Chemistry Assignment: Core Principles of Chemistry - TAQ 1-6
VerifiedAdded on 2023/01/18
|11
|2290
|66
Homework Assignment
AI Summary
This comprehensive chemistry assignment delves into several core principles. It begins with an analysis of periodic trends, examining the properties of alkali metals (Group 1) and halogens (Group 7), and trends across Period III, including atomic radius, ionization energy, melting and boiling points. The assignment then explores redox reactions, identifying oxidized and reduced reactants in various equations. Further, it covers transition metals, defining their characteristics, differentiating them from other metals, and exploring complex formation and color changes. Isomerism is also discussed, including chain, position, and functional group isomerism, with examples and structural formulas. The assignment continues with an examination of reaction rates and the concentration of reagents, including determining reaction orders and rate equations. Finally, the assignment concludes with an exploration of acids and bases, including definitions, differences between strong and weak acids, the use of universal indicators, pH calculations, and buffer solutions. The assignment provides a solid foundation in fundamental chemical concepts.

Core Principles of Chemistry
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
TAQ 1: Trends across periodic table and in group 1 and 7
Trends down group 1 (Alkali Metals)
Group 1 elements (Alkali metals) are a group of chemical elements from the s-block of
the periodic table having similar physical and chemical properties. They are silvery in color,
shiny and soft. Group 1 element are highly reactive at STP (standard temperature and pressure)
and ready to lose their outermost electron to form cation. The group 1 elements includes; lithium
(Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) (Kostiner,
2003). Table 1 below shows the atomic radius, ionization energy, melting and boiling point of
Alkali metals
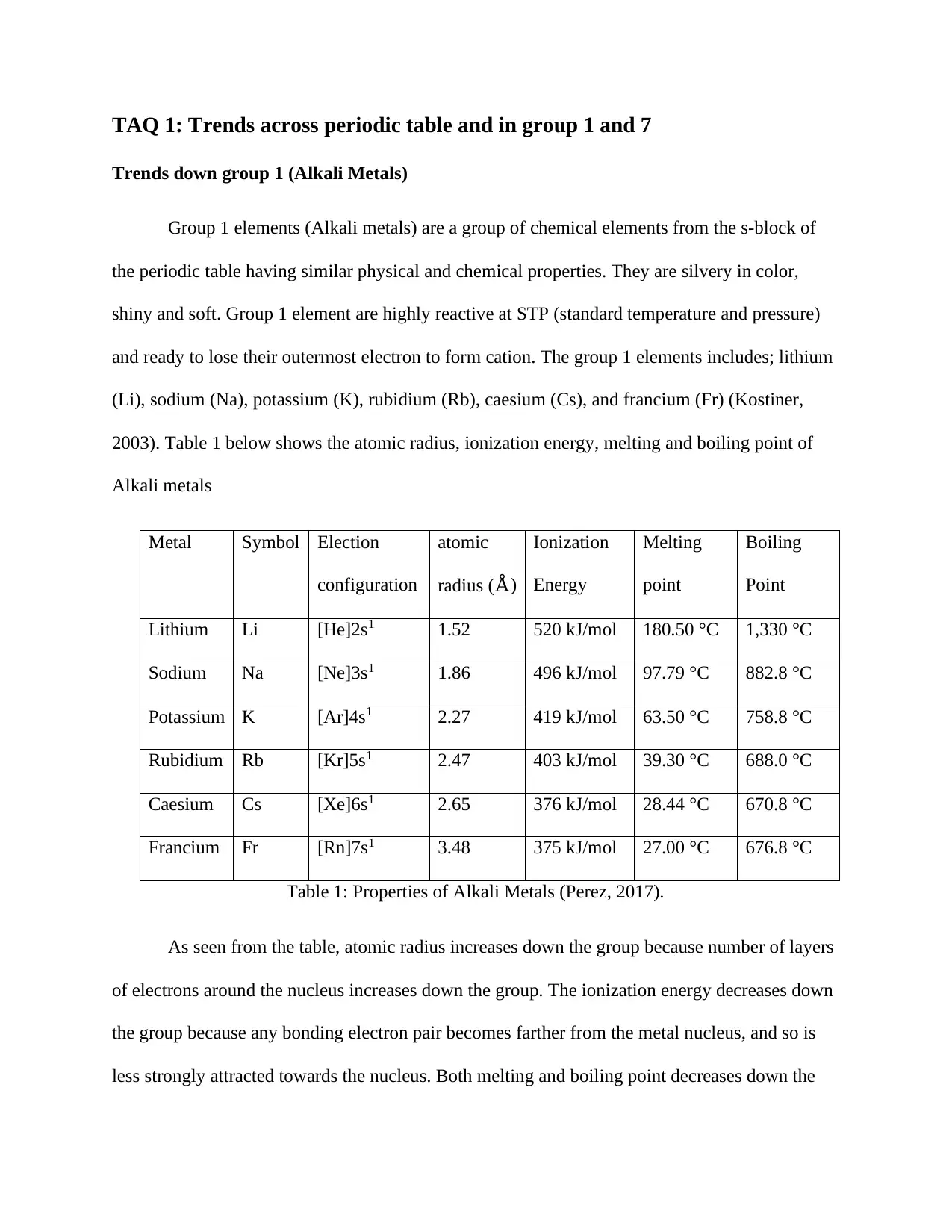
Metal Symbol Election
configuration
atomic
radius (Å)
Ionization
Energy
Melting
point
Boiling
Point
Lithium Li [He]2s1 1.52 520 kJ/mol 180.50 °C 1,330 °C
Sodium Na [Ne]3s1 1.86 496 kJ/mol 97.79 °C 882.8 °C
Potassium K [Ar]4s1 2.27 419 kJ/mol 63.50 °C 758.8 °C
Rubidium Rb [Kr]5s1 2.47 403 kJ/mol 39.30 °C 688.0 °C
Caesium Cs [Xe]6s1 2.65 376 kJ/mol 28.44 °C 670.8 °C
Francium Fr [Rn]7s1 3.48 375 kJ/mol 27.00 °C 676.8 °C
Table 1: Properties of Alkali Metals (Perez, 2017).
