Chemistry 350, Organic Chemistry I: Assignment 2 Covering Units 10-16
VerifiedAdded on 2022/09/21
|11
|1215
|21
Homework Assignment
AI Summary
This document provides comprehensive solutions for Chemistry 350 Assignment 2, focusing on organic chemistry concepts. The assignment addresses IUPAC nomenclature, requiring students to name compounds with stereochemistry. It includes multi-step synthesis problems starting from butane and benzene, demonstrating knowledge of reaction mechanisms. Further, the assignment encompasses predicting NMR spectra and analyzing compounds using mass spectrometry, 1H NMR and IR spectroscopy. The document provides detailed answers to each question, including chemical equations, structural representations, and spectroscopic data interpretations, reflecting a strong understanding of organic chemistry principles.
Answers to the given questions
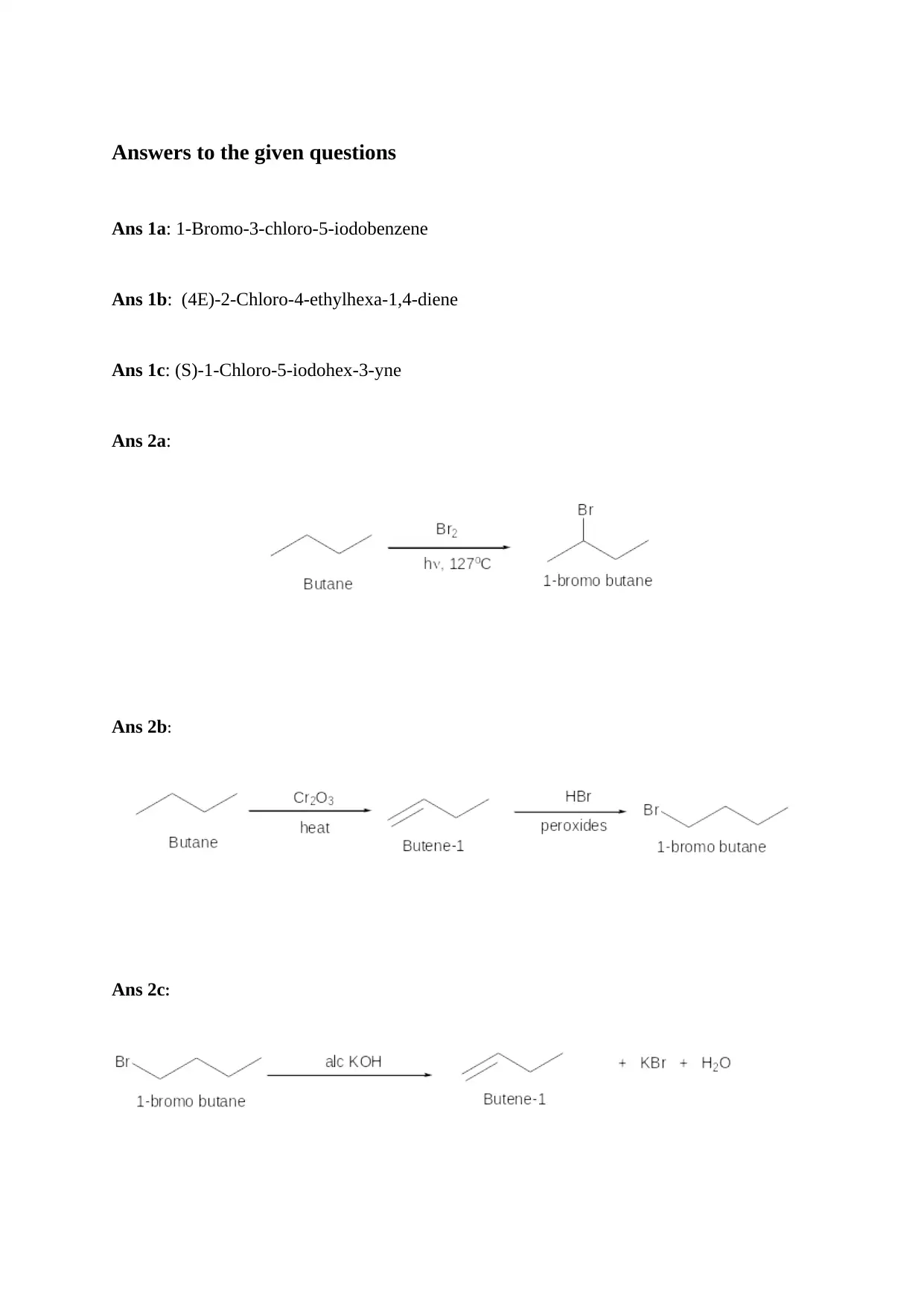
Ans 1a: 1-Bromo-3-chloro-5-iodobenzene
Ans 1b: (4E)-2-Chloro-4-ethylhexa-1,4-diene
Ans 1c: (S)-1-Chloro-5-iodohex-3-yne
Ans 2a:
Ans 2b:
Ans 2c:
