BTEC Applied Science: Aspirin Synthesis and Purity Analysis Report
VerifiedAdded on 2023/01/23
|9
|2131
|48
Report
AI Summary
This laboratory report details the synthesis, testing, and analysis of aspirin (acetylsalicylic acid). The experiment involves the esterification of salicylic acid using acetic anhydride and a sulfuric acid catalyst. The report outlines the methodology, including the weighing of reactants, the reaction procedure, and the purification process involving recrystallization. The results section presents the calculated theoretical yield, experimental yield, and percentage yield, along with a discussion of factors affecting the yield, such as reaction conditions, mass loss, and side reactions. The report further compares industrial and laboratory manufacturing processes, highlighting differences in reagents, testing methods, and production scales. The conclusion summarizes the experiment's success, acknowledges the low percentage yield, and suggests improvements for future experiments. Finally, the report includes a bibliography of cited sources.

Laboratory Report on Preparation of Aspirin
By(Name)
Institutional Affiliation
By(Name)
Institutional Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Introduction
Aspirin is a common name to 2-acetoxybenzoic acid. It is a white crystalline substance
that is characterized by pain-relieving properties1 . Acetylsalicylic acid is a derivative of salicylic
acid. It is a prodrug that initiates active metabolism of salicylic acid. Aspirin is an ester of
salicylic acid. It is also an analgestics.
The primary active gradient in aspirin is acetylsalicylic that is manufactured from the
principles of esterification. Esterification process in this case involves the use of acetic anhydride
and salicylic acid as the primary reagents. This procedure encompasses a chemical reaction
between acetic anhydride and carboxylate ester. This acid (salicylic acid) is a weak acid with
alkanol as the functional group it is attached to2. The reaction produces both acetylsalicylic acid
and acetic acid as the products.
The general mechanism for the reaction for aspirin preparation in this case is an
esterification. This reaction happens when an alkanoic acid and an alkanol reacts to produce an
ester. The water molecule split off to form and an alkanoic acid and alkanol forms an ester
instead. In this reaction, a phenoxide ion which is just a hydroxide ion attached to a ring
stabilizes by the electron that withdraws a carbonyl group on the salicyclic acid hence forming a
stable nucleophile. This property makes these reagents an excellent electrophile since the leaving
group undergoes stabilization because of the acidic media.
Industrial manufacturer of Aspirin
Industrially, aspirin is manufactured by reacting acetic anhydride with salicylic acid in
approximate or the same stoichiometric ratios, ZnO and CaO3. The reaction yields a mixture of
acetyl salicylic acid, zinc or calcium acetate. A maximum of 2% free salicylic acid is produced in
the process4. This process is an exothermic reaction and does not require recrystallization. The
aspirin produced is dense and is easily mixed with the excipients of acetyl salicylic acid which is
immediately compressed into the desired tabled once it is synthesized.
1 Paul Knochel, Viet A. Vu, and Ilan Marek, "Stereoselective Preparationof Functionalized Unsaturated Lactones and
Esters via FunctionalizedMagnesium Carbenoids," Synthesis, no. 12 (2013): 1799
2 Frederick A. Bettelheim and Joseph M. Landesberg, Laboratory Experiments for Introduction to General, Organic
and Biochemistry (Boston: Cengage Learning, 2012), 9
3 Knochel, Vu, and Marek, "Preparationof Functionalized Unsaturated Lactones and Esters" 1775.
4 Rachel M. Stevens, Gale Researcher Guide for: Natural Resources and Industrial Production (Gale, Cengage
Learning, 2018), 21
Aspirin is a common name to 2-acetoxybenzoic acid. It is a white crystalline substance
that is characterized by pain-relieving properties1 . Acetylsalicylic acid is a derivative of salicylic
acid. It is a prodrug that initiates active metabolism of salicylic acid. Aspirin is an ester of
salicylic acid. It is also an analgestics.
The primary active gradient in aspirin is acetylsalicylic that is manufactured from the
principles of esterification. Esterification process in this case involves the use of acetic anhydride
and salicylic acid as the primary reagents. This procedure encompasses a chemical reaction
between acetic anhydride and carboxylate ester. This acid (salicylic acid) is a weak acid with
alkanol as the functional group it is attached to2. The reaction produces both acetylsalicylic acid
and acetic acid as the products.