As seen from the table, atomic radius increases down the group because number of layers
of electrons around the nucleus increases down the group. The ionization energy decreases down
the group because any bonding electron pair becomes farther from the metal nucleus, and so is
less strongly attracted towards the nucleus. Both melting and boiling point decreases down the
Trends down group 1 (Alkali Metals)
Group 1 elements (Alkali metals) are a group of chemical elements from the s-block of
the periodic table having similar physical and chemical properties. They are silvery in color,
shiny and soft. Group 1 element are highly reactive at STP (standard temperature and pressure)
and ready to lose their outermost electron to form cation. The group 1 elements includes; lithium
(Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) (Kostiner,
2003). Table 1 below shows the atomic radius, ionization energy, melting and boiling point of
Alkali metals
Metal Symbol Election
configuration
atomic
radius (Å)
Ionization
Energy
Melting
point
Boiling
Point
Lithium Li [He]2s1 1.52 520 kJ/mol 180.50 °C 1,330 °C
Sodium Na [Ne]3s1 1.86 496 kJ/mol 97.79 °C 882.8 °C
Potassium K [Ar]4s1 2.27 419 kJ/mol 63.50 °C 758.8 °C
Rubidium Rb [Kr]5s1 2.47 403 kJ/mol 39.30 °C 688.0 °C
Caesium Cs [Xe]6s1 2.65 376 kJ/mol 28.44 °C 670.8 °C
Francium Fr [Rn]7s1 3.48 375 kJ/mol 27.00 °C 676.8 °C
Table 1: Properties of Alkali Metals (Perez, 2017).
As seen from the table, atomic radius increases down the group because number of layers
of electrons around the nucleus increases down the group. The ionization energy decreases down
the group because any bonding electron pair becomes farther from the metal nucleus, and so is
less strongly attracted towards the nucleus. Both melting and boiling point decreases down the
group since the metals have metallic bond which depends on the nucleus attraction. As the size
of atoms increase down the group, the distance between the nuclei and delocalized electrons
increases hence decreasing the electronic bond, thus MP and BP decreases down the group
(Anon., n.d.).
Trends down group 7 (Halogens)
Group 7 elements (Halogens) are the family of chemical elements which include fluorine
(F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). This element exists as diatomic
molecules. Table 2 below shows some physical and chemical trends down the group.
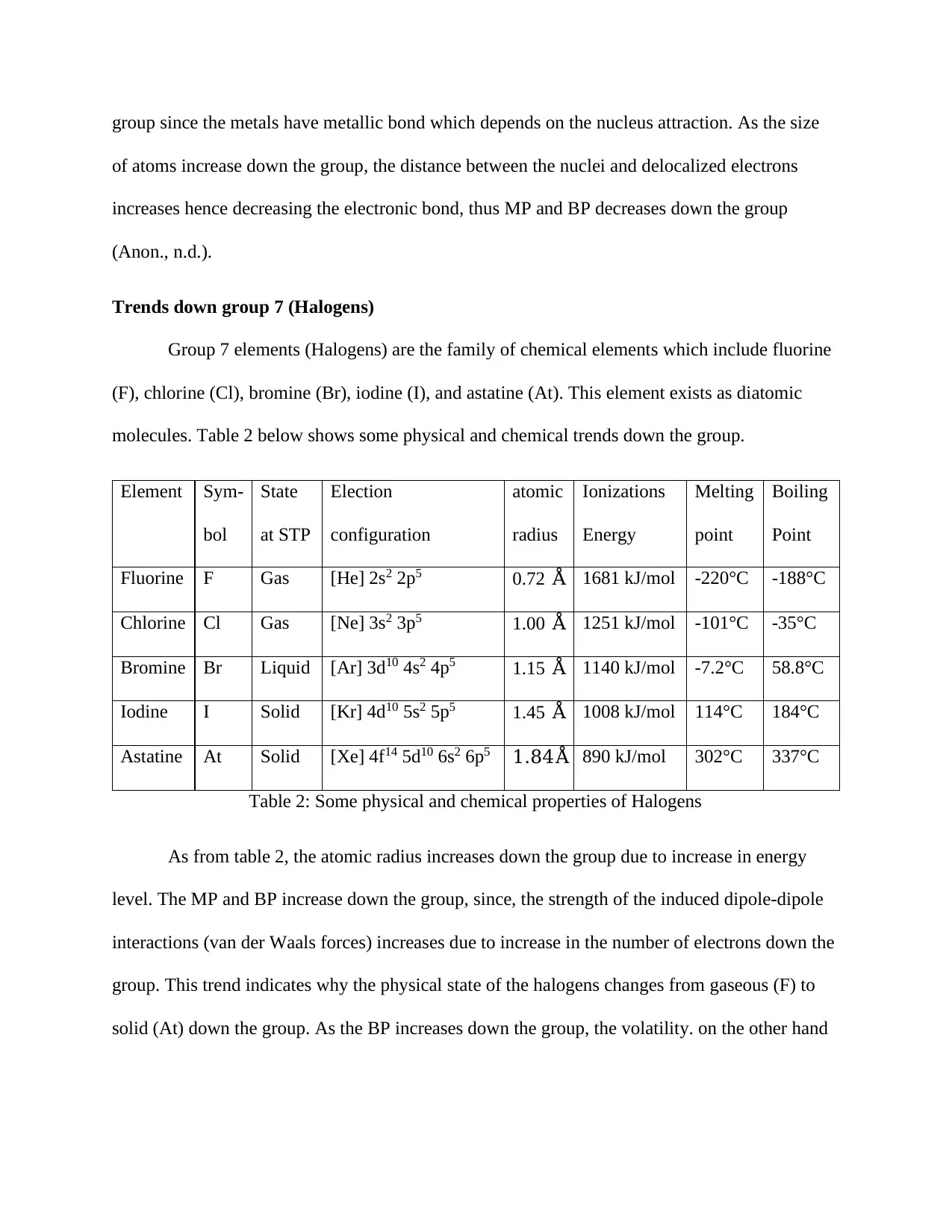
Element Sym-
bol
State
at STP
Election
configuration
atomic
radius
Ionizations
Energy
Melting
point
Boiling
Point
Fluorine F Gas [He] 2s2 2p5 0.72 Å 1681 kJ/mol -220°C -188°C
Chlorine Cl Gas [Ne] 3s2 3p5 1.00 Å 1251 kJ/mol -101°C -35°C
Bromine Br Liquid [Ar] 3d10 4s2 4p5 1.15 Å 1140 kJ/mol -7.2°C 58.8°C
Iodine I Solid [Kr] 4d10 5s2 5p5 1.45 Å 1008 kJ/mol 114°C 184°C
Astatine At Solid [Xe] 4f14 5d10 6s2 6p5 1.84Å 890 kJ/mol 302°C 337°C
Table 2: Some physical and chemical properties of Halogens
As from table 2, the atomic radius increases down the group due to increase in energy
level. The MP and BP increase down the group, since, the strength of the induced dipole-dipole
interactions (van der Waals forces) increases due to increase in the number of electrons down the
group. This trend indicates why the physical state of the halogens changes from gaseous (F) to
solid (At) down the group. As the BP increases down the group, the volatility. on the other hand
of atoms increase down the group, the distance between the nuclei and delocalized electrons
increases hence decreasing the electronic bond, thus MP and BP decreases down the group
(Anon., n.d.).