Ans 1a: 1-Bromo-3-chloro-5-iodobenzene
Ans 1b: (4E)-2-Chloro-4-ethylhexa-1,4-diene
Ans 1c: (S)-1-Chloro-5-iodohex-3-yne
Ans 2a:
Ans 2b:
Ans 2c:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
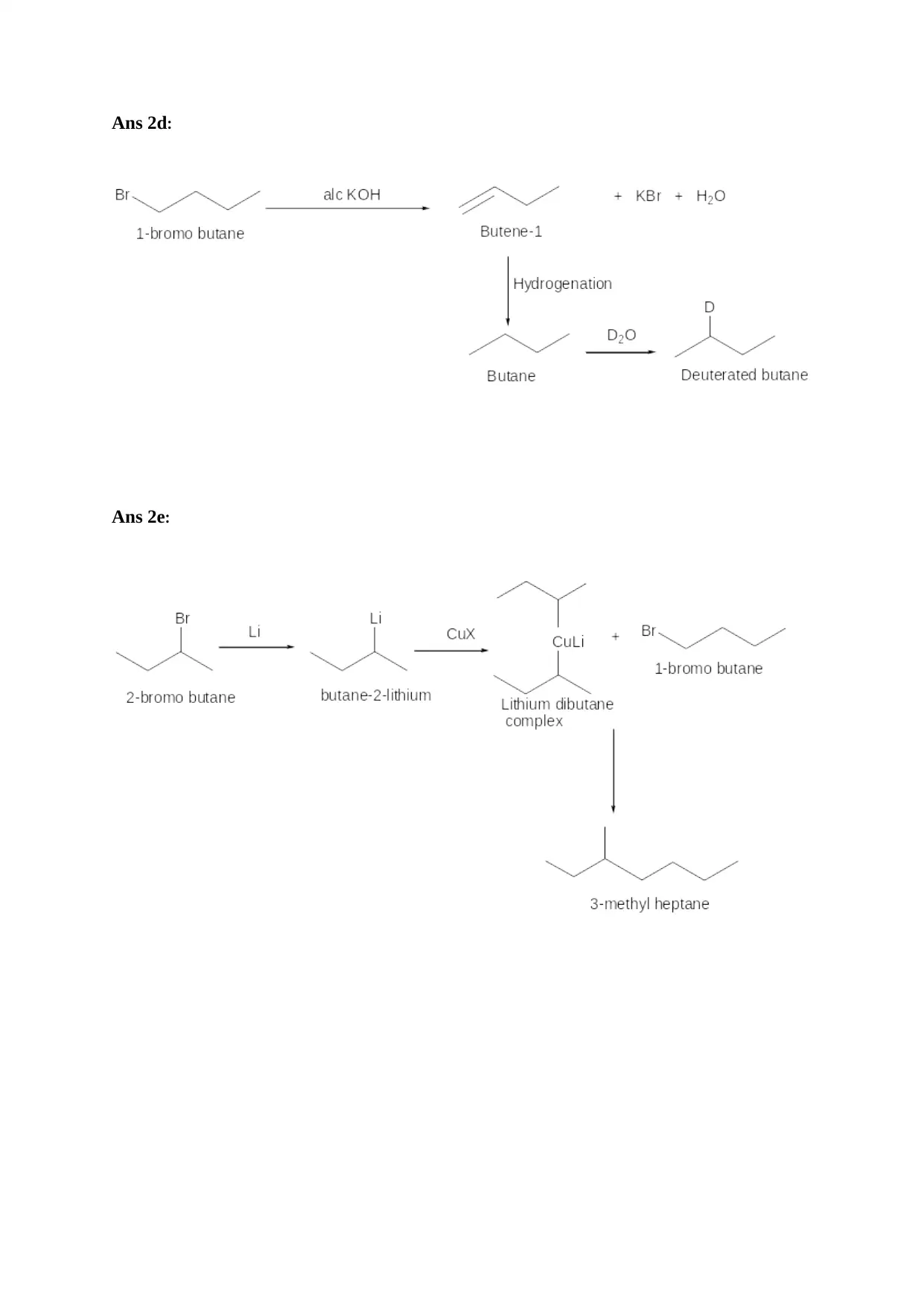
Ans 2d:
Ans 2e:
Ans 2e:
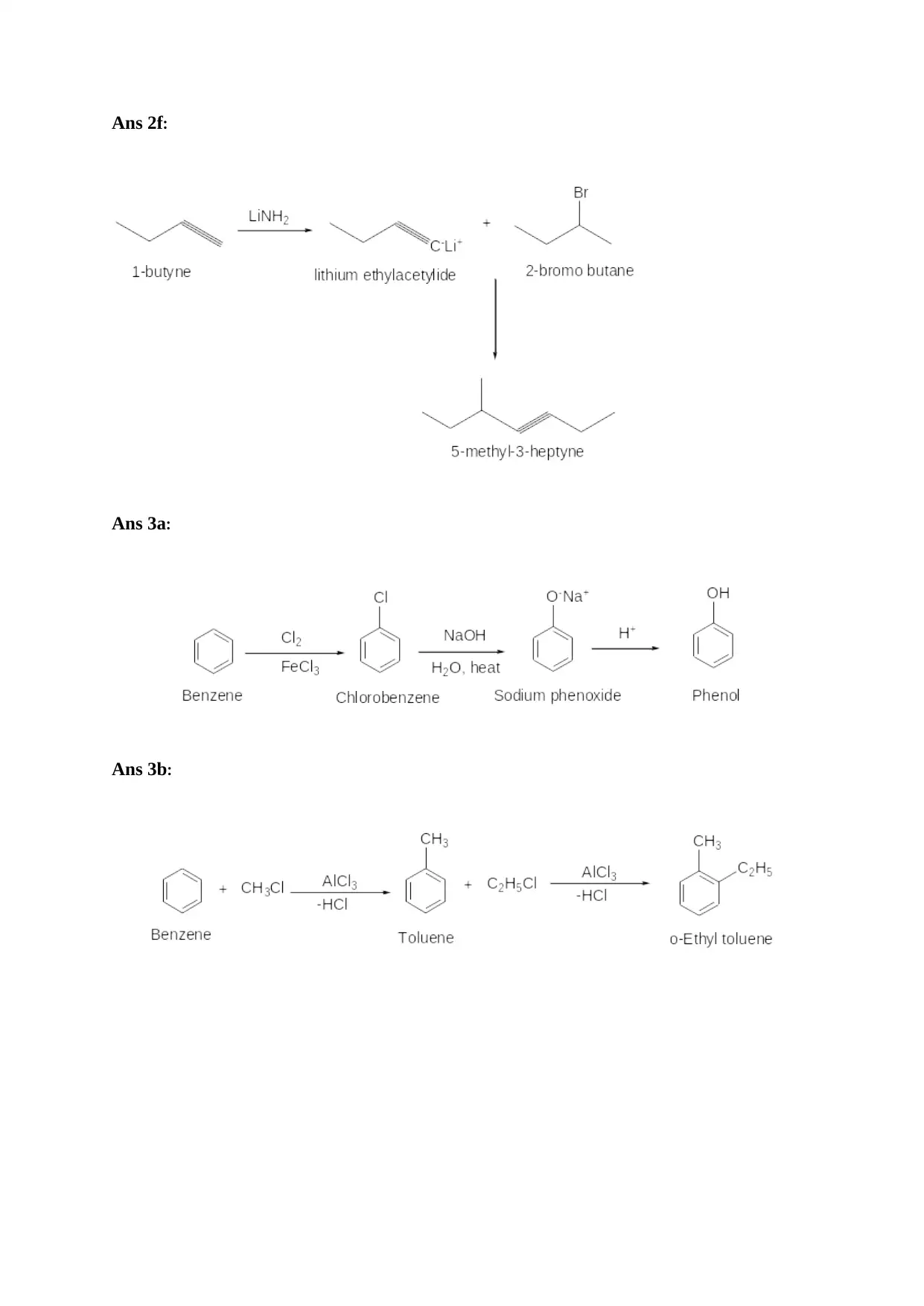
Ans 2f:
Ans 3a:
Ans 3b:
Ans 3a:
Ans 3b:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
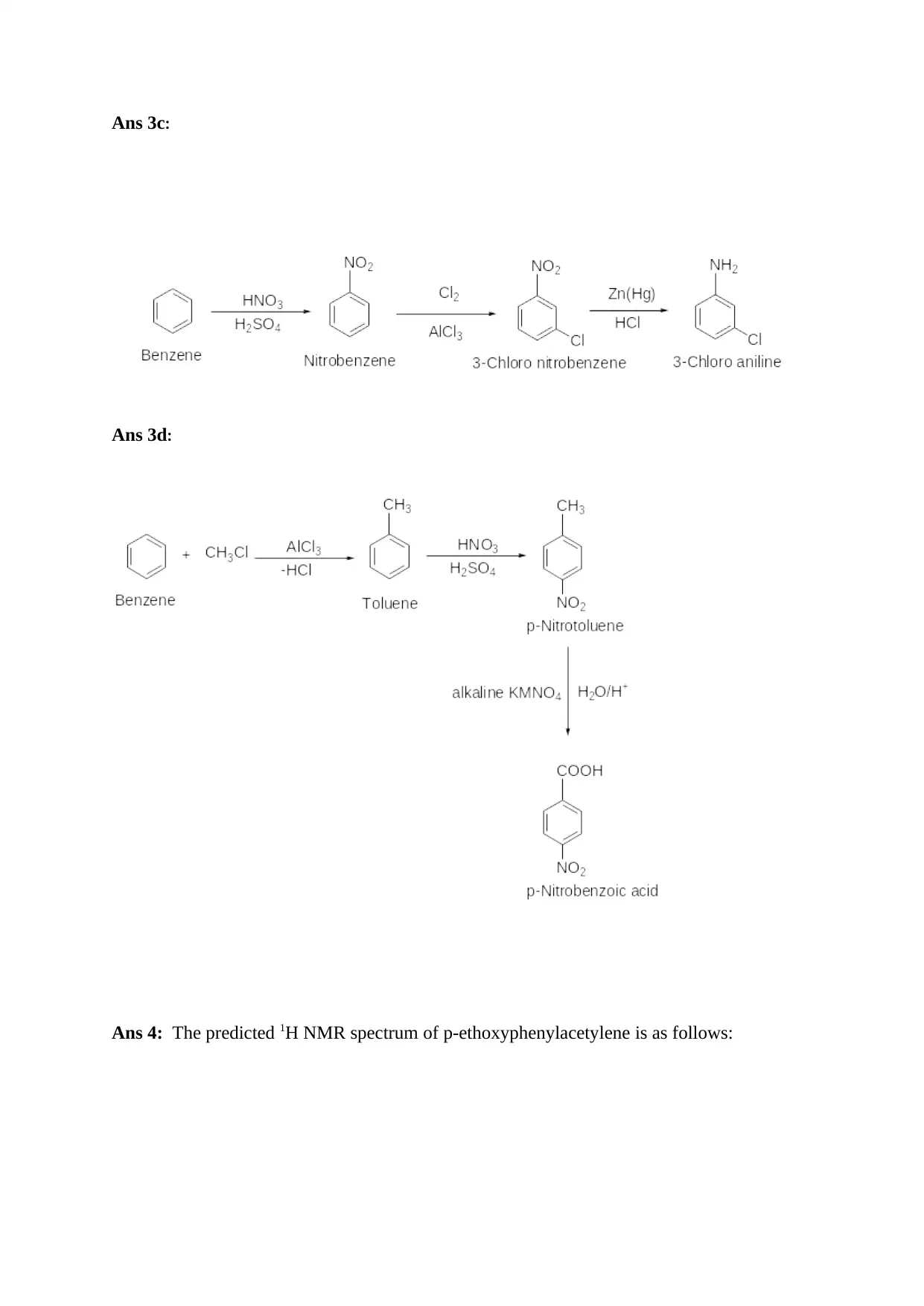
Ans 3c:
Ans 3d:
Ans 4: The predicted 1H NMR spectrum of p-ethoxyphenylacetylene is as follows:
Ans 3d:
Ans 4: The predicted 1H NMR spectrum of p-ethoxyphenylacetylene is as follows:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
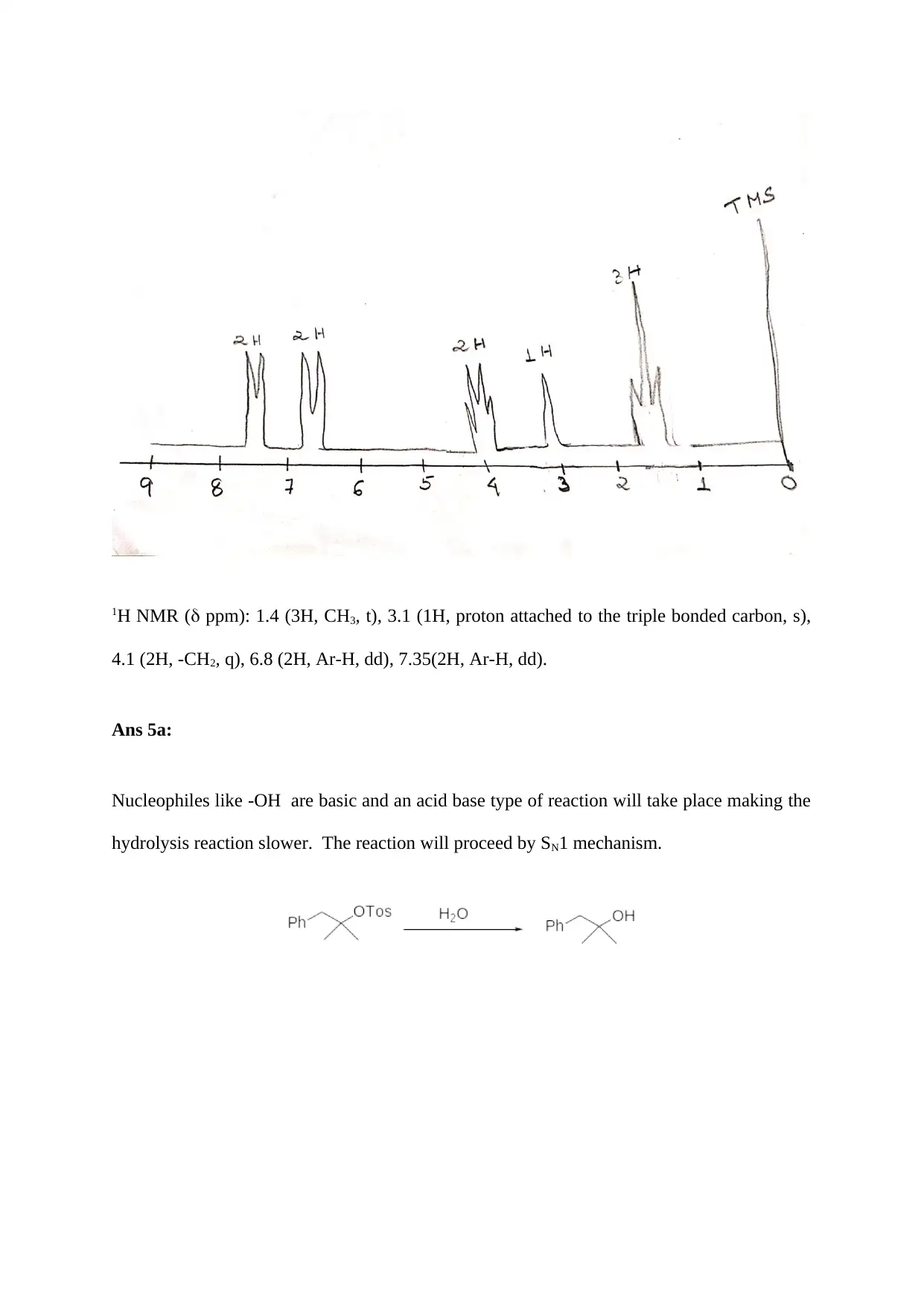
1H NMR ( ppm): 1.4 (3H, CH3, t), 3.1 (1H, proton attached to the triple bonded carbon, s),
4.1 (2H, -CH2, q), 6.8 (2H, Ar-H, dd), 7.35(2H, Ar-H, dd).
Ans 5a:
Nucleophiles like -OH are basic and an acid base type of reaction will take place making the
hydrolysis reaction slower. The reaction will proceed by SN1 mechanism.
4.1 (2H, -CH2, q), 6.8 (2H, Ar-H, dd), 7.35(2H, Ar-H, dd).
