Essay on Atomic Structure, Identification, and Properties (Chemistry)
VerifiedAdded on 2021/02/22
|9
|1607
|421
Essay
AI Summary
This essay, designed for A-Level Chemistry students, delves into the fundamental concepts of atomic structure and bonding. It begins by outlining the basic components of an atom, including protons, neutrons, and electrons, and explains the significance of the atomic number. The essay then addresses the identification of elements based on their atomic numbers, providing examples for Fluorine, Krypton, Silver, and Barium. It clarifies the concepts of mass number and relative atomic mass, highlighting their differences and importance. Furthermore, the essay explores the electronic structure of atoms, detailing the arrangement of electrons in s, p, d, and f orbitals, and provides examples of electronic configurations for the aforementioned elements. The essay references relevant sources, including books, journals, and online resources, to support its explanations and findings.

Atomic Structure and Bonding
(Chemistry A-Level)
(Chemistry A-Level)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
1. Structure of Atom....................................................................................................................1
2. Identification of element from their Atomic number...............................................................2
3. Mass Number and Nucleon Number of Data...........................................................................2
4. Relative Atomic Mass..............................................................................................................2
5. Electronic Structure As Per S, P, D and F Orbit......................................................................3
6. Electronic Structure.................................................................................................................5
REFERENCES................................................................................................................................7
1. Structure of Atom....................................................................................................................1
2. Identification of element from their Atomic number...............................................................2
3. Mass Number and Nucleon Number of Data...........................................................................2
4. Relative Atomic Mass..............................................................................................................2
5. Electronic Structure As Per S, P, D and F Orbit......................................................................3
6. Electronic Structure.................................................................................................................5
REFERENCES................................................................................................................................7