Trends down group 7 (Halogens)
Group 7 elements (Halogens) are the family of chemical elements which include fluorine
(F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). This element exists as diatomic
molecules. Table 2 below shows some physical and chemical trends down the group.
Element Sym-
bol
State
at STP
Election
configuration
atomic
radius
Ionizations
Energy
Melting
point
Boiling
Point
Fluorine F Gas [He] 2s2 2p5 0.72 Å 1681 kJ/mol -220°C -188°C
Chlorine Cl Gas [Ne] 3s2 3p5 1.00 Å 1251 kJ/mol -101°C -35°C
Bromine Br Liquid [Ar] 3d10 4s2 4p5 1.15 Å 1140 kJ/mol -7.2°C 58.8°C
Iodine I Solid [Kr] 4d10 5s2 5p5 1.45 Å 1008 kJ/mol 114°C 184°C
Astatine At Solid [Xe] 4f14 5d10 6s2 6p5 1.84Å 890 kJ/mol 302°C 337°C
Table 2: Some physical and chemical properties of Halogens
As from table 2, the atomic radius increases down the group due to increase in energy
level. The MP and BP increase down the group, since, the strength of the induced dipole-dipole
interactions (van der Waals forces) increases due to increase in the number of electrons down the
group. This trend indicates why the physical state of the halogens changes from gaseous (F) to
solid (At) down the group. As the BP increases down the group, the volatility. on the other hand
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
decreases down the group. As the atomic radius increases down the group, the force required to
remove an electron decreases, thus the ionization energy decreases down the group (Anon., n.d.).
Trends across period III
Table 3 shows some physical and chemical properties of period 3 elements
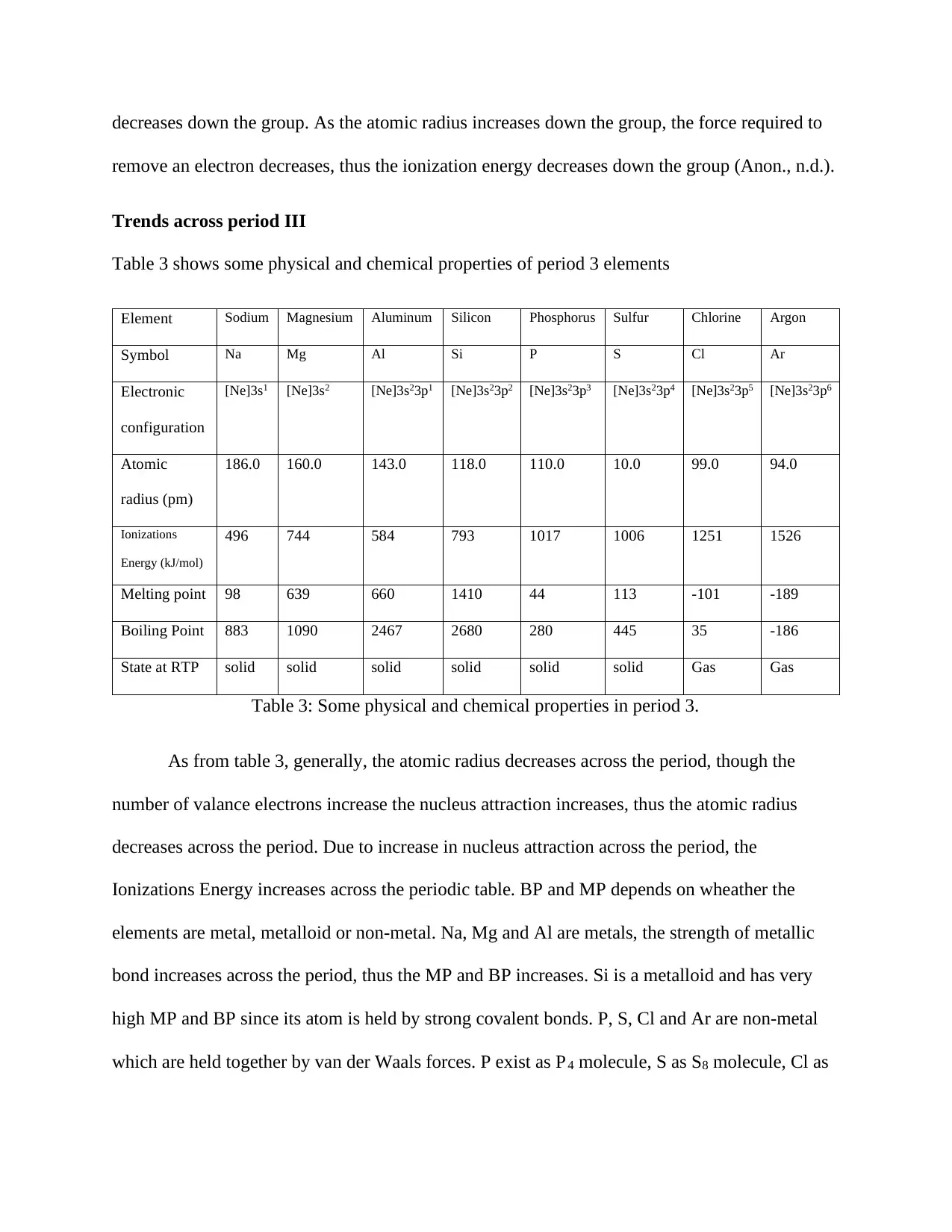
Element Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Symbol Na Mg Al Si P S Cl Ar
Electronic
configuration
[Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6
Atomic
radius (pm)
186.0 160.0 143.0 118.0 110.0 10.0 99.0 94.0
Ionizations
Energy (kJ/mol)
496 744 584 793 1017 1006 1251 1526
Melting point 98 639 660 1410 44 113 -101 -189
Boiling Point 883 1090 2467 2680 280 445 35 -186
State at RTP solid solid solid solid solid solid Gas Gas
Table 3: Some physical and chemical properties in period 3.