Ans 5a:
Nucleophiles like -OH are basic and an acid base type of reaction will take place making the
hydrolysis reaction slower. The reaction will proceed by SN1 mechanism.
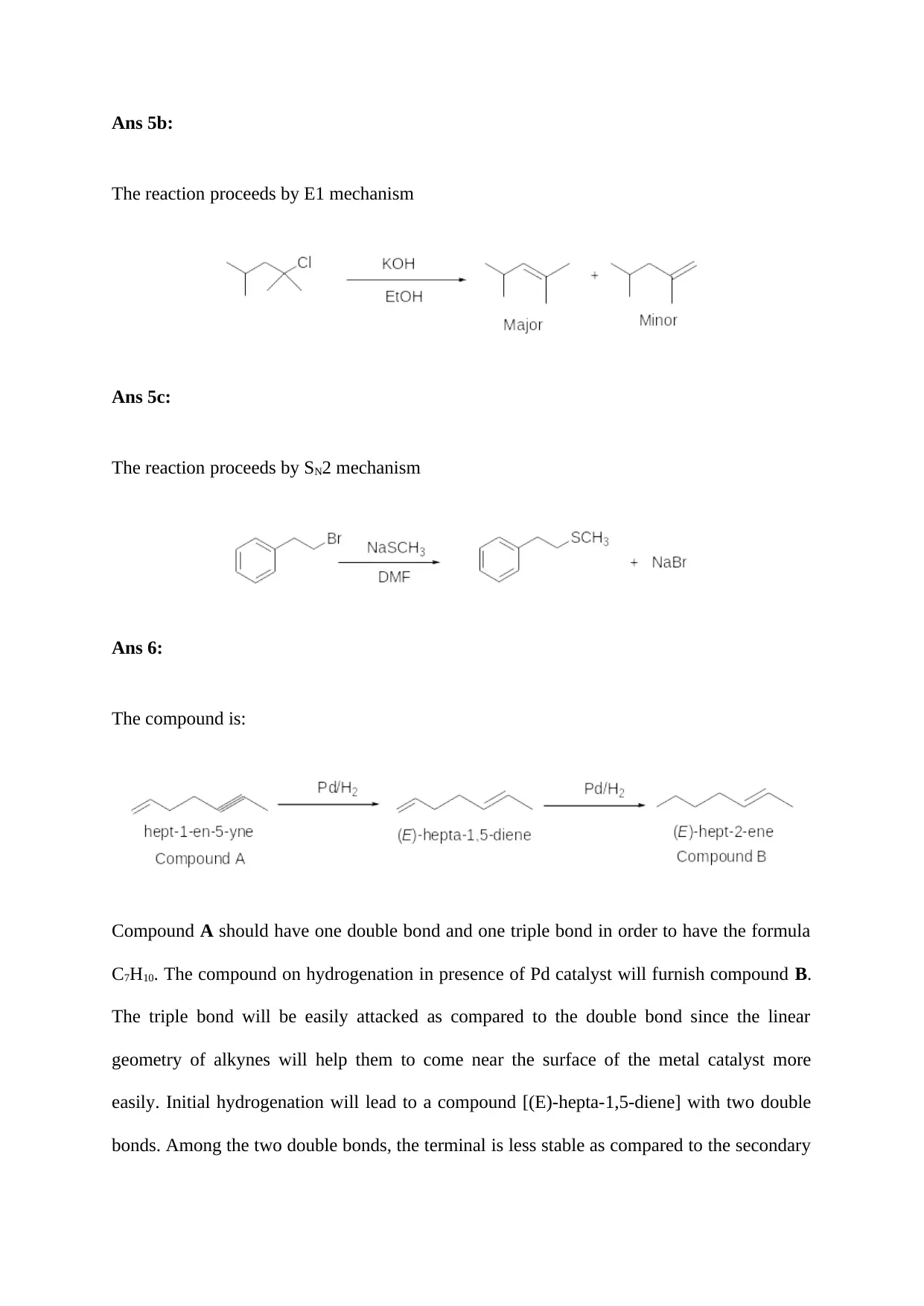
Ans 5b:
The reaction proceeds by E1 mechanism
Ans 5c:
The reaction proceeds by SN2 mechanism
Ans 6:
The compound is:
Compound A should have one double bond and one triple bond in order to have the formula
C7H10. The compound on hydrogenation in presence of Pd catalyst will furnish compound B.