The general mechanism for the reaction for aspirin preparation in this case is an
esterification. This reaction happens when an alkanoic acid and an alkanol reacts to produce an
ester. The water molecule split off to form and an alkanoic acid and alkanol forms an ester
instead. In this reaction, a phenoxide ion which is just a hydroxide ion attached to a ring
stabilizes by the electron that withdraws a carbonyl group on the salicyclic acid hence forming a
stable nucleophile. This property makes these reagents an excellent electrophile since the leaving
group undergoes stabilization because of the acidic media.
Industrial manufacturer of Aspirin
Industrially, aspirin is manufactured by reacting acetic anhydride with salicylic acid in
approximate or the same stoichiometric ratios, ZnO and CaO3. The reaction yields a mixture of
acetyl salicylic acid, zinc or calcium acetate. A maximum of 2% free salicylic acid is produced in
the process4. This process is an exothermic reaction and does not require recrystallization. The
aspirin produced is dense and is easily mixed with the excipients of acetyl salicylic acid which is
immediately compressed into the desired tabled once it is synthesized.
1 Paul Knochel, Viet A. Vu, and Ilan Marek, "Stereoselective Preparationof Functionalized Unsaturated Lactones and
Esters via FunctionalizedMagnesium Carbenoids," Synthesis, no. 12 (2013): 1799
2 Frederick A. Bettelheim and Joseph M. Landesberg, Laboratory Experiments for Introduction to General, Organic
and Biochemistry (Boston: Cengage Learning, 2012), 9
3 Knochel, Vu, and Marek, "Preparationof Functionalized Unsaturated Lactones and Esters" 1775.
4 Rachel M. Stevens, Gale Researcher Guide for: Natural Resources and Industrial Production (Gale, Cengage
Learning, 2018), 21
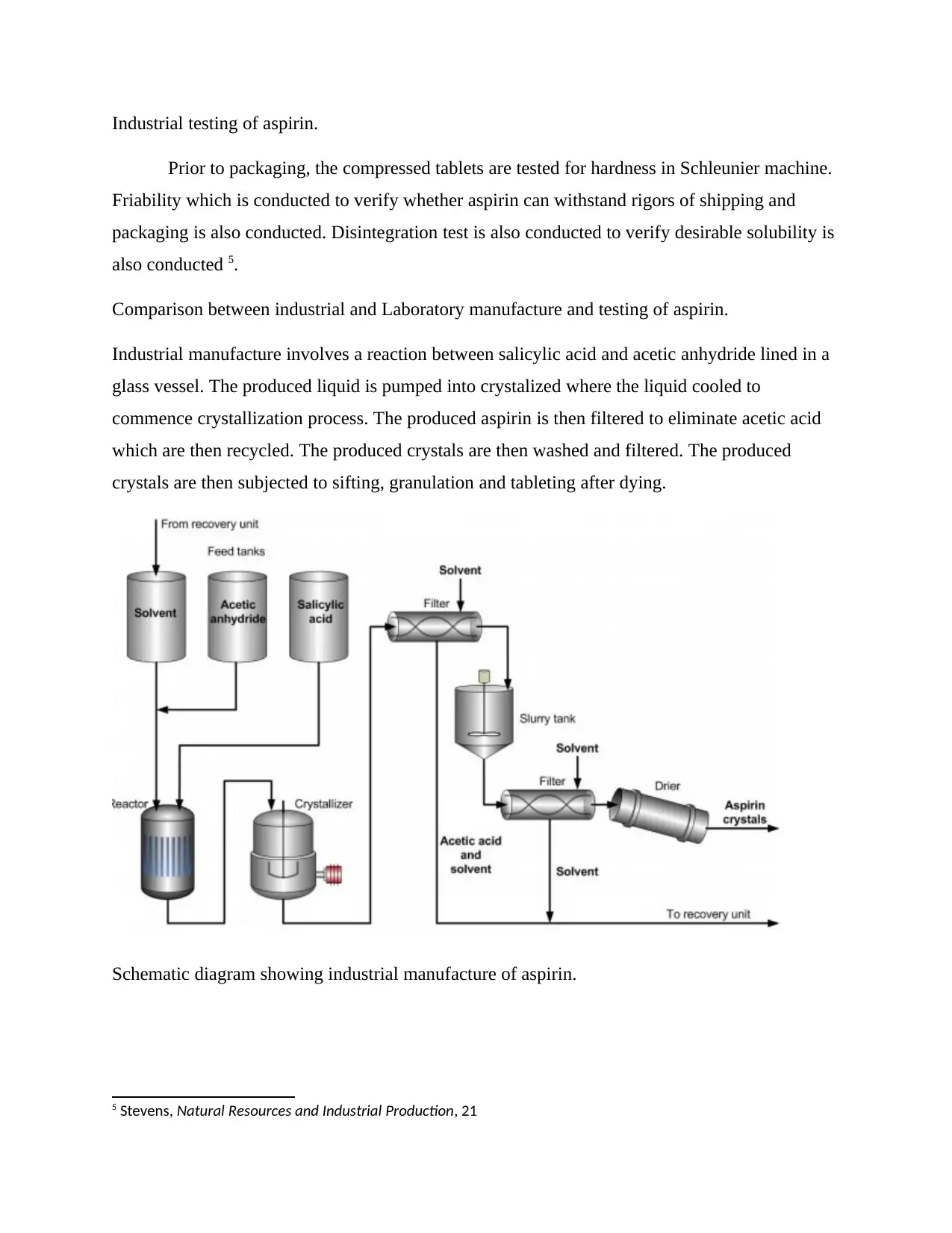
Industrial testing of aspirin.