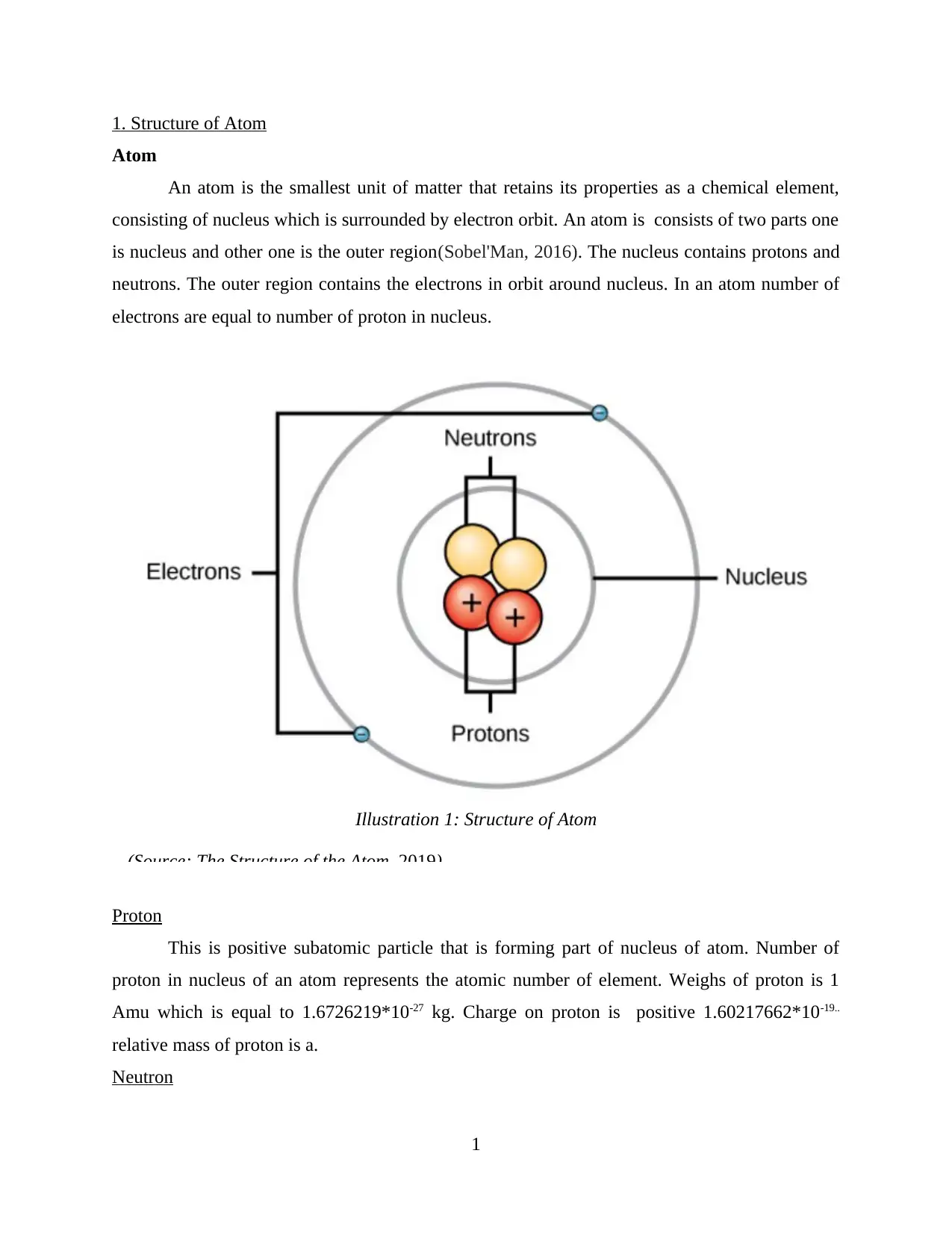
1. Structure of Atom
Atom
An atom is the smallest unit of matter that retains its properties as a chemical element,
consisting of nucleus which is surrounded by electron orbit. An atom is consists of two parts one
is nucleus and other one is the outer region(Sobel'Man, 2016). The nucleus contains protons and
neutrons. The outer region contains the electrons in orbit around nucleus. In an atom number of
electrons are equal to number of proton in nucleus.
Proton
This is positive subatomic particle that is forming part of nucleus of atom. Number of
proton in nucleus of an atom represents the atomic number of element. Weighs of proton is 1
Amu which is equal to 1.6726219*10-27 kg. Charge on proton is positive 1.60217662*10-19..
relative mass of proton is a.
Neutron
1
Illustration 1: Structure of Atom
(Source: The Structure of the Atom, 2019)
Atom
An atom is the smallest unit of matter that retains its properties as a chemical element,
consisting of nucleus which is surrounded by electron orbit. An atom is consists of two parts one
is nucleus and other one is the outer region(Sobel'Man, 2016). The nucleus contains protons and
neutrons. The outer region contains the electrons in orbit around nucleus. In an atom number of
electrons are equal to number of proton in nucleus.
Proton
This is positive subatomic particle that is forming part of nucleus of atom. Number of
proton in nucleus of an atom represents the atomic number of element. Weighs of proton is 1
Amu which is equal to 1.6726219*10-27 kg. Charge on proton is positive 1.60217662*10-19..
relative mass of proton is a.
Neutron
1
Illustration 1: Structure of Atom
(Source: The Structure of the Atom, 2019)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

It is also subatomic particle and second forming particle of atom. It is neutral in terms of
charge. The mass of neutron is equal to proton that is 1 Amu. Mass of neutron in kilograms is
1.674929*10-27 kg. Relative mass of neutron is also 1.
Electron
This is the third sub atomic particle in atom which is negatively charged. The mass of
electron is 9.109390*10-31 kg. Charge on electron is negative 1.60217662*10-19 coulombs.
Relative mass on electron is .0005.
2. Identification of element from their Atomic number
“The atomic number is known as the number of protons in the nucleus of atom.”
The Atomic Number 9 is Fluorine and its symbol is F (Roca, 2018). the element with Atomic
Number 36 Krypton. Silver's Atomic Number is 47 cause there are 47 protons in nucleus of
proton. Atomic Number 56 is of Barium and number of proton in nucleus are 56.
3. Mass Number and Nucleon Number of Data
“Atomic mass is known as sum of number of proton and neutron in nucleus of an atom.”
Fluorine's atomic mass number is 18.99843 u. the Atomic mass of the Krypton is 83.798
u and Atomic mass of Silver is 107.8682. Barium is the element with 56 protons in nucleus and
its Atomic mass is 137.327.