The triple bond will be easily attacked as compared to the double bond since the linear
geometry of alkynes will help them to come near the surface of the metal catalyst more
easily. Initial hydrogenation will lead to a compound [(E)-hepta-1,5-diene] with two double
bonds. Among the two double bonds, the terminal is less stable as compared to the secondary
The reaction proceeds by E1 mechanism
Ans 5c:
The reaction proceeds by SN2 mechanism
Ans 6:
The compound is:
Compound A should have one double bond and one triple bond in order to have the formula
C7H10. The compound on hydrogenation in presence of Pd catalyst will furnish compound B.
The triple bond will be easily attacked as compared to the double bond since the linear
geometry of alkynes will help them to come near the surface of the metal catalyst more
easily. Initial hydrogenation will lead to a compound [(E)-hepta-1,5-diene] with two double
bonds. Among the two double bonds, the terminal is less stable as compared to the secondary
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
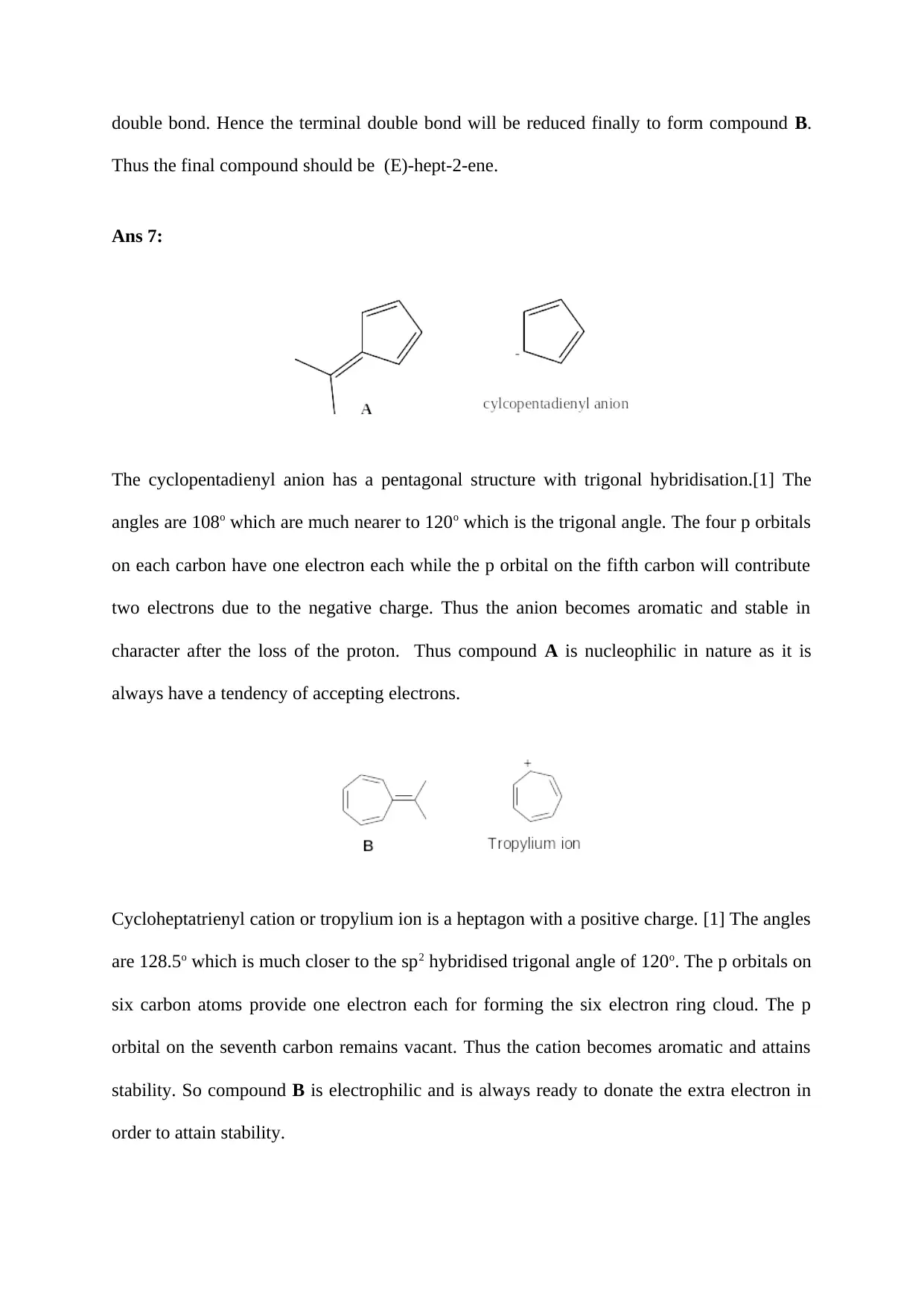
double bond. Hence the terminal double bond will be reduced finally to form compound B.