Prior to packaging, the compressed tablets are tested for hardness in Schleunier machine.
Friability which is conducted to verify whether aspirin can withstand rigors of shipping and
packaging is also conducted. Disintegration test is also conducted to verify desirable solubility is
also conducted 5.
Comparison between industrial and Laboratory manufacture and testing of aspirin.
Industrial manufacture involves a reaction between salicylic acid and acetic anhydride lined in a
glass vessel. The produced liquid is pumped into crystalized where the liquid cooled to
commence crystallization process. The produced aspirin is then filtered to eliminate acetic acid
which are then recycled. The produced crystals are then washed and filtered. The produced
crystals are then subjected to sifting, granulation and tableting after dying.
Schematic diagram showing industrial manufacture of aspirin.
5 Stevens, Natural Resources and Industrial Production, 21
Prior to packaging, the compressed tablets are tested for hardness in Schleunier machine.
Friability which is conducted to verify whether aspirin can withstand rigors of shipping and
packaging is also conducted. Disintegration test is also conducted to verify desirable solubility is
also conducted 5.
Comparison between industrial and Laboratory manufacture and testing of aspirin.
Industrial manufacture involves a reaction between salicylic acid and acetic anhydride lined in a
glass vessel. The produced liquid is pumped into crystalized where the liquid cooled to
commence crystallization process. The produced aspirin is then filtered to eliminate acetic acid
which are then recycled. The produced crystals are then washed and filtered. The produced
crystals are then subjected to sifting, granulation and tableting after dying.
Schematic diagram showing industrial manufacture of aspirin.
5 Stevens, Natural Resources and Industrial Production, 21
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
The primary reagents in manufacturing process are similar for both processes. In
industries however, besides anhydride and salicylic acid, zinc and calcium oxides are added as
the reagents. The laboratory process involves recrystallization process which is not conducted in
the industries. The various testing procedures in the industries such as friability, disintegration
and hardness are not conducted in the Laboratory. In the Laboratory, testing is only done on the
purity which is based on experimenting on melting point. In terms of production scale, industrial
process produces larger volumes of aspirin which cannot be produced in the Laboratory.