4. Relative Atomic Mass
The relative atomic mass of an atom is compared with the mass of an atom of the Carbon
12 whose mass is exactly 12. The atomic mass of all isotopes are different and the relative
atomic mass can be considered as the average of weighted of the masses of Isotopes of the
2
Illustration 2: Atomic Number
and Atomic Mass
(Source:ATOMIC NUMBER AND MASS NUMBERS, 2019 )
charge. The mass of neutron is equal to proton that is 1 Amu. Mass of neutron in kilograms is
1.674929*10-27 kg. Relative mass of neutron is also 1.
Electron
This is the third sub atomic particle in atom which is negatively charged. The mass of
electron is 9.109390*10-31 kg. Charge on electron is negative 1.60217662*10-19 coulombs.
Relative mass on electron is .0005.
2. Identification of element from their Atomic number
“The atomic number is known as the number of protons in the nucleus of atom.”
The Atomic Number 9 is Fluorine and its symbol is F (Roca, 2018). the element with Atomic
Number 36 Krypton. Silver's Atomic Number is 47 cause there are 47 protons in nucleus of
proton. Atomic Number 56 is of Barium and number of proton in nucleus are 56.
3. Mass Number and Nucleon Number of Data
“Atomic mass is known as sum of number of proton and neutron in nucleus of an atom.”
Fluorine's atomic mass number is 18.99843 u. the Atomic mass of the Krypton is 83.798
u and Atomic mass of Silver is 107.8682. Barium is the element with 56 protons in nucleus and
its Atomic mass is 137.327.
4. Relative Atomic Mass
The relative atomic mass of an atom is compared with the mass of an atom of the Carbon
12 whose mass is exactly 12. The atomic mass of all isotopes are different and the relative
atomic mass can be considered as the average of weighted of the masses of Isotopes of the
2
Illustration 2: Atomic Number
and Atomic Mass
(Source:ATOMIC NUMBER AND MASS NUMBERS, 2019 )
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

elements. The relative atomic mass is dimension less quantity so it doesn't have any unit. The
measurement of this performed with spectrometer.
The Carbon 12 is the standard atom against which masses of other atoms are compared
with. The relative Atomic mass of element is the average mass of its atoms compared to 1/12th
the mass of a carbon -2 atom.
5. Electronic Structure As Per S, P, D and F Orbit
As stated in the definition of atom there are two parts nucleus and other one is outer
space where electrons moves in different types of orbits (Liao and et. al., 2016). There are four
types of orbits in chemistry. These orbits are S, P, D and F. this orbits are classified as per the
movement of electron around nucleus.
3
Illustration 3: Relative Atomic
Mass
(Source: What is Relative Atomic Mass, 2019)
measurement of this performed with spectrometer.
The Carbon 12 is the standard atom against which masses of other atoms are compared
with. The relative Atomic mass of element is the average mass of its atoms compared to 1/12th
the mass of a carbon -2 atom.
5. Electronic Structure As Per S, P, D and F Orbit
As stated in the definition of atom there are two parts nucleus and other one is outer
space where electrons moves in different types of orbits (Liao and et. al., 2016). There are four
types of orbits in chemistry. These orbits are S, P, D and F. this orbits are classified as per the
movement of electron around nucleus.
