University Chemistry Lab: Chromatography Experiment Analysis
VerifiedAdded on 2020/04/21
|5
|1339
|161
Practical Assignment
AI Summary
This document presents a comprehensive chromatography experiment, encompassing column, paper, and thin-layer chromatography techniques. The experiment details the separation of dye mixtures and plant pigments, including the determination of Rf values for various pigments like Xanthophyll, Chlorophyll a and b, and β-Carotene. The report includes observations on solvent behavior, such as ethanol and water, and their effects on dye migration. Furthermore, it addresses theoretical questions regarding the principles of chromatography, comparing and contrasting the three methods in terms of sample size, ease of use, and versatility. The analysis covers the identification of pigments in tomato paste and discusses factors influencing separation, such as adhesion, cohesion, and solubility. The conclusion summarizes the successful achievement of experimental objectives, acknowledges potential sources of error, and highlights the critical role of component properties in the separation process.

Running head: CHROMATOGRAPHY EXPERIMENT. 1
Chromatography Experiment
Name
Institution Affiliation
Chromatography Experiment
Name
Institution Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CHROMATOGRAPHY EXPERIMENT 2
Chromatography Experiment.
Solvent Observations
Ethanol On addition of ethanol as a solvent
approximately 13 ml of methylene blue dye
drained out of the column shown by the dark
blue eluent. During this time the fluorescein
dye only moved very slightly.
Water When water was added as a solvent caused it
caused the migration a greenish yellow liquid
which is most likely to be the fluorescein dye.
The eluent collected was around 14 ml.
Observations/Results:
Part A: Separation of a dye mixture by solid-liquid (column) chromatography.
Table 1: Separation of a dye mixture by solid-liquid (column) chromatography
Part B: Separation of plant pigments by paper chromatography
Table 2: Rf values of the different pigments in spinach.
Identify the pigments and their Rf values.
Distance travelled by solvent front: 10 cm
Rf = Distance travelled between the starting point and pigment line
Distance between the starting point and solvent front
Pigment Distance Travelled by Rf Value
Chromatography Experiment.
Solvent Observations
Ethanol On addition of ethanol as a solvent
approximately 13 ml of methylene blue dye
drained out of the column shown by the dark
blue eluent. During this time the fluorescein
dye only moved very slightly.