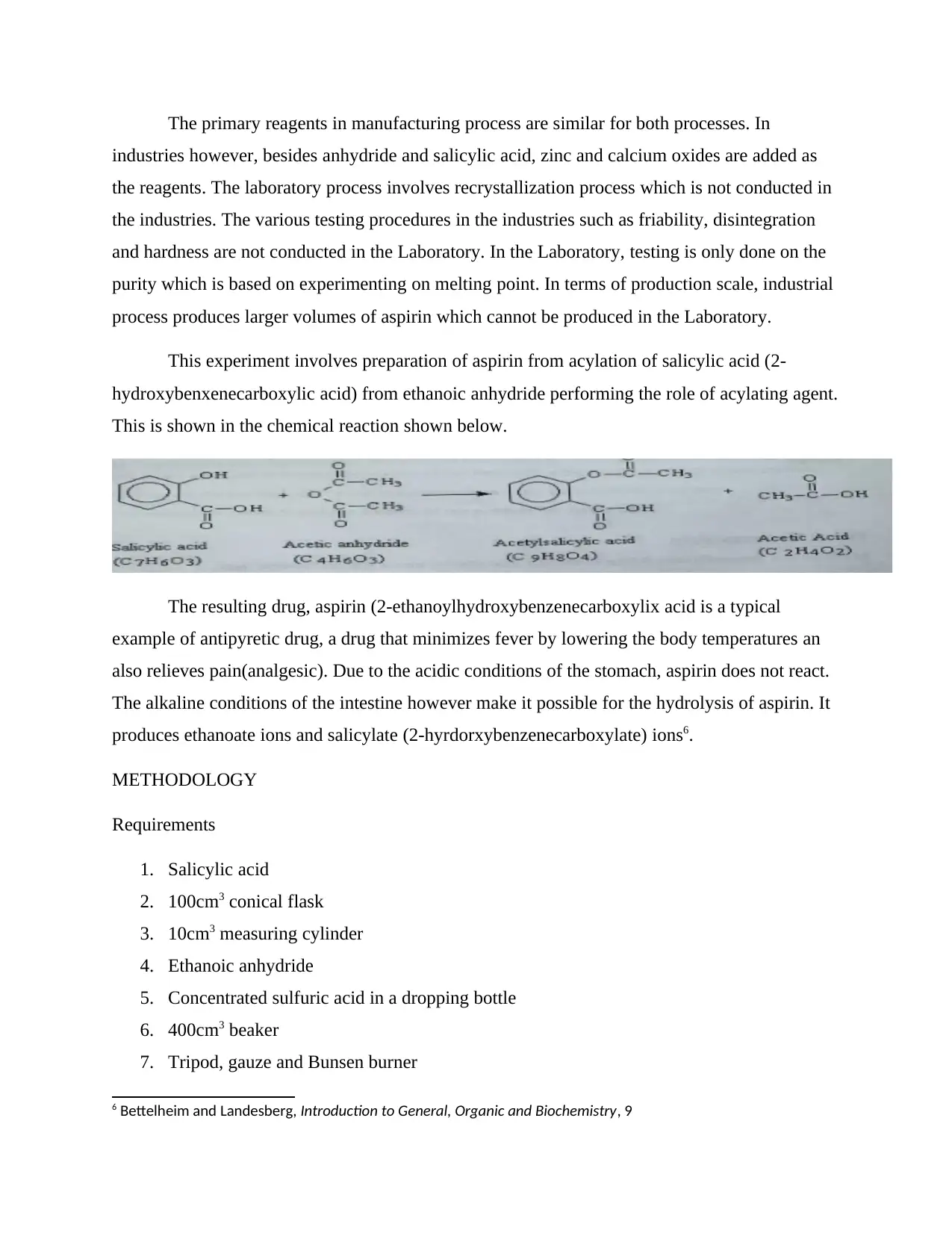
This experiment involves preparation of aspirin from acylation of salicylic acid (2-
hydroxybenxenecarboxylic acid) from ethanoic anhydride performing the role of acylating agent.
This is shown in the chemical reaction shown below.
The resulting drug, aspirin (2-ethanoylhydroxybenzenecarboxylix acid is a typical
example of antipyretic drug, a drug that minimizes fever by lowering the body temperatures an
also relieves pain(analgesic). Due to the acidic conditions of the stomach, aspirin does not react.
The alkaline conditions of the intestine however make it possible for the hydrolysis of aspirin. It
produces ethanoate ions and salicylate (2-hyrdorxybenzenecarboxylate) ions6.
METHODOLOGY
Requirements
1. Salicylic acid
2. 100cm3 conical flask
3. 10cm3 measuring cylinder
4. Ethanoic anhydride
5. Concentrated sulfuric acid in a dropping bottle
6. 400cm3 beaker
7. Tripod, gauze and Bunsen burner
6 Bettelheim and Landesberg, Introduction to General, Organic and Biochemistry, 9
industries however, besides anhydride and salicylic acid, zinc and calcium oxides are added as
the reagents. The laboratory process involves recrystallization process which is not conducted in
the industries. The various testing procedures in the industries such as friability, disintegration
and hardness are not conducted in the Laboratory. In the Laboratory, testing is only done on the
purity which is based on experimenting on melting point. In terms of production scale, industrial
process produces larger volumes of aspirin which cannot be produced in the Laboratory.