3
Illustration 3: Relative Atomic
Mass
(Source: What is Relative Atomic Mass, 2019)
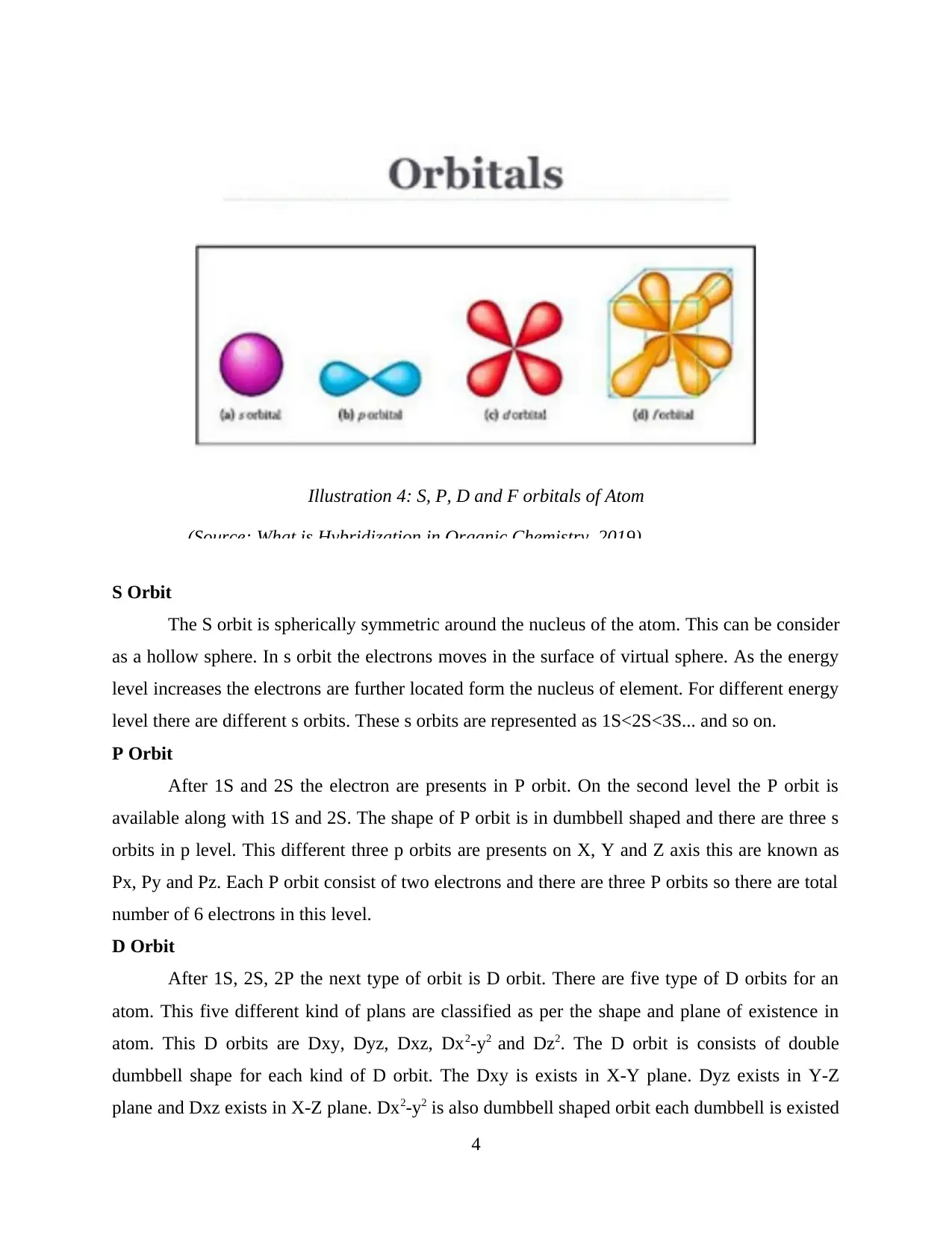
S Orbit
The S orbit is spherically symmetric around the nucleus of the atom. This can be consider
as a hollow sphere. In s orbit the electrons moves in the surface of virtual sphere. As the energy
level increases the electrons are further located form the nucleus of element. For different energy
level there are different s orbits. These s orbits are represented as 1S<2S<3S... and so on.
P Orbit
After 1S and 2S the electron are presents in P orbit. On the second level the P orbit is
available along with 1S and 2S. The shape of P orbit is in dumbbell shaped and there are three s
orbits in p level. This different three p orbits are presents on X, Y and Z axis this are known as
Px, Py and Pz. Each P orbit consist of two electrons and there are three P orbits so there are total
number of 6 electrons in this level.