As from table 3, generally, the atomic radius decreases across the period, though the
number of valance electrons increase the nucleus attraction increases, thus the atomic radius
decreases across the period. Due to increase in nucleus attraction across the period, the
Ionizations Energy increases across the periodic table. BP and MP depends on wheather the
elements are metal, metalloid or non-metal. Na, Mg and Al are metals, the strength of metallic
bond increases across the period, thus the MP and BP increases. Si is a metalloid and has very
high MP and BP since its atom is held by strong covalent bonds. P, S, Cl and Ar are non-metal
which are held together by van der Waals forces. P exist as P4 molecule, S as S8 molecule, Cl as
remove an electron decreases, thus the ionization energy decreases down the group (Anon., n.d.).
Trends across period III
Table 3 shows some physical and chemical properties of period 3 elements
Element Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Symbol Na Mg Al Si P S Cl Ar
Electronic
configuration
[Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6
Atomic
radius (pm)
186.0 160.0 143.0 118.0 110.0 10.0 99.0 94.0
Ionizations
Energy (kJ/mol)
496 744 584 793 1017 1006 1251 1526
Melting point 98 639 660 1410 44 113 -101 -189
Boiling Point 883 1090 2467 2680 280 445 35 -186
State at RTP solid solid solid solid solid solid Gas Gas
Table 3: Some physical and chemical properties in period 3.
As from table 3, generally, the atomic radius decreases across the period, though the
number of valance electrons increase the nucleus attraction increases, thus the atomic radius
decreases across the period. Due to increase in nucleus attraction across the period, the
Ionizations Energy increases across the periodic table. BP and MP depends on wheather the
elements are metal, metalloid or non-metal. Na, Mg and Al are metals, the strength of metallic
bond increases across the period, thus the MP and BP increases. Si is a metalloid and has very
high MP and BP since its atom is held by strong covalent bonds. P, S, Cl and Ar are non-metal
which are held together by van der Waals forces. P exist as P4 molecule, S as S8 molecule, Cl as
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Cl2 molecule and Ar as monatomic molecule. The strength of the van der Waals forces decrease
as the molecular size decreases, thus the MP and BP decreases from P to Ar.
TAQ 2: Redox Reactions
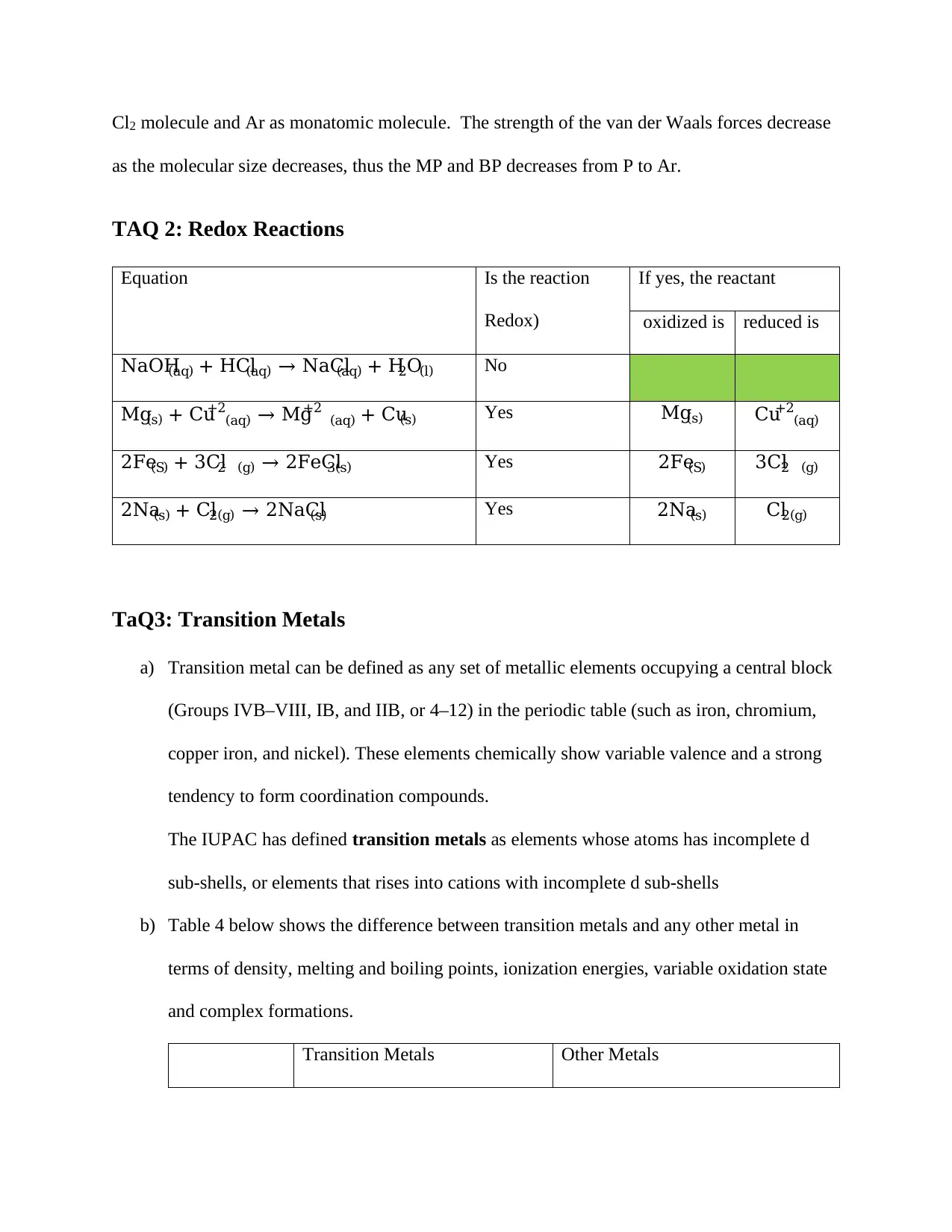
Equation Is the reaction
Redox)
If yes, the reactant
oxidized is reduced is
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) No
Mg(s) + Cu (aq)
