Year 10 Chemistry Practical: Hypo Concentration & Reaction Rate
VerifiedAdded on 2023/06/14
|8
|1429
|259
Practical Assignment
AI Summary
This chemistry practical report investigates the effect of hypo solution concentration on the rate of reaction with hydrochloric acid. The experiment involves varying the concentration of hypo solution and measuring the time taken for a visual indicator (a cross) to disappear, indicating the reaction's progress. The results, presented in a table and graph, demonstrate the relationship between concentration and reaction rate. The discussion analyzes the data, explaining how the concentration of reactants affects the reaction rate based on collision theory. The report concludes that increasing the concentration of hypo solution generally increases the reaction rate, supported by the experimental findings. The document includes a detailed methodology, results, and a comprehensive bibliography. Desklib provides access to similar documents and study resources for students.

Chemistry 1
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 2
Title:
How does the concentration of hypo solution affect the rate of reaction with hydrochloric acid.
Aim:
To investigate how the concentration of hypo solution affects the rate of reaction time with
hydrochloric acid.
THEORY
The viability and level of reaction are very significant in any chemical reaction. The viability of
a chemical reaction can be anticipated by thermodynamics and the level can be obtained from
chemical equilibrium. Such as extent and feasibility, it is significant to understand that the
factors and the rate influencing the chemical reaction rate1.
In any chemical reaction, the reactant is used up, as a result, new products will be produced.
The chemical reaction is shown below
Sodium Thiosulphate + Hydrochloric acid Sulphur + Sodium Chloride + Sodium
Dioxide + Water.
Na2S203+2HCL S +2NaCl +SO2+H2O
Some of the factors which affect this type of reaction include the following
Temperature
1 Mohamed, Ahmed. 2011. Chemistry of enthalpy. 3rd. London: Springer.
Title:
How does the concentration of hypo solution affect the rate of reaction with hydrochloric acid.
Aim:
To investigate how the concentration of hypo solution affects the rate of reaction time with
hydrochloric acid.
THEORY
The viability and level of reaction are very significant in any chemical reaction. The viability of
a chemical reaction can be anticipated by thermodynamics and the level can be obtained from
chemical equilibrium. Such as extent and feasibility, it is significant to understand that the
factors and the rate influencing the chemical reaction rate1.
In any chemical reaction, the reactant is used up, as a result, new products will be produced.
The chemical reaction is shown below
Sodium Thiosulphate + Hydrochloric acid Sulphur + Sodium Chloride + Sodium
Dioxide + Water.
Na2S203+2HCL S +2NaCl +SO2+H2O
Some of the factors which affect this type of reaction include the following
Temperature
1 Mohamed, Ahmed. 2011. Chemistry of enthalpy. 3rd. London: Springer.

Chemistry 3
The concentration of the reactant
Catalyst
Physical state
A spontaneous chemical reaction between the hypo solution and the hydro chloric acid can be
illustrated by the following diagram.
Fig 1: Showing the spontaneous reaction diagram
For this reaction, the concentration of the hypo-solution is the independent variable while the
dependent variable is the rate of reaction as measured by the time the cross took to disappear.
But this reaction is controlled by the following variables2.
The volume of hydrochloric acid
The volume of hypo solution
Materials:
2 Lvov, Boris. 2012. Thermal Decomposition of Solids and Melts: New Thermochemical Approach
to the Mechanism, Kinetics, and Methodology. 3rd. Hull: Springer Science & Business Media.
The concentration of the reactant
Catalyst
Physical state
A spontaneous chemical reaction between the hypo solution and the hydro chloric acid can be
illustrated by the following diagram.
Fig 1: Showing the spontaneous reaction diagram
For this reaction, the concentration of the hypo-solution is the independent variable while the
dependent variable is the rate of reaction as measured by the time the cross took to disappear.
But this reaction is controlled by the following variables2.
The volume of hydrochloric acid
The volume of hypo solution
Materials:
2 Lvov, Boris. 2012. Thermal Decomposition of Solids and Melts: New Thermochemical Approach
to the Mechanism, Kinetics, and Methodology. 3rd. Hull: Springer Science & Business Media.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry 4
Hydrochloric acid (2m) solution
Sodium thiosulfate (hypo solution)
20 ml and 100 ml measuring cylinders
100 ml flask
Large mat
Pen
Stopwatch
White paper
Different volumes of Water
250 ml beaker
Method:
The required amount of hypo solution was put inside a 250 ml beaker, a 100 ml measuring
cylinder was used to measure 50 ml of hypo solution. A cross was drawn on a white piece of
paper. The 50 ml of hypo solution were transferred to a 100 ml flask, the flask was positioned so
it’s center was directly above the cross. 5 ml of hydrochloric acid were added to the hypo
solution and swirled twice to mix it’s contents at the same time starting the stopwatch. When
viewed down the mouth of the flask. The stopwatch was stopped when the cross disappeared.
The process was repeated and the concentration of the hypo solution (sodium thiosulphate) was
changed by adding water to it. The hypo solution and water were added of equal amount to 50
ml. For instance, if 30 ml of hypo solution were in the cylinder, 20 ml of water should be added
Hydrochloric acid (2m) solution
Sodium thiosulfate (hypo solution)
20 ml and 100 ml measuring cylinders
100 ml flask
Large mat
Pen
Stopwatch
White paper
Different volumes of Water
250