Water When water was added as a solvent caused it
caused the migration a greenish yellow liquid
which is most likely to be the fluorescein dye.
The eluent collected was around 14 ml.
Observations/Results:
Part A: Separation of a dye mixture by solid-liquid (column) chromatography.
Table 1: Separation of a dye mixture by solid-liquid (column) chromatography
Part B: Separation of plant pigments by paper chromatography
Table 2: Rf values of the different pigments in spinach.
Identify the pigments and their Rf values.
Distance travelled by solvent front: 10 cm
Rf = Distance travelled between the starting point and pigment line
Distance between the starting point and solvent front
Pigment Distance Travelled by Rf Value
CHROMATOGRAPHY EXPERIMENT 3
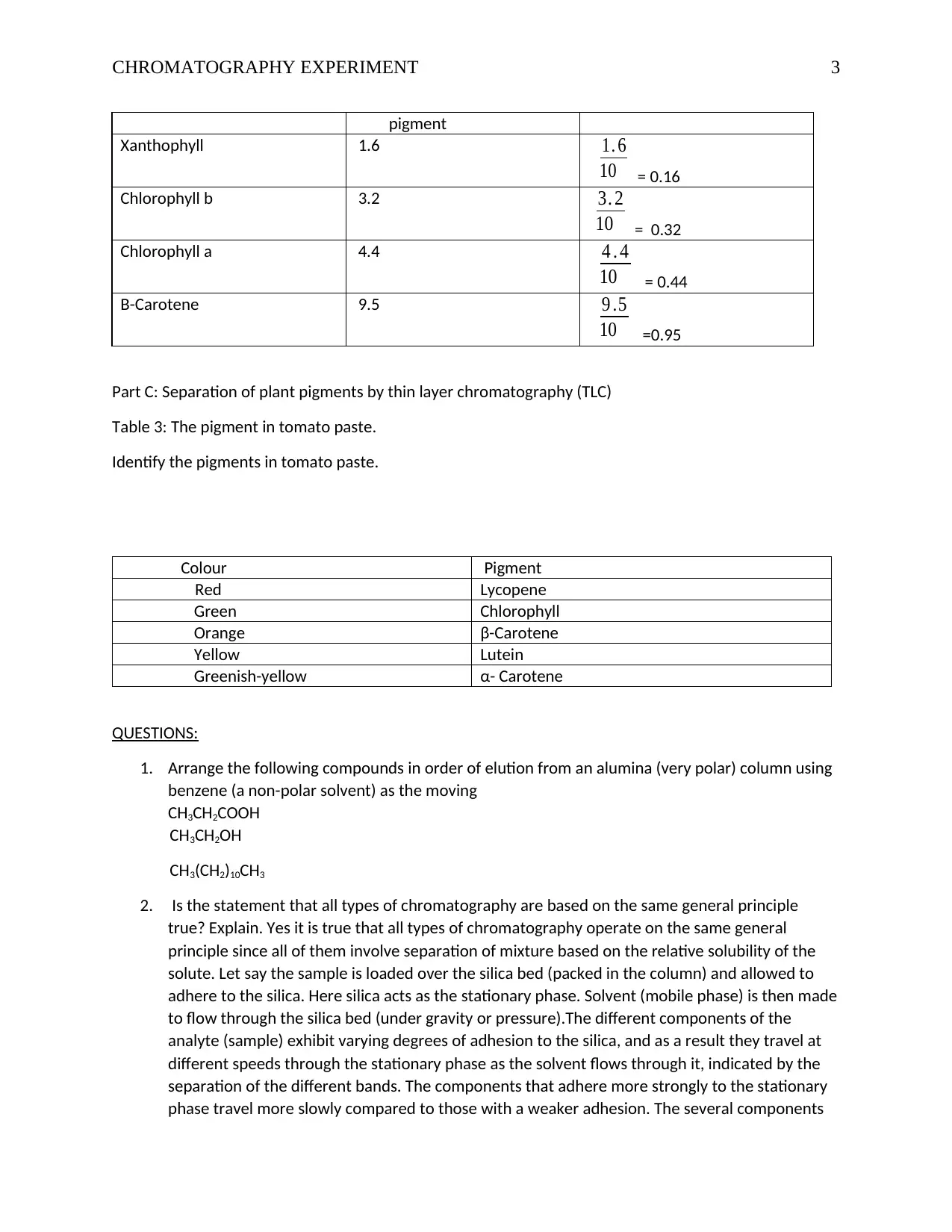
pigment
Xanthophyll 1.6 1. 6
10 = 0.16
Chlorophyll b 3.2 3. 2
10 = 0.32
Chlorophyll a 4.4 4 . 4
10 = 0.44
B-Carotene 9.5 9 .5
10 =0.95
Part C: Separation of plant pigments by thin layer chromatography (TLC)
Table 3: The pigment in tomato paste.
Identify the pigments in tomato paste.
Colour Pigment
Red Lycopene
Green Chlorophyll
Orange β-Carotene
Yellow Lutein
Greenish-yellow α- Carotene
QUESTIONS:
1. Arrange the following compounds in order of elution from an alumina (very polar) column using
benzene (a non-polar solvent) as the moving
CH3CH2COOH
CH3CH2OH
CH3(CH2)10CH3
2. Is the statement that all types of chromatography are based on the same general principle
true? Explain. Yes it is true that all types of chromatography operate on the same general
principle since all of them involve separation of mixture based on the relative solubility of the
solute. Let say the sample is loaded over the silica bed (packed in the column) and allowed to
adhere to the silica. Here silica acts as the stationary phase. Solvent (mobile phase) is then made
to flow through the silica bed (under gravity or pressure).The different components of the
analyte (sample) exhibit varying degrees of adhesion to the silica, and as a result they travel at
different speeds through the stationary phase as the solvent flows through it, indicated by the
separation of the different bands. The components that adhere more strongly to the stationary
phase travel more slowly compared to those with a weaker adhesion. The several components
pigment
Xanthophyll 1.6 1. 6
10 = 0.16
Chlorophyll b 3.2 3. 2
10 = 0.32
Chlorophyll a 4.4 4 . 4
10 = 0.44
B-Carotene 9.5 9 .5
10 =0.95
Part C: Separation of plant pigments by thin layer chromatography (TLC)
Table 3: The pigment in tomato paste.
Identify the pigments in tomato paste.