+2 → Mg (aq)
+2 + Cu(s) Yes Mg(s) Cu (aq)
+2
2Fe(S) + 3Cl2 (g) → 2FeCl3(s) Yes 2Fe(S) 3Cl2 (g)
2Na(s) + Cl2(g) → 2NaCl(s) Yes 2Na(s) Cl2(g)
TaQ3: Transition Metals
a) Transition metal can be defined as any set of metallic elements occupying a central block
(Groups IVB–VIII, IB, and IIB, or 4–12) in the periodic table (such as iron, chromium,
copper iron, and nickel). These elements chemically show variable valence and a strong
tendency to form coordination compounds.
The IUPAC has defined transition metals as elements whose atoms has incomplete d
sub-shells, or elements that rises into cations with incomplete d sub-shells
b) Table 4 below shows the difference between transition metals and any other metal in
terms of density, melting and boiling points, ionization energies, variable oxidation state
and complex formations.
Transition Metals Other Metals
as the molecular size decreases, thus the MP and BP decreases from P to Ar.
TAQ 2: Redox Reactions
Equation Is the reaction
Redox)
If yes, the reactant
oxidized is reduced is
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) No
Mg(s) + Cu (aq)
+2 → Mg (aq)
+2 + Cu(s) Yes Mg(s) Cu (aq)
+2
2Fe(S) + 3Cl2 (g) → 2FeCl3(s) Yes 2Fe(S) 3Cl2 (g)
2Na(s) + Cl2(g) → 2NaCl(s) Yes 2Na(s) Cl2(g)
TaQ3: Transition Metals
a) Transition metal can be defined as any set of metallic elements occupying a central block
(Groups IVB–VIII, IB, and IIB, or 4–12) in the periodic table (such as iron, chromium,
copper iron, and nickel). These elements chemically show variable valence and a strong
tendency to form coordination compounds.
The IUPAC has defined transition metals as elements whose atoms has incomplete d
sub-shells, or elements that rises into cations with incomplete d sub-shells
b) Table 4 below shows the difference between transition metals and any other metal in
terms of density, melting and boiling points, ionization energies, variable oxidation state
and complex formations.
Transition Metals Other Metals
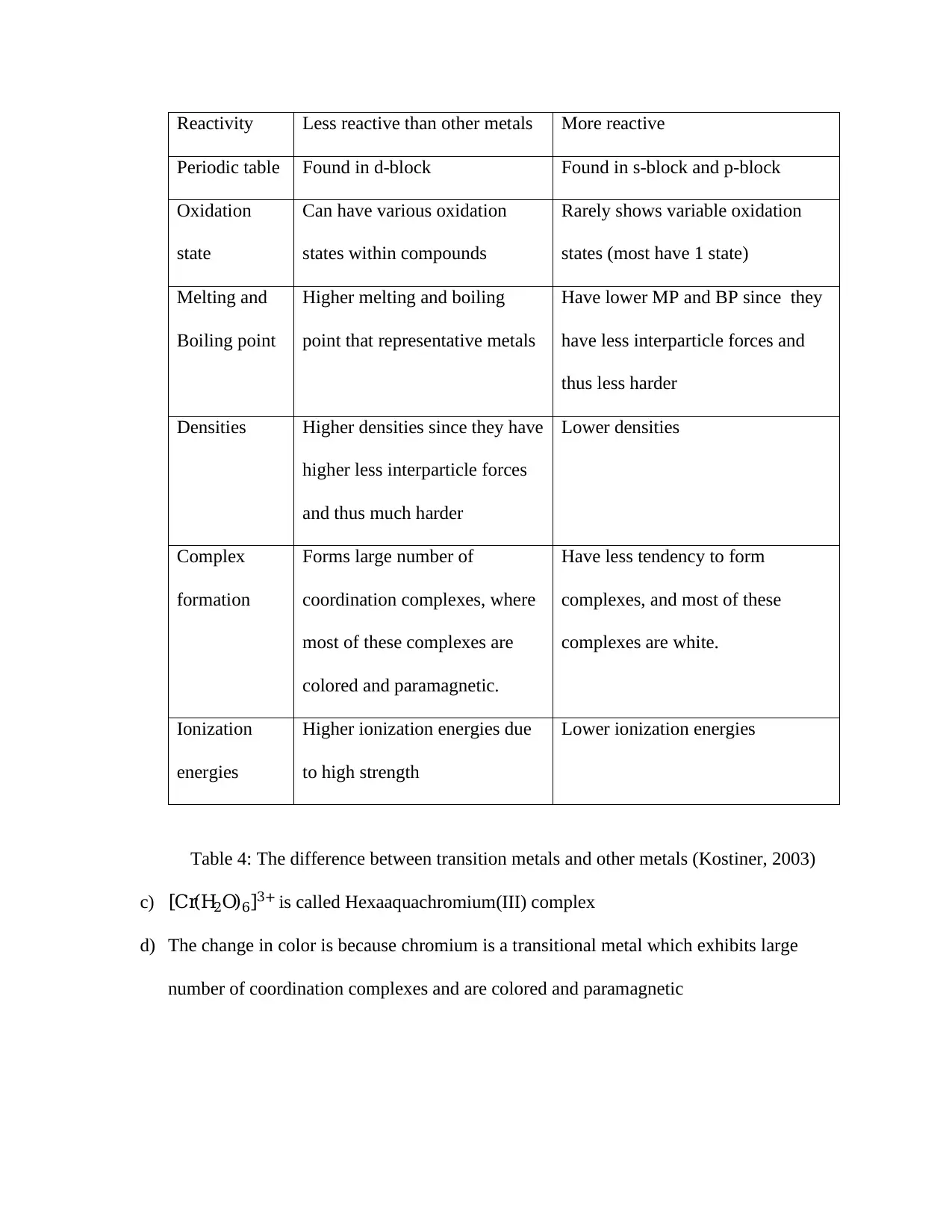
Reactivity Less reactive than other metals More reactive
Periodic table Found in d-block Found in s-block and p-block
Oxidation
state
Can have various oxidation
states within compounds
Rarely shows variable oxidation
states (most have 1 state)
Melting and
Boiling point
Higher melting and boiling
point that representative metals
Have lower MP and BP since they
have less interparticle forces and
thus less harder
Densities Higher densities since they have
higher less interparticle forces
and thus much harder
Lower densities
Complex
formation
Forms large number of
coordination complexes, where
most of these complexes are
