
Title (this should be in italics)
Series title and number (if part of a series)
Edition (if not the first edition)
Place of publication (if there is more than one place listed, use the first named)
Publisher
Simons, N. E., Menzies, B. & Matthews, M. (2001) A Short Course in Soil and Rock Slope
Engineering. London, Thomas Telford Publishing.
Banwell, C. N. & McCash, E. M. (1995) Fundamentals of molecular spectroscopy. 5th
edition ,Tata McGraw- Hill.
Keeler, J ( 2010 ) Understanding NMR spectroscopy. 2nd edition, John Wiley & Sons Ltd
publications.
Gunther, H (2013) NMR spectroscopy: Basic principles, concepts and applications in
chemistry, 3rd edition, Wiley VCH Verlag.
Morrison, R. T., Boyd, R. N. & Bhattacharjee, S. K. (2011) Organic Chemistry, 7th edition,
Pearson press.
Book: print
Author/Editor (if it is an editor always put (ed.) after the name)
(Year of publication)
Title (this should be in italics)
Series title and number (if part of a series)
Edition (if not the first edition)
Place of publication (if there is more than one place listed, use the first named)
Publisher
Simons, N. E., Menzies, B. & Matthews, M. (2001) A Short Course in Soil and Rock Slope
Engineering. London, Thomas Telford Publishing.
1 out of 5
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