Colour Pigment
Red Lycopene
Green Chlorophyll
Orange β-Carotene
Yellow Lutein
Greenish-yellow α- Carotene
QUESTIONS:
1. Arrange the following compounds in order of elution from an alumina (very polar) column using
benzene (a non-polar solvent) as the moving
CH3CH2COOH
CH3CH2OH
CH3(CH2)10CH3
2. Is the statement that all types of chromatography are based on the same general principle
true? Explain. Yes it is true that all types of chromatography operate on the same general
principle since all of them involve separation of mixture based on the relative solubility of the
solute. Let say the sample is loaded over the silica bed (packed in the column) and allowed to
adhere to the silica. Here silica acts as the stationary phase. Solvent (mobile phase) is then made
to flow through the silica bed (under gravity or pressure).The different components of the
analyte (sample) exhibit varying degrees of adhesion to the silica, and as a result they travel at
different speeds through the stationary phase as the solvent flows through it, indicated by the
separation of the different bands. The components that adhere more strongly to the stationary
phase travel more slowly compared to those with a weaker adhesion. The several components
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CHROMATOGRAPHY EXPERIMENT 4
of the mixture separate because they travel at different speeds. Every component of the
mixture occupies certain time absorbed on the surface of the stationary phase and certain time
in the moving phase. The speed at which each of the component travel is dependent on the
fraction of time spent in each phase. A component that is highly absorbed by the stationary
phase will spend more time on the stationary phase hence move slowly over the stationary
phase compared to a component that is feebly absorbed by the stationary phase. This principle
is applied in all types of chromatography namely column chromatography, paper
chromatography, thin layer chromatography and gas chromatography. The proper choice of the
moving phase component and the stationary phase component determine the success of any
type of chromatography.
3. Compare and contrast the three types of chromatography you studied with respect to the size
of the sample that can be separated, the case of use, and the versatility of the method.
The chromatography methods I have studied in the above experiment are column chromatography,
paper chromatography and thin layer chromatography. Like any other methods used in qualitative
and quantitative analysis in chemistry, they all have their advantages and disadvantages. It’s
therefore upon the student or researcher to consider the various disadvantages and advantages
before settling on which type of chromatography to use.
Column chromatography is used in chemistry to purify individual chemical components from a
mixture. It is mostly used for preparative applications on measures from micrograms to kilograms.
The comparative low cost and the disposability of the stationary phase stand out as the key
advantages of this technique. The disposability of the stationary phase prevents its cross-
contamination and degradation due to recycling. This type of chromatography is also divided into
two methods according to the technique of preparing the column namely the wet method and the
dry method. This gives the researcher a choice on which method to use considering the properties
of the mixture to be separated. Running several columns at a time is one way of increasing the
productivity of this particular method. In conclusion, this type of chromatography is useful not only
in identifying the individual components on the mixture but also in separating the components. The
eluents of coloured compounds can be seen as moving bands through the glass wall.
Paper chromatography as one of the types of chromatography can be used for very less quantitative
materials therefore it will be ineffective to use it to analyse large amounts of compound mixtures.
Paper chromatography provides a simple and cost effective means for separation of components of
mixtures. The technique was simple to understand and did not require any expensive equipment. It
provided a visual understanding of the consept of separation science. Another advantage of paper
chromatography is that it can be used to identify both inorganic and organic compounds thus giving
it a wide variety of applications. One of the short comings of this method is that it is not an accurate
compared to other techniques. As mentioned earlier, it cannot be applied for large quantity of
sample .Paper chromatography is also not effective when applied in quantitative analysis. This
technique cannot be used to separate complex mixture.
Thin layer chromatography is a commonly used technique for detection and separation of
components from a mixture of compounds. Non-volatile compounds are mostly separated using this
technique. As the name suggests, it is performed on thin aluminium, glass or plastic sheet that is
covered with different media. The frequently used media is silica gel and cellulose. Thin layer
chromatography has the advantage of using very few equipment .It is a simple and very sensitive
of the mixture separate because they travel at different speeds. Every component of the
mixture occupies certain time absorbed on the surface of the stationary phase and certain time
in the moving phase. The speed at which each of the component travel is dependent on the
fraction of time spent in each phase. A component that is highly absorbed by the stationary
phase will spend more time on the stationary phase hence move slowly over the stationary
phase compared to a component that is feebly absorbed by the stationary phase. This principle
is applied in all types of chromatography namely column chromatography, paper
chromatography, thin layer chromatography and gas chromatography. The proper choice of the
moving phase component and the stationary phase component determine the success of any
type of chromatography.