This experiment involves preparation of aspirin from acylation of salicylic acid (2-
hydroxybenxenecarboxylic acid) from ethanoic anhydride performing the role of acylating agent.
This is shown in the chemical reaction shown below.
The resulting drug, aspirin (2-ethanoylhydroxybenzenecarboxylix acid is a typical
example of antipyretic drug, a drug that minimizes fever by lowering the body temperatures an
also relieves pain(analgesic). Due to the acidic conditions of the stomach, aspirin does not react.
The alkaline conditions of the intestine however make it possible for the hydrolysis of aspirin. It
produces ethanoate ions and salicylate (2-hyrdorxybenzenecarboxylate) ions6.
METHODOLOGY
Requirements
1. Salicylic acid
2. 100cm3 conical flask
3. 10cm3 measuring cylinder
4. Ethanoic anhydride
5. Concentrated sulfuric acid in a dropping bottle
6. 400cm3 beaker
7. Tripod, gauze and Bunsen burner
6 Bettelheim and Landesberg, Introduction to General, Organic and Biochemistry, 9
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8. Thermometer (-10 oC to 110 oC)
9. 250 cm3 beaker
10. Reduced pressure filtration apparatus
11. Filter paper
12. Glass stirring rod
13. Distilled water in a wash bottle
14. Spatula
15. Procedure
preparation
About 3.00 g of salicylic acid was weighed directly into a 100cm3 conical flask. The mass of
salicylic acid used was recorded. Using a 10 cm3 measuring cylinder, 6 cm3 of ethanoic
anhydride was added to the flask and the content swirled. 5 drops of concentrated sulfuric acid
was then added to the flask and the flask’s mixture swirled to ensure proper mixing. The flask
was then warmed for 10 minutes in a 400 cm3 beaker of hot water at a temperature of about 60oC
but not exceeding 65oC. 10cm3 of cold water was then added. The flask was then allowed to cool
in an ice bath and proper stirring done to precipitate the solid. Aspirin was then filtered off and
pressure reduced to limit skin contact. The resulting crude aspirin was then collected on a double
thickness filter paper and then allowed to dry.
Purification process.
A 25cm3 of measuring cylinder was used to measure 15cm3 of ethanol into a boiling tube.
A beaker half-filled with hot water at a temperature of about 75oC was then prepared by adding
boiling water from a kettle to cold water until temperature reached about 75oC. A spatula was
used to add crude aspirin to the boiling tube and the tube placed in a beaker of hot water. The
boiling tube was then stirred until all the aspirin dissolved.
The hot solution containing dissolved aspirin was then poured into approximately 40cm3
of water in a 100cm3 conical flask. The conical flask was then allowed to slowly cool and white
needles of aspirin separated. Ice bath was used in situations where no aspirin crystals was formed
after allowing the solution to cool at room temperature. The purified solution under a reduced
pressure was filtered off and then allowed to dry on a filter.
9. 250 cm3 beaker
10. Reduced pressure filtration apparatus
11. Filter paper
12. Glass stirring rod
13. Distilled water in a wash bottle
14. Spatula
15. Procedure
preparation
About 3.00 g of salicylic acid was weighed directly into a 100cm3 conical flask. The mass of
salicylic acid used was recorded. Using a 10 cm3 measuring cylinder, 6 cm3 of ethanoic
anhydride was added to the flask and the content swirled. 5 drops of concentrated sulfuric acid
was then added to the flask and the flask’s mixture swirled to ensure proper mixing. The flask
was then warmed for 10 minutes in a 400 cm3 beaker of hot water at a temperature of about 60oC
but not exceeding 65oC. 10cm3 of cold water was then added. The flask was then allowed to cool
in an ice bath and proper stirring done to precipitate the solid. Aspirin was then filtered off and
pressure reduced to limit skin contact. The resulting crude aspirin was then collected on a double
thickness filter paper and then allowed to dry.
Purification process.
A 25cm3 of measuring cylinder was used to measure 15cm3 of ethanol into a boiling tube.
A beaker half-filled with hot water at a temperature of about 75oC was then prepared by adding
boiling water from a kettle to cold water until temperature reached about 75oC. A spatula was
used to add crude aspirin to the boiling tube and the tube placed in a beaker of hot water. The
boiling tube was then stirred until all the aspirin dissolved.
