Chemistry Homework Solutions
VerifiedAdded on 2019/10/09
|3
|456
|194
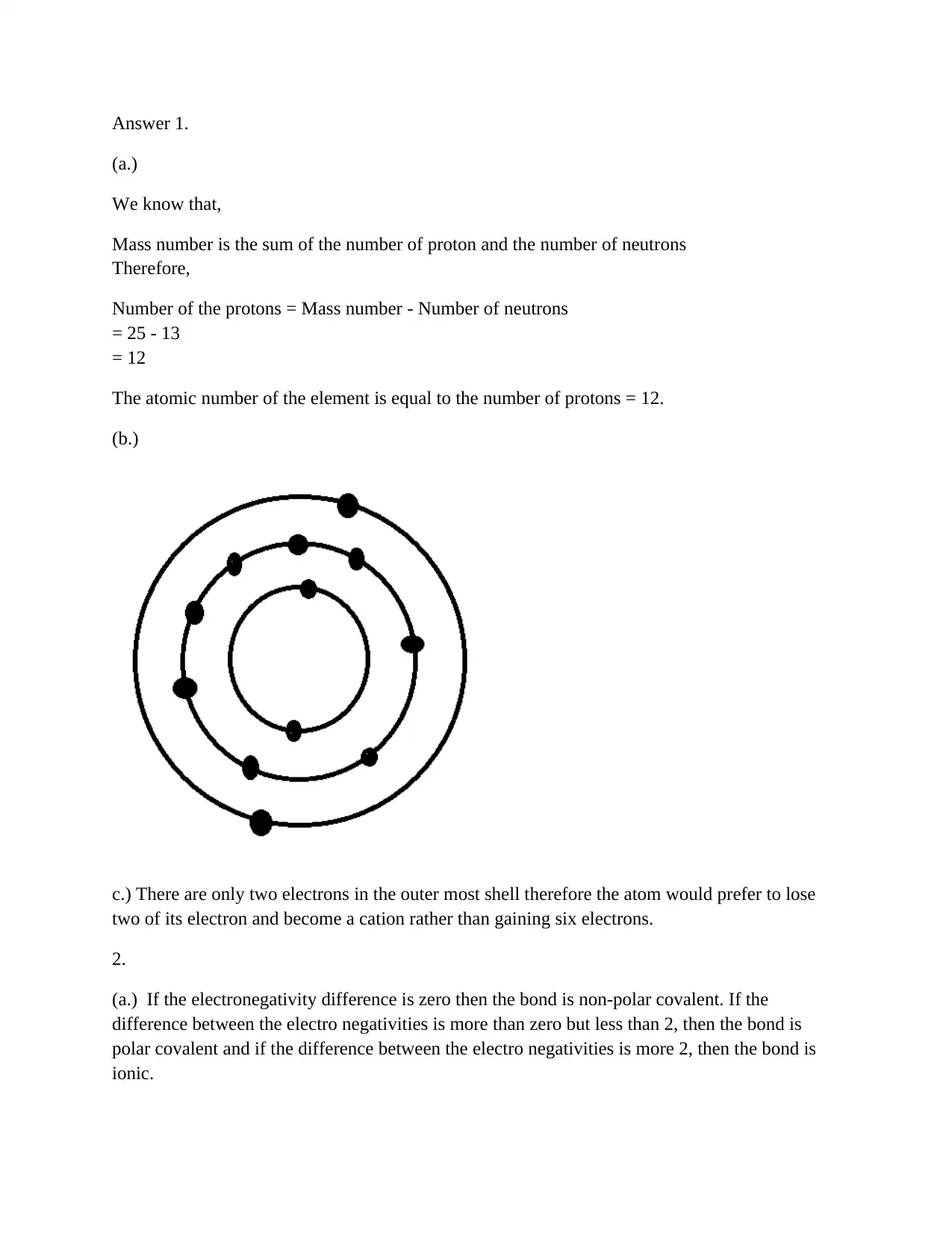
Homework Assignment
AI Summary
This document presents solutions to a chemistry homework assignment. The solutions cover several topics, including determining the number of protons in an element given its mass number and number of neutrons, identifying the type of bond (polar covalent, non-polar covalent, or ionic) based on electronegativity differences, and explaining the solubility of NaCl in water and methanol. The solutions provide detailed explanations and calculations for each problem.
1 out of 3







![[object Object]](/_next/static/media/star-bottom.7253800d.svg)