colored and paramagnetic.
Have less tendency to form
complexes, and most of these
complexes are white.
Ionization
energies
Higher ionization energies due
to high strength
Lower ionization energies
Table 4: The difference between transition metals and other metals (Kostiner, 2003)
c) [Cr(H2O)6]3+ is called Hexaaquachromium(III) complex
d) The change in color is because chromium is a transitional metal which exhibits large
number of coordination complexes and are colored and paramagnetic
Periodic table Found in d-block Found in s-block and p-block
Oxidation
state
Can have various oxidation
states within compounds
Rarely shows variable oxidation
states (most have 1 state)
Melting and
Boiling point
Higher melting and boiling
point that representative metals
Have lower MP and BP since they
have less interparticle forces and
thus less harder
Densities Higher densities since they have
higher less interparticle forces
and thus much harder
Lower densities
Complex
formation
Forms large number of
coordination complexes, where
most of these complexes are
colored and paramagnetic.
Have less tendency to form
complexes, and most of these
complexes are white.
Ionization
energies
Higher ionization energies due
to high strength
Lower ionization energies
Table 4: The difference between transition metals and other metals (Kostiner, 2003)
c) [Cr(H2O)6]3+ is called Hexaaquachromium(III) complex
d) The change in color is because chromium is a transitional metal which exhibits large
number of coordination complexes and are colored and paramagnetic
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
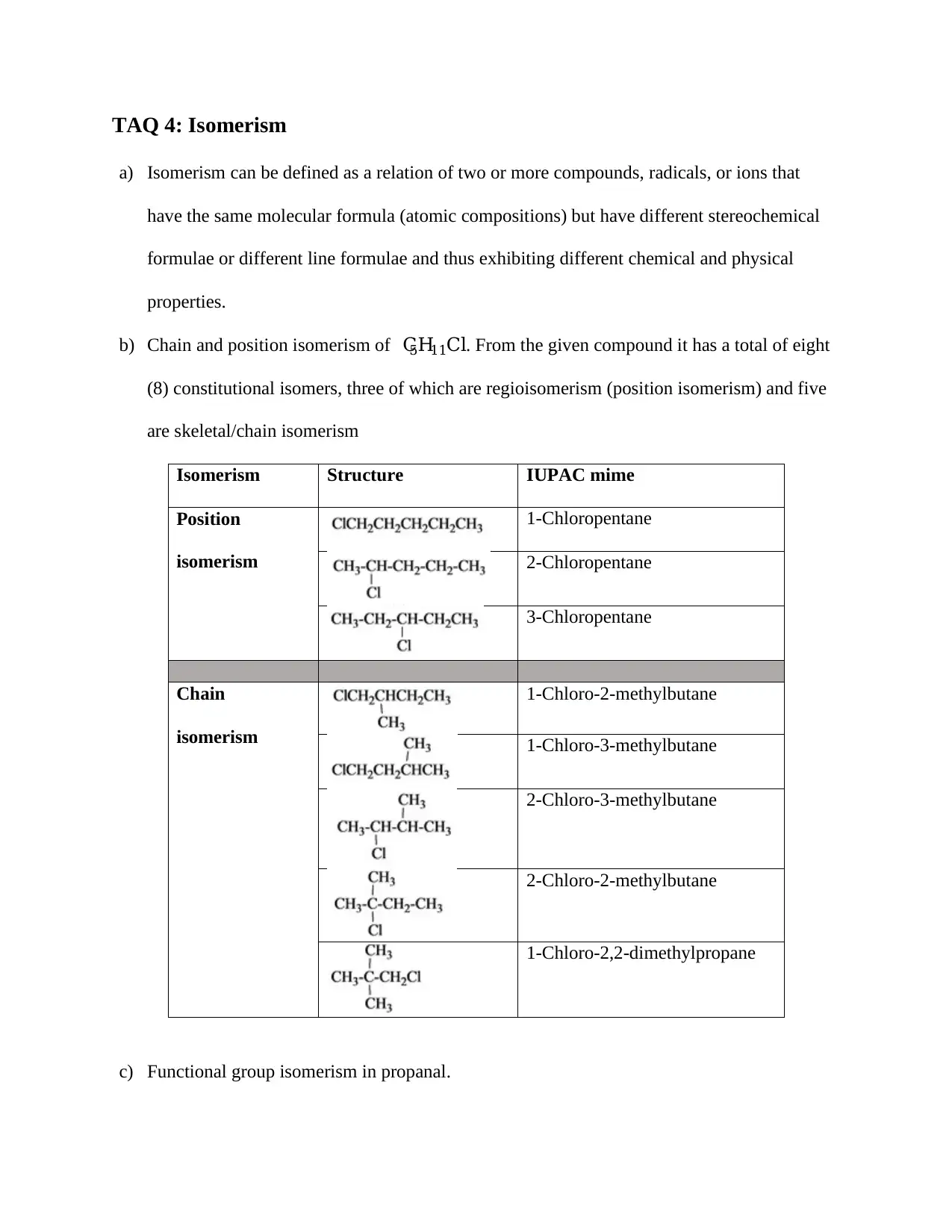
TAQ 4: Isomerism
a) Isomerism can be defined as a relation of two or more compounds, radicals, or ions that
have the same molecular formula (atomic compositions) but have different stereochemical
formulae or different line formulae and thus exhibiting different chemical and physical
properties.
b) Chain and position isomerism of C5H11Cl. From the given compound it has a total of eight
(8) constitutional isomers, three of which are regioisomerism (position isomerism) and five
are skeletal/chain isomerism
Isomerism Structure IUPAC mime
Position
isomerism
1-Chloropentane
2-Chloropentane
3-Chloropentane
Chain
isomerism
1-Chloro-2-methylbutane
1-Chloro-3-methylbutane
2-Chloro-3-methylbutane
2-Chloro-2-methylbutane
1-Chloro-2,2-dimethylpropane
c) Functional group isomerism in propanal.
a) Isomerism can be defined as a relation of two or more compounds, radicals, or ions that
have the same molecular formula (atomic compositions) but have different stereochemical