D Orbit
After 1S, 2S, 2P the next type of orbit is D orbit. There are five type of D orbits for an
atom. This five different kind of plans are classified as per the shape and plane of existence in
atom. This D orbits are Dxy, Dyz, Dxz, Dx2-y2 and Dz2. The D orbit is consists of double
dumbbell shape for each kind of D orbit. The Dxy is exists in X-Y plane. Dyz exists in Y-Z
plane and Dxz exists in X-Z plane. Dx2-y2 is also dumbbell shaped orbit each dumbbell is existed
4
Illustration 4: S, P, D and F orbitals of Atom
(Source: What is Hybridization in Organic Chemistry, 2019)
The S orbit is spherically symmetric around the nucleus of the atom. This can be consider
as a hollow sphere. In s orbit the electrons moves in the surface of virtual sphere. As the energy
level increases the electrons are further located form the nucleus of element. For different energy
level there are different s orbits. These s orbits are represented as 1S<2S<3S... and so on.
P Orbit
After 1S and 2S the electron are presents in P orbit. On the second level the P orbit is
available along with 1S and 2S. The shape of P orbit is in dumbbell shaped and there are three s
orbits in p level. This different three p orbits are presents on X, Y and Z axis this are known as
Px, Py and Pz. Each P orbit consist of two electrons and there are three P orbits so there are total
number of 6 electrons in this level.
D Orbit
After 1S, 2S, 2P the next type of orbit is D orbit. There are five type of D orbits for an
atom. This five different kind of plans are classified as per the shape and plane of existence in
atom. This D orbits are Dxy, Dyz, Dxz, Dx2-y2 and Dz2. The D orbit is consists of double
dumbbell shape for each kind of D orbit. The Dxy is exists in X-Y plane. Dyz exists in Y-Z
plane and Dxz exists in X-Z plane. Dx2-y2 is also dumbbell shaped orbit each dumbbell is existed
4
Illustration 4: S, P, D and F orbitals of Atom
(Source: What is Hybridization in Organic Chemistry, 2019)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

in XY horizon and the last type of D orbit is Dz2 is presents on Z plane and a ring is existed in
XY plane. There are total five type of D orbits and each type is consists of two electrons. So
there are total number of 10 electrons are arranged in D orbit electronic structure.
F Orbit
There are seven type of F orbits and total 14 number of electrons are present in F orbit the
shape of F orbit is given in Diagram. The parts of fX3, FY3, FZ3, FZ(X2-Y2), FX(Y2-Z2), FY(Z2-X2) and FXYZ..
6. Electronic Structure
For example the electronic Structure of Fluorine is considered below(Inglesfield, 2018)
. There are 9 electrons in oxygen so the electronic structure will be-
Fluorine Electronic Structure= 1S2, 2S2, 2P5
5
Illustration 5: Electronic Structure
(Source: Electronic Structure of Atoms (Electron Configurations), 2019)
XY plane. There are total five type of D orbits and each type is consists of two electrons. So
there are total number of 10 electrons are arranged in D orbit electronic structure.
F Orbit
There are seven type of F orbits and total 14 number of electrons are present in F orbit the
shape of F orbit is given in Diagram. The parts of fX3, FY3, FZ3, FZ(X2-Y2), FX(Y2-Z2), FY(Z2-X2) and FXYZ..
6. Electronic Structure
For example the electronic Structure of Fluorine is considered below(Inglesfield, 2018)
. There are 9 electrons in oxygen so the electronic structure will be-
Fluorine Electronic Structure= 1S2, 2S2, 2P5
5
Illustration 5: Electronic Structure
(Source: Electronic Structure of Atoms (Electron Configurations), 2019)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

In the Electronic Structure there are 9 electrons. The first and second S orbit consists of
two each so there are four electrons remaining. This remaining five electrons will be filled in P
orbit.
Krypton Electronic Structure- [Ar] d10 4s2 4p6
Silver Electronic Structure- [Kr]4d10 5s1
Barium Electronic Structure – [Xe] 6s2
the electronic Structure of four element whose Atomic Number are 9, 36, 47 and 56. To write
electronic structure for large Atomic Number is difficult. To make it easy some element are used
as base whose last orbit is complete filled and further electrons are filled in further orbits.
6
two each so there are four electrons remaining. This remaining five electrons will be filled in P
orbit.
