Chemistry Homework: Comparing Boiling Points and Intermolecular Forces
VerifiedAdded on 2020/03/04
|4
|634
|132
Homework Assignment
AI Summary
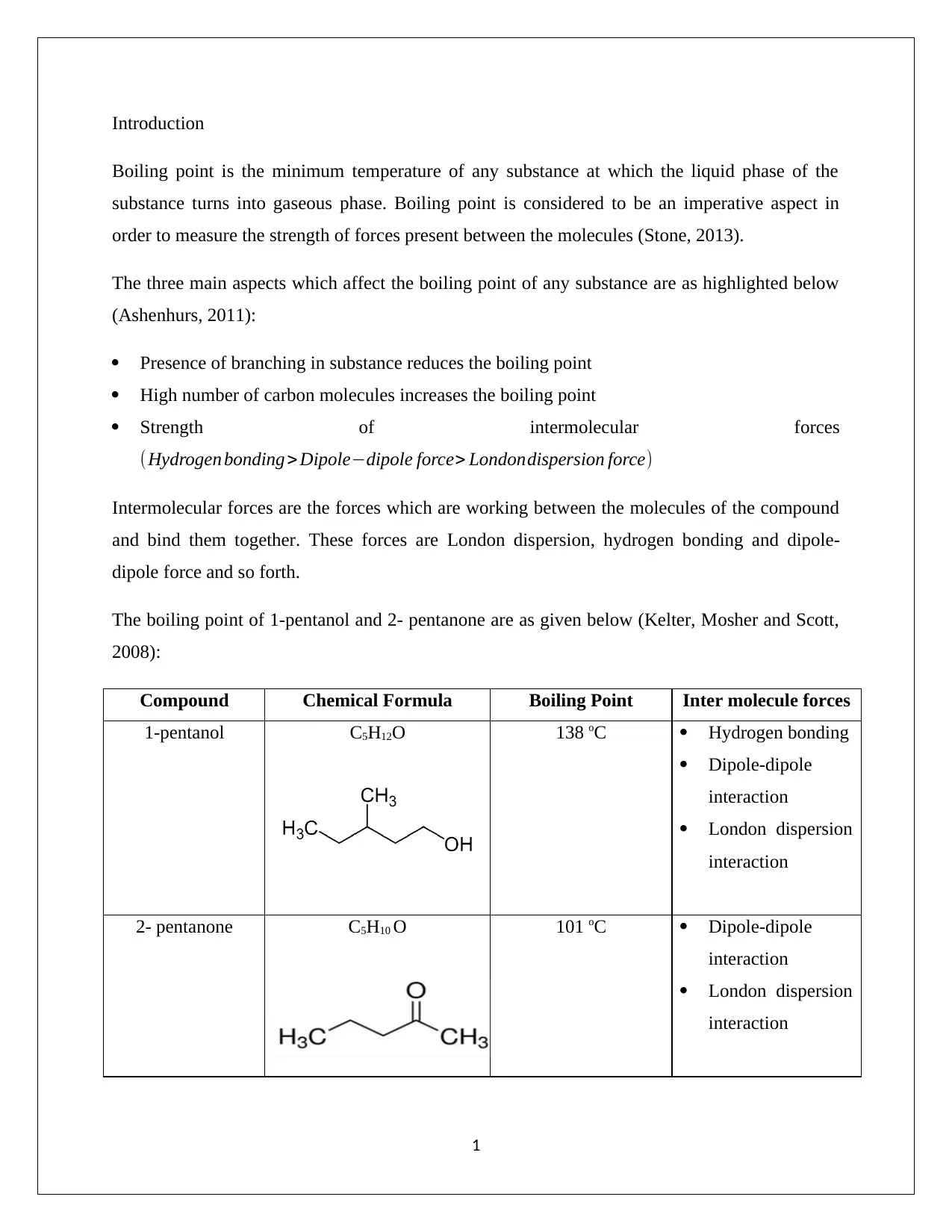
This chemistry assignment analyzes the boiling points of 1-pentanol and 2-pentanone, focusing on the influence of intermolecular forces. The introduction defines boiling point and highlights the factors affecting it, including branching, carbon chain length, and intermolecular forces. The assignment compares the boiling points of the two compounds, explaining that 1-pentanol has a higher boiling point due to the presence of hydrogen bonding, dipole-dipole interactions, and London dispersion forces, making its intermolecular forces stronger. The assignment contrasts this with 2-pentanone, which lacks hydrogen bonding, and therefore has weaker intermolecular forces. The conclusion reiterates that the strong intermolecular forces in 1-pentanol require more energy to break, resulting in a higher boiling point. The document includes references to relevant chemistry resources.
1 out of 4










![[object Object]](/_next/static/media/star-bottom.7253800d.svg)