The hot solution containing dissolved aspirin was then poured into approximately 40cm3
of water in a 100cm3 conical flask. The conical flask was then allowed to slowly cool and white
needles of aspirin separated. Ice bath was used in situations where no aspirin crystals was formed
after allowing the solution to cool at room temperature. The purified solution under a reduced
pressure was filtered off and then allowed to dry on a filter.

RESULTS AND ANALYSIS
The mass of aspirin from the experiment----------1.09g
Calculation of theoretical yield of aspirin.
Consider the chemical equation used in the reaction used in the preparation,
HOOCC6H4OH +(CH3CO)2O → HOOCC6H4OCOCH3+CH3COOH
The mass of salicylic used----------3.09g
The molar mass of salicylic =C*7 +H*1+O*3= (12*7) +(1*6) +(16*3) =138
Number of moles of salicylic=mass/molar mass= (3.09/138) =0.02239130
From the mole ratios, salicylic: aspirin=1:1
Moles of aspirin = moles of salicylic =0.02239130
Mass=mole*molar mass
Molar mass of aspirin=(C*9) +(H*8) +(O*4) = (12*4) +(1*8) +(16*4) =180
Mass=180*0.0224=3.919 g
Percentage yield= Actual mass
Theoritical mass × 100= 1.09
3.919 ×100=27.813 %
DISCUSSION
A table showing theoretical yield, percent error and the percent yield
Theoretical yield (g) 3.919g
Experimental Yield (g) 1.09g
Percent error 72.187%
Percent yield 27.813%
The hot water bath of (60-65oC) was aimed at increasing the rate of reaction because
addition of more heat into the system increase the rate of reaction because addition of more heat
into this system increases the energy present making the particles to move faster thus increasing
The mass of aspirin from the experiment----------1.09g
Calculation of theoretical yield of aspirin.
Consider the chemical equation used in the reaction used in the preparation,
HOOCC6H4OH +(CH3CO)2O → HOOCC6H4OCOCH3+CH3COOH
The mass of salicylic used----------3.09g
The molar mass of salicylic =C*7 +H*1+O*3= (12*7) +(1*6) +(16*3) =138
Number of moles of salicylic=mass/molar mass= (3.09/138) =0.02239130
From the mole ratios, salicylic: aspirin=1:1
Moles of aspirin = moles of salicylic =0.02239130
Mass=mole*molar mass
Molar mass of aspirin=(C*9) +(H*8) +(O*4) = (12*4) +(1*8) +(16*4) =180
Mass=180*0.0224=3.919 g
Percentage yield= Actual mass
Theoritical mass × 100= 1.09
3.919 ×100=27.813 %
DISCUSSION
A table showing theoretical yield, percent error and the percent yield
Theoretical yield (g) 3.919g
Experimental Yield (g) 1.09g
Percent error 72.187%
Percent yield 27.813%
The hot water bath of (60-65oC) was aimed at increasing the rate of reaction because
addition of more heat into the system increase the rate of reaction because addition of more heat
into this system increases the energy present making the particles to move faster thus increasing
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

chances of successful collisions. The computed theoretical yield was 3.919g yielding a
percentage error of 72.187% and percentage yield of 27.813%.
Factors that affected yield.
Achievement of optimal and higher collision rates during the reaction is key factor
affecting aspirin yield. In this experiment, there was a possibility of a failure to achieve the
above condition because the experimental procedure was more of a reflux reaction. In the
industry, care is taken to avoid too much temperature which would cause gas escape thus
lowering yield. There is also a possibility of mass loss while transferring solutions. Mass loss
results into a decline in the overall yield. This is avoided in the industries by having a sealed
system that does not allow spillage. Despite remaining at equilibria, there is a possibility of
unexpected side reaction leading to contamination of the precursors of the final product. This
leads to side reactions. In the industry, this occurs as result of excess or improper use of the
reagents.
Improper washing of the crystals in cold water might have also contained the
experimental results thus leading a lower yield. In the industry, washing process is thorough and
is only done after the second filtration process to ensure higher yields. Another possible source
of this large error could a possibility of failing to wash the crystal with cold water. In the
industry, washing procedure cannot be skipped. It is part of the plant system ensuring higher
yields. The rinsing process might have been less thorough. This could have increased percentage
error to 72.187% since the large amount of acetic anhydride could have been eliminated from the
reaction thus contaminating the entire process leading to erroneous results. Using the entire crude
product in hot water and crystallized instead of just a portion of the product is another possible
source of error. Using the entire crystal would have maximized the total losses. Using a different
samples and then summing up the amount of aspirin formed would have limited the losses.