Krypton Electronic Structure- [Ar] d10 4s2 4p6
Silver Electronic Structure- [Kr]4d10 5s1
Barium Electronic Structure – [Xe] 6s2
the electronic Structure of four element whose Atomic Number are 9, 36, 47 and 56. To write
electronic structure for large Atomic Number is difficult. To make it easy some element are used
as base whose last orbit is complete filled and further electrons are filled in further orbits.
6

REFERENCES
Books and Journals
Sobel'Man, I.I., 2016. Introduction to the Theory of Atomic Spectra: International Series of
Monographs in Natural Philosophy (Vol. 40). Elsevier.
Roca, M., 2018. Is It a Meteorite? Applying Problem-Solving Strategy to Mass Spectra and
Atomic Mass.
Liao and et. al., 2016. Tailoring thermal conductivity of single-stranded carbon-chain polymers
through atomic mass modification. Scientific reports. 6. p.34999.
Stone, A.J., 2017. Natural bond orbitals and the nature of the hydrogen bond. The Journal of
Physical Chemistry A. 121(7). pp.1531-1534.
Inglesfield, J.E., 2018. Surface electronic structure. In Electronic Properties of Surfaces (pp. 1-
69). Routledge.
Online
The Structure of the Atom, 2019. [Online]. Available through :
<https://courses.lumenlearning.com/boundless-chemistry/chapter/the-structure-of-the-
atom/>.
Relative Atomic Mass, 2019. [Online]. Available through :
<https://www.senecalearning.com/definitions/isotopes/>.
ATOMIC NUMBER AND MASS NUMBERS, 2019.[Online]. Available through :
<http://www.ndeed.org/EducationResources/HighSchool/Radiography/atomicmassnumber.
htm>.
What is Relative Atomic Mass, 2019. [Online]. Available through :
<https://www.aplustopper.com/relative-atomic-mass-relative-molecular-mass-element/>.
What is Hybridization in Organic Chemistry, 2019. [Online]. Available through : <
http://theinfoscience.blogspot.com/2016/02/what-is-hybridization-in-organic.html>.
Electronic Structure of Atoms (Electron Configurations), 2019. [Online]. Available through : <
https://opentextbc.ca/chemistry/chapter/6-4-electronic-structure-of-atoms-electron-
configurations/>.
7
Books and Journals
Sobel'Man, I.I., 2016. Introduction to the Theory of Atomic Spectra: International Series of
Monographs in Natural Philosophy (Vol. 40). Elsevier.
Roca, M., 2018. Is It a Meteorite? Applying Problem-Solving Strategy to Mass Spectra and
Atomic Mass.
Liao and et. al., 2016. Tailoring thermal conductivity of single-stranded carbon-chain polymers
through atomic mass modification. Scientific reports. 6. p.34999.
Stone, A.J., 2017. Natural bond orbitals and the nature of the hydrogen bond. The Journal of
Physical Chemistry A. 121(7). pp.1531-1534.
Inglesfield, J.E., 2018. Surface electronic structure. In Electronic Properties of Surfaces (pp. 1-
69). Routledge.
Online
The Structure of the Atom, 2019. [Online]. Available through :
<https://courses.lumenlearning.com/boundless-chemistry/chapter/the-structure-of-the-
atom/>.
Relative Atomic Mass, 2019. [Online]. Available through :
<https://www.senecalearning.com/definitions/isotopes/>.
ATOMIC NUMBER AND MASS NUMBERS, 2019.[Online]. Available through :
<http://www.ndeed.org/EducationResources/HighSchool/Radiography/atomicmassnumber.
htm>.
What is Relative Atomic Mass, 2019. [Online]. Available through :
<https://www.aplustopper.com/relative-atomic-mass-relative-molecular-mass-element/>.
What is Hybridization in Organic Chemistry, 2019. [Online]. Available through : <
http://theinfoscience.blogspot.com/2016/02/what-is-hybridization-in-organic.html>.
Electronic Structure of Atoms (Electron Configurations), 2019. [Online]. Available through : <
https://opentextbc.ca/chemistry/chapter/6-4-electronic-structure-of-atoms-electron-
configurations/>.
7
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