formulae or different line formulae and thus exhibiting different chemical and physical
properties.
b) Chain and position isomerism of C5H11Cl. From the given compound it has a total of eight
(8) constitutional isomers, three of which are regioisomerism (position isomerism) and five
are skeletal/chain isomerism
Isomerism Structure IUPAC mime
Position
isomerism
1-Chloropentane
2-Chloropentane
3-Chloropentane
Chain
isomerism
1-Chloro-2-methylbutane
1-Chloro-3-methylbutane
2-Chloro-3-methylbutane
2-Chloro-2-methylbutane
1-Chloro-2,2-dimethylpropane
c) Functional group isomerism in propanal.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Functional group isomerism is an example of structural isomerism. Functional isomerism
occurs when substances having the same molecular formula but different functional groups.
For instant the functional group isomerism of propanal, C3H6O is given below
i) aldehyde
propionaldehyde
ii) ketone
Acetone
iii) Enol form
1-propene-ol
d) Given the first four members of alkane homologous series, the general formular for the
series can be given as:
CnH2n+2 for n = 1, 2, 3, 4 … … …
The formula for the 5Th and 9Th member of the series are:
5TH C5H(5×2+2) ⟹ C5H12 ⇒ CH3CH2CH2CH2CH3 Pentane
5TH C9H(9×2+2) ⟹ C10H20 ⇒ CH3CH2CH2CH2CH2CH2CH2CH2CH3 nonane
TAQ 5: Concentration of Reagents.
a) Orders of Reaction with respect to A
rate = k[A]x[b]y
3.2 × 10−2
1.6 × 10−2 = [0.8
0.4
]
𝑥
⟹ 𝑥 = 1
b) Orders of Reaction with respect to A
occurs when substances having the same molecular formula but different functional groups.
For instant the functional group isomerism of propanal, C3H6O is given below
i) aldehyde
propionaldehyde
ii) ketone
Acetone
iii) Enol form
1-propene-ol
d) Given the first four members of alkane homologous series, the general formular for the
series can be given as:
CnH2n+2 for n = 1, 2, 3, 4 … … …
The formula for the 5Th and 9Th member of the series are:
5TH C5H(5×2+2) ⟹ C5H12 ⇒ CH3CH2CH2CH2CH3 Pentane
5TH C9H(9×2+2) ⟹ C10H20 ⇒ CH3CH2CH2CH2CH2CH2CH2CH2CH3 nonane
TAQ 5: Concentration of Reagents.
a) Orders of Reaction with respect to A
rate = k[A]x[b]y
3.2 × 10−2
1.6 × 10−2 = [0.8
0.4
]
𝑥
⟹ 𝑥 = 1
b) Orders of Reaction with respect to A

rate = k[A]x[b]y
0.4 × 10−2
1.6 × 10−2 = [0.2
0.4
]
𝑦
⟹ 𝑦 = 2
c) Overall order of the reaction
𝑛 = 1 + 2 = 3
d) Rate equation for the reaction
rate = k[A]1[b]2
e) The value of rate constant.
𝑘 = 𝑟𝑎𝑡𝑒
[A]1[b]2
Taking any reaction, let say the second
k = 1.6 × 10−2
[0.4]1[0.04]2 = 25 𝑚𝑜𝑙 𝑑𝑚−3 𝑠−1
TAQ 6: Acids and Bases
a) Definition of acid and base
According to Brønsted-Lowry Theory
Acids is a proton (hydrogen ion) donor.