Besides, a large amount of crystals might have been lost during the occasional transfer of
masses.
CONCLUSION
A total of 1.09g out of the possible yield of 3.919g was realized. This translates into a
percentage yield of 27.813% and percentage error 72.187%. The reaction involved esterification
percentage error of 72.187% and percentage yield of 27.813%.
Factors that affected yield.
Achievement of optimal and higher collision rates during the reaction is key factor
affecting aspirin yield. In this experiment, there was a possibility of a failure to achieve the
above condition because the experimental procedure was more of a reflux reaction. In the
industry, care is taken to avoid too much temperature which would cause gas escape thus
lowering yield. There is also a possibility of mass loss while transferring solutions. Mass loss
results into a decline in the overall yield. This is avoided in the industries by having a sealed
system that does not allow spillage. Despite remaining at equilibria, there is a possibility of
unexpected side reaction leading to contamination of the precursors of the final product. This
leads to side reactions. In the industry, this occurs as result of excess or improper use of the
reagents.
Improper washing of the crystals in cold water might have also contained the
experimental results thus leading a lower yield. In the industry, washing process is thorough and
is only done after the second filtration process to ensure higher yields. Another possible source
of this large error could a possibility of failing to wash the crystal with cold water. In the
industry, washing procedure cannot be skipped. It is part of the plant system ensuring higher
yields. The rinsing process might have been less thorough. This could have increased percentage
error to 72.187% since the large amount of acetic anhydride could have been eliminated from the
reaction thus contaminating the entire process leading to erroneous results. Using the entire crude
product in hot water and crystallized instead of just a portion of the product is another possible
source of error. Using the entire crystal would have maximized the total losses. Using a different
samples and then summing up the amount of aspirin formed would have limited the losses.
Besides, a large amount of crystals might have been lost during the occasional transfer of
masses.
CONCLUSION
A total of 1.09g out of the possible yield of 3.919g was realized. This translates into a
percentage yield of 27.813% and percentage error 72.187%. The reaction involved esterification
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

as one functional group was converted into an ester. Formation of an ester makes aspirin lesser
acidic thus limiting the possibility of damaging human digestive system. The low percentage
yield is attributed to the various sources of errors discussed in the previous chapter. Because of
the low yield realized, care should be taken to thoroughly wash of the product (crystals) with
cold water in order to improve the percentage yield. Despite the above mentioned sources of
errors and the low percentage yield, the experiment was a success because we were not only able
to prepare aspirin in the laboratory but also purify aspirin from its crude form.
Bibliography
acidic thus limiting the possibility of damaging human digestive system. The low percentage
yield is attributed to the various sources of errors discussed in the previous chapter. Because of
the low yield realized, care should be taken to thoroughly wash of the product (crystals) with
cold water in order to improve the percentage yield. Despite the above mentioned sources of
errors and the low percentage yield, the experiment was a success because we were not only able
to prepare aspirin in the laboratory but also purify aspirin from its crude form.
Bibliography

Bettelheim, Frederick A., and Joseph M. Landesberg. Laboratory Experiments for Introduction
to General, Organic and Biochemistry. Boston: Cengage Learning, 2012.
Knochel, Paul, Viet A. Vu, and Ilan Marek. "Stereoselective Preparationof
Functionalized Unsaturated Lactones and Esters via FunctionalizedMagnesium Carbenoids."
Synthesis, no. 12 (2013), 1797-1802
Stevens, Rachel M. Gale Researcher Guide for: Natural Resources and Industrial Production.
Gale, Cengage Learning, 2018.
to General, Organic and Biochemistry. Boston: Cengage Learning, 2012.
Knochel, Paul, Viet A. Vu, and Ilan Marek. "Stereoselective Preparationof
Functionalized Unsaturated Lactones and Esters via FunctionalizedMagnesium Carbenoids."
Synthesis, no. 12 (2013), 1797-1802
Stevens, Rachel M. Gale Researcher Guide for: Natural Resources and Industrial Production.
Gale, Cengage Learning, 2018.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.