Thus the final compound should be (E)-hept-2-ene.
Ans 7:
The cyclopentadienyl anion has a pentagonal structure with trigonal hybridisation.[1] The
angles are 108o which are much nearer to 120o which is the trigonal angle. The four p orbitals
on each carbon have one electron each while the p orbital on the fifth carbon will contribute
two electrons due to the negative charge. Thus the anion becomes aromatic and stable in
character after the loss of the proton. Thus compound A is nucleophilic in nature as it is
always have a tendency of accepting electrons.
Cycloheptatrienyl cation or tropylium ion is a heptagon with a positive charge. [1] The angles
are 128.5o which is much closer to the sp2 hybridised trigonal angle of 120o. The p orbitals on
six carbon atoms provide one electron each for forming the six electron ring cloud. The p
orbital on the seventh carbon remains vacant. Thus the cation becomes aromatic and attains
stability. So compound B is electrophilic and is always ready to donate the extra electron in
order to attain stability.
Thus the final compound should be (E)-hept-2-ene.
Ans 7:
The cyclopentadienyl anion has a pentagonal structure with trigonal hybridisation.[1] The
angles are 108o which are much nearer to 120o which is the trigonal angle. The four p orbitals
on each carbon have one electron each while the p orbital on the fifth carbon will contribute
two electrons due to the negative charge. Thus the anion becomes aromatic and stable in
character after the loss of the proton. Thus compound A is nucleophilic in nature as it is
always have a tendency of accepting electrons.
Cycloheptatrienyl cation or tropylium ion is a heptagon with a positive charge. [1] The angles
are 128.5o which is much closer to the sp2 hybridised trigonal angle of 120o. The p orbitals on
six carbon atoms provide one electron each for forming the six electron ring cloud. The p
orbital on the seventh carbon remains vacant. Thus the cation becomes aromatic and attains
stability. So compound B is electrophilic and is always ready to donate the extra electron in
order to attain stability.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Ans 8a:
The mass spectroscopy is used to identify the molecules with the aid of the ratio of the mass
to charge (m/e) of the compound. Each of the ion of a particular compound will have a
specific m/e ratio. Each value of m/e will generate a peak in the spectrum. The most
prominent peak is termed as the base peak. The m/e vales of both the given compound are
shown as follows [2]:
Compound m/e Fragments
2-Heptanone 71, 58, 43 -COCH3, -(CH2)4, -CH3
3-Heptanone 85, 72, 57 -C2H5, - COCH3, -(CH2)3
Thus in 2-heptanone the first m/z peak is for the acetyl fragment while for 3-heptanone the
the first m/z peak is for the ethyl fragment.
Ans 8b:
In 1H NMR spectrum, 2-heptanone will exhibit a singlet at 2.1 ppm and the other –CH2
groups will appear as multiplet within 08-1.2 ppm.
In 1H NMR spectrum, heptanal will exhibit a broad singlet at around 9.9 ppm and the other
–CH2 groups will appear as multiplet within 08-1.2 ppm.
Ans 8c:
In IR spectra ethyl phenyl ketone will show a sharp absorption peak at 1720 cm-1 while the
aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active due
to the conjugation of double bonds present in the structure.
The mass spectroscopy is used to identify the molecules with the aid of the ratio of the mass
to charge (m/e) of the compound. Each of the ion of a particular compound will have a
specific m/e ratio. Each value of m/e will generate a peak in the spectrum. The most
prominent peak is termed as the base peak. The m/e vales of both the given compound are
shown as follows [2]:
Compound m/e Fragments
2-Heptanone 71, 58, 43 -COCH3, -(CH2)4, -CH3
3-Heptanone 85, 72, 57 -C2H5, - COCH3, -(CH2)3
Thus in 2-heptanone the first m/z peak is for the acetyl fragment while for 3-heptanone the
the first m/z peak is for the ethyl fragment.
Ans 8b:
In 1H NMR spectrum, 2-heptanone will exhibit a singlet at 2.1 ppm and the other –CH2
groups will appear as multiplet within 08-1.2 ppm.