Base is a proton (hydrogen ion) accepter
b) The difference between a strong acid and a weak acid is that a strong acid like
Hydrochloric acid (HCl) dissociate completely in aqueous solution or in water. Weak
acid like ethanoic acid (CH3COOH) dissociate only slightly in water
c) The universal indicator can be used to determine the pH of a solution in reference to
hydrogen ions and Hydronium ions:
0.4 × 10−2
1.6 × 10−2 = [0.2
0.4
]
𝑦
⟹ 𝑦 = 2
c) Overall order of the reaction
𝑛 = 1 + 2 = 3
d) Rate equation for the reaction
rate = k[A]1[b]2
e) The value of rate constant.
𝑘 = 𝑟𝑎𝑡𝑒
[A]1[b]2
Taking any reaction, let say the second
k = 1.6 × 10−2
[0.4]1[0.04]2 = 25 𝑚𝑜𝑙 𝑑𝑚−3 𝑠−1
TAQ 6: Acids and Bases
a) Definition of acid and base
According to Brønsted-Lowry Theory
Acids is a proton (hydrogen ion) donor.
Base is a proton (hydrogen ion) accepter
b) The difference between a strong acid and a weak acid is that a strong acid like
Hydrochloric acid (HCl) dissociate completely in aqueous solution or in water. Weak
acid like ethanoic acid (CH3COOH) dissociate only slightly in water
c) The universal indicator can be used to determine the pH of a solution in reference to
hydrogen ions and Hydronium ions:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The indicator has different colors that indicate the pH of a given solution. It shows the
molecules interaction and separation into the H3O+ ion and the OH− ion
d) Calculating the pH of 0.10 mol/dm3 solution ethanoic acid having a dissociation constant
1.8 × 10−5 mol/dm3
𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞) + 𝐻2𝑂(𝑙) ⇌ 𝐻3𝑂 (𝑎𝑞)
+ + 𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
−
ka = [𝐻3𝑂 (𝑎𝑞)
+ ][𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
− ]
[𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞)]
[𝐻3𝑂 (𝑎𝑞)
+ ] = [𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
− ] = 𝑥
1.8 × 105 = 𝑥2
0.1
𝑥 =√1.8 × 10−5 × 0.1 = 1.34164 × 10−3
𝑝𝐻 = − log(𝐻3𝑂 (𝑎𝑞)
+ ) = − log(1.34164 × 10−3) = 2.9
e) A buffer solution is one which resists changes in pH when small quantities of an acid or
an alkali are added to it
For example a mixture of ethanoic acid and sodium ethanoate in solution. Here, if the
solution contained equal molar concentrations of both the acid and the salt, it remains at a
constant pH
H3COOH(aq) ⇌ H3COO (aq)
− + H (aq)
+
Adding acid in the equilibrium above does not affect much the equilibrium point.
molecules interaction and separation into the H3O+ ion and the OH− ion
d) Calculating the pH of 0.10 mol/dm3 solution ethanoic acid having a dissociation constant
1.8 × 10−5 mol/dm3
𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞) + 𝐻2𝑂(𝑙) ⇌ 𝐻3𝑂 (𝑎𝑞)
+ + 𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
−
ka = [𝐻3𝑂 (𝑎𝑞)
+ ][𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
− ]
[𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞)]
[𝐻3𝑂 (𝑎𝑞)
+ ] = [𝐶𝐻3𝐶𝑂𝑂 (𝑎𝑞)
− ] = 𝑥
1.8 × 105 = 𝑥2
0.1
𝑥 =√1.8 × 10−5 × 0.1 = 1.34164 × 10−3
𝑝𝐻 = − log(𝐻3𝑂 (𝑎𝑞)
+ ) = − log(1.34164 × 10−3) = 2.9
e) A buffer solution is one which resists changes in pH when small quantities of an acid or
an alkali are added to it
For example a mixture of ethanoic acid and sodium ethanoate in solution. Here, if the
solution contained equal molar concentrations of both the acid and the salt, it remains at a
constant pH
H3COOH(aq) ⇌ H3COO (aq)
− + H (aq)
+
Adding acid in the equilibrium above does not affect much the equilibrium point.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Anon., n.d. Group 1: Properties of Alkali Metals. [Online]
Available at:
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorgani
c_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_s-
Block_Elements/Group__1%3A_The_Alkali_Metals/1Group_1%3A_Physical_Properties_of_Al
kali_Metals
[Accessed 25 April 2019].
Anon., n.d. Group 17: General Properties of Halogens. [Online]
Available at:
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorgani
c_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-
Block_Elements/Group_17%3A_The_Halogens/0Group_17%3A_Physical_Properties_of_the_H
alogens/Group_17%
[Accessed 25 April 2019].
Kostiner, E., 2003. Chemistry. Hauppauge, N.Y.: Barron's.
Perez, J. C., 2017. Symmetry and Asymmetry in the MENDELEEV’s Periodic Table Predictive
EQUATION. SDRP Journal of Computational Chemistry & Molecular Modelling, 2(1).
Anon., n.d. Group 1: Properties of Alkali Metals. [Online]
Available at:
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorgani
c_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_s-
Block_Elements/Group__1%3A_The_Alkali_Metals/1Group_1%3A_Physical_Properties_of_Al
kali_Metals
[Accessed 25 April 2019].
Anon., n.d. Group 17: General Properties of Halogens. [Online]
Available at:
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorgani
c_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-
Block_Elements/Group_17%3A_The_Halogens/0Group_17%3A_Physical_Properties_of_the_H
alogens/Group_17%
[Accessed 25 April 2019].
Kostiner, E., 2003. Chemistry. Hauppauge, N.Y.: Barron's.
Perez, J. C., 2017. Symmetry and Asymmetry in the MENDELEEV’s Periodic Table Predictive
EQUATION. SDRP Journal of Computational Chemistry & Molecular Modelling, 2(1).
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