3. Compare and contrast the three types of chromatography you studied with respect to the size
of the sample that can be separated, the case of use, and the versatility of the method.
The chromatography methods I have studied in the above experiment are column chromatography,
paper chromatography and thin layer chromatography. Like any other methods used in qualitative
and quantitative analysis in chemistry, they all have their advantages and disadvantages. It’s
therefore upon the student or researcher to consider the various disadvantages and advantages
before settling on which type of chromatography to use.
Column chromatography is used in chemistry to purify individual chemical components from a
mixture. It is mostly used for preparative applications on measures from micrograms to kilograms.
The comparative low cost and the disposability of the stationary phase stand out as the key
advantages of this technique. The disposability of the stationary phase prevents its cross-
contamination and degradation due to recycling. This type of chromatography is also divided into
two methods according to the technique of preparing the column namely the wet method and the
dry method. This gives the researcher a choice on which method to use considering the properties
of the mixture to be separated. Running several columns at a time is one way of increasing the
productivity of this particular method. In conclusion, this type of chromatography is useful not only
in identifying the individual components on the mixture but also in separating the components. The
eluents of coloured compounds can be seen as moving bands through the glass wall.
Paper chromatography as one of the types of chromatography can be used for very less quantitative
materials therefore it will be ineffective to use it to analyse large amounts of compound mixtures.
Paper chromatography provides a simple and cost effective means for separation of components of
mixtures. The technique was simple to understand and did not require any expensive equipment. It
provided a visual understanding of the consept of separation science. Another advantage of paper
chromatography is that it can be used to identify both inorganic and organic compounds thus giving
it a wide variety of applications. One of the short comings of this method is that it is not an accurate
compared to other techniques. As mentioned earlier, it cannot be applied for large quantity of
sample .Paper chromatography is also not effective when applied in quantitative analysis. This
technique cannot be used to separate complex mixture.
Thin layer chromatography is a commonly used technique for detection and separation of
components from a mixture of compounds. Non-volatile compounds are mostly separated using this
technique. As the name suggests, it is performed on thin aluminium, glass or plastic sheet that is
covered with different media. The frequently used media is silica gel and cellulose. Thin layer
chromatography has the advantage of using very few equipment .It is a simple and very sensitive
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CHROMATOGRAPHY EXPERIMENT 5
method of separating mixtures. The components elute out quickly hence the components will be
separated in a short period of time. Quantitative separation of zones is possible because separating
and recovering of the components in the mixture is easily done by scratching the powdery coat on
the thin layer plate.
Thin layer chromatography also has various disadvantages. In this technique the length of the plate
is limited making separation to occur up to only given length. However, since this technique is
carried out in an open environment other physical properties may affect the separation of the
mixture .The properties that may affect the separation are temperature and humidity of the
surrounding environment.
Conclusion
The experiment was a success since all the objectives were met. The Rf values of the various
components were calculated successful. Any deviation from the theoretical values learnt in class
may be due to errors in determining the centre of the dot of the sample, wrong reading of the
distances or errors in calculation process. We were able to carry out the three types of
chromatography to separate various mixtures. The experiment illustrated how adhesion and
cohesion properties of component play a critical role on the separation technique. Solubility of the
various components in the solvent determine the rate of sepration.
method of separating mixtures. The components elute out quickly hence the components will be
separated in a short period of time. Quantitative separation of zones is possible because separating
and recovering of the components in the mixture is easily done by scratching the powdery coat on
the thin layer plate.
Thin layer chromatography also has various disadvantages. In this technique the length of the plate
is limited making separation to occur up to only given length. However, since this technique is
carried out in an open environment other physical properties may affect the separation of the
mixture .The properties that may affect the separation are temperature and humidity of the
surrounding environment.
Conclusion
The experiment was a success since all the objectives were met. The Rf values of the various
components were calculated successful. Any deviation from the theoretical values learnt in class
may be due to errors in determining the centre of the dot of the sample, wrong reading of the
distances or errors in calculation process. We were able to carry out the three types of
chromatography to separate various mixtures. The experiment illustrated how adhesion and
cohesion properties of component play a critical role on the separation technique. Solubility of the
various components in the solvent determine the rate of sepration.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