In 1H NMR spectrum, heptanal will exhibit a broad singlet at around 9.9 ppm and the other
–CH2 groups will appear as multiplet within 08-1.2 ppm.
Ans 8c:
In IR spectra ethyl phenyl ketone will show a sharp absorption peak at 1720 cm-1 while the
aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active due
to the conjugation of double bonds present in the structure.

In IR spectra ethyl 5-cyclohexenyl ketone will show a sharp absorption peak at 1720 cm-1
while the saturated aliphatic stretch will be observed at around 2900 cm-1. A weak peak will
be observed at around 3040 cm-1 for the alkene present in the ring. This compound will be uv
inactive due to the absence of conjugated double bonds.[3]
Ans 8d:
In IR spectra o-xylene will show a sharp absorption peak in the range of 735-770 cm-1 while
the aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active
due to the conjugation of double bonds present in the structure.
In IR spectra p-xylene will show a sharp absorption peak in the range of 814-840 cm-1 while
the aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active
due to the conjugation of double bonds present in the structure.[3]
Ans 9:
The compound should be
The strong absorption peak at 1672 cm-1 in the IR spectrum indicates the presence of an ester
group. The peaks at 2994 cm-1 indicates the presence of aromatic ring while the broad
absorption band at 3218 cm-1 indicates the presence of a hydroxyl group.
while the saturated aliphatic stretch will be observed at around 2900 cm-1. A weak peak will
be observed at around 3040 cm-1 for the alkene present in the ring. This compound will be uv
inactive due to the absence of conjugated double bonds.[3]
Ans 8d:
In IR spectra o-xylene will show a sharp absorption peak in the range of 735-770 cm-1 while
the aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active
due to the conjugation of double bonds present in the structure.
In IR spectra p-xylene will show a sharp absorption peak in the range of 814-840 cm-1 while
the aromatic stretch will be observed at around 3100 cm-1. This compound will be uv-active
due to the conjugation of double bonds present in the structure.[3]
Ans 9:
The compound should be
The strong absorption peak at 1672 cm-1 in the IR spectrum indicates the presence of an ester
group. The peaks at 2994 cm-1 indicates the presence of aromatic ring while the broad
absorption band at 3218 cm-1 indicates the presence of a hydroxyl group.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The 1H NMR spectrum exhibits a triplet at 1.3 ppm with integration of 3 protons, indicating
the presence of a methyl group which is attached to a –CH2 group. The presence of a quartet
at 4.35 indicated the presence of –CH2 group attached to a methyl group and to an oxygen
atom. The singlet at 6.6 ppm denotes the presence of the hydroxyl proton. The functional
groups are attached to the para position of the benzene ring since the four protons on the
benzene rings are exhibiting two doublets at 6.8 ppm amd 8 ppm respectively.
The mass spectrum demonstrates that the m/z peak is at 166 which is the total mass of the
compound. The other mass peak at 121 indicates the loss of ester group.
Thus the compound U is 4-hydroxyphenyl propionate.
the presence of a methyl group which is attached to a –CH2 group. The presence of a quartet
at 4.35 indicated the presence of –CH2 group attached to a methyl group and to an oxygen
atom. The singlet at 6.6 ppm denotes the presence of the hydroxyl proton. The functional
groups are attached to the para position of the benzene ring since the four protons on the
benzene rings are exhibiting two doublets at 6.8 ppm amd 8 ppm respectively.
The mass spectrum demonstrates that the m/z peak is at 166 which is the total mass of the
compound. The other mass peak at 121 indicates the loss of ester group.
Thus the compound U is 4-hydroxyphenyl propionate.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
[1] R. T. Morrison, R. N. Boyd and S. K. Bhattacharjee, Organic Chemistry. Pearson, India,
7th edition, 2011.
[2] S. Erhart, A. Amann, E. Haberlandt, G. Edlinger, A. Schmid, W. Filipiak, K. Schwarz, P.
Mochalski, K. Rostasy, D. Karall, and S Scholl-Burgi. “3-Heptanone as a potential new
marker for valproic acid therapy” J. Breath Res. 3 , 016004 ,6pp, 2009.
[3] C. N. Banwell and E. M. McCash, Fundamentals of molecular spectroscopy. Tata
McGraw- Hill, 4th edition, 1995.
[1] R. T. Morrison, R. N. Boyd and S. K. Bhattacharjee, Organic Chemistry. Pearson, India,
7th edition, 2011.
[2] S. Erhart, A. Amann, E. Haberlandt, G. Edlinger, A. Schmid, W. Filipiak, K. Schwarz, P.
Mochalski, K. Rostasy, D. Karall, and S Scholl-Burgi. “3-Heptanone as a potential new
marker for valproic acid therapy” J. Breath Res. 3 , 016004 ,6pp, 2009.
[3] C. N. Banwell and E. M. McCash, Fundamentals of molecular spectroscopy. Tata
McGraw- Hill, 4th edition, 1995.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.