University Chemistry Assignment: Thermodynamics and Equilibrium
VerifiedAdded on 2022/12/19
|6
|1218
|49
Homework Assignment
AI Summary
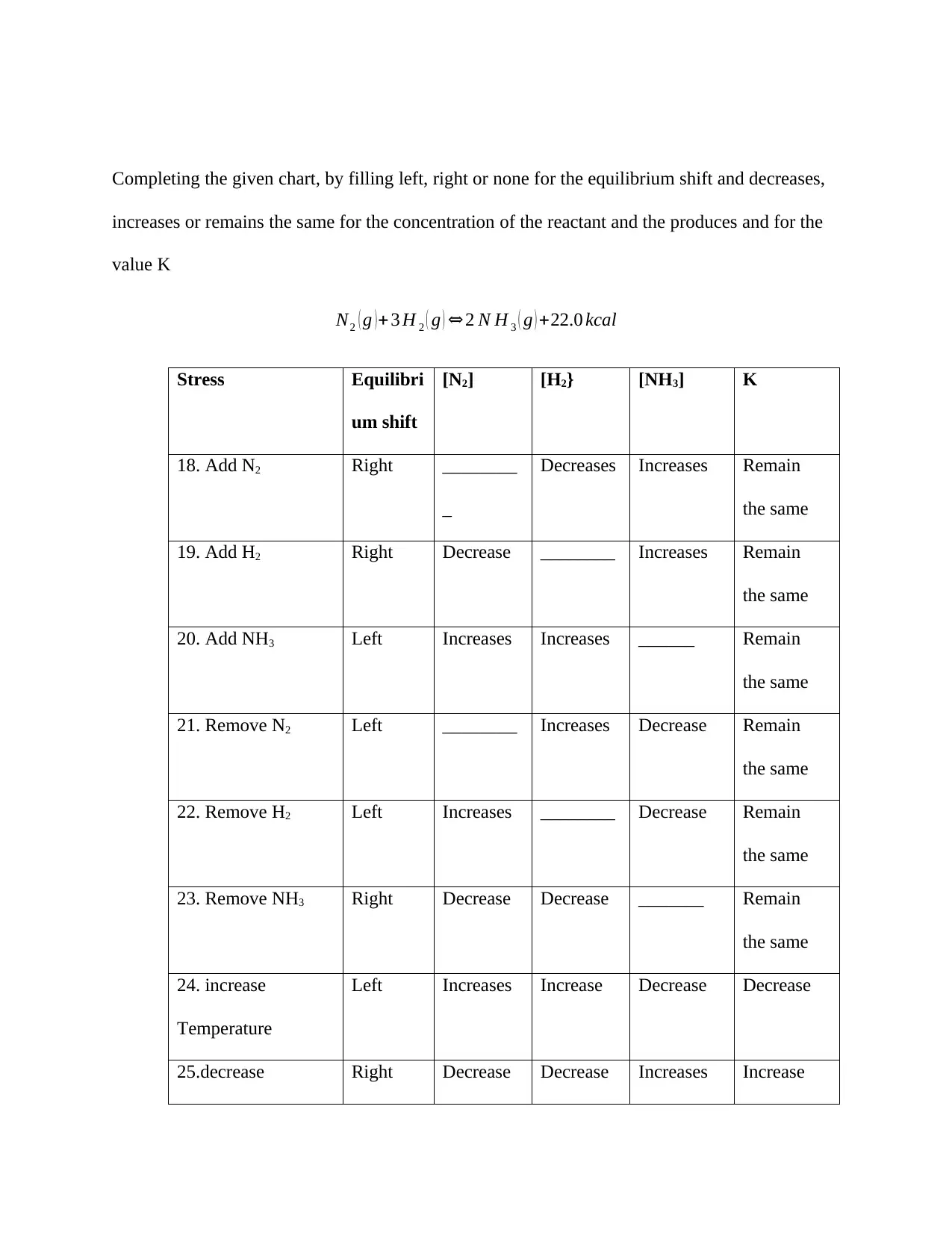
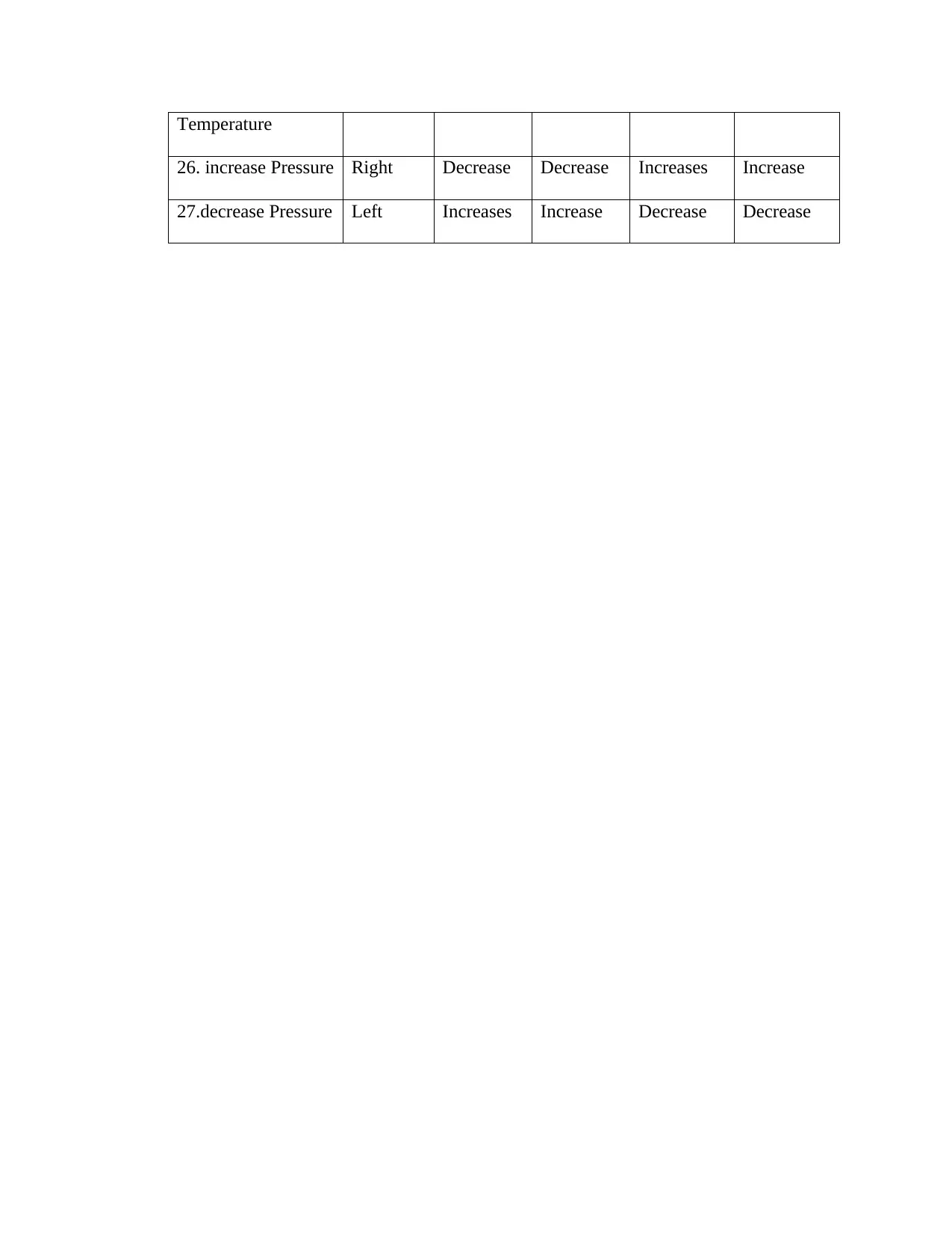
This document presents a comprehensive solution to a chemistry assignment, addressing key concepts in thermodynamics and chemical equilibrium. It begins with calculations involving enthalpy changes, determining energy released or absorbed during the combustion of methane and related reactions, including calculations for energy released based on moles and mass of reactants. The assignment then delves into heat capacity calculations, determining the heat capacity of water and calculating final temperatures in scenarios involving heat transfer between substances. Furthermore, the document explores equilibrium principles, calculating equilibrium constants (Keq) and reaction quotients (Q) to predict the direction of reaction shifts under various conditions. The assignment also includes a detailed analysis of Le Chatelier's principle, predicting the effects of changes in concentration, temperature, and pressure on equilibrium position and reactant/product concentrations for several chemical reactions. The solutions are presented with step-by-step calculations and explanations, providing a thorough understanding of the concepts involved.
1 out of 6











![[object Object]](/_next/static/media/star-bottom.7253800d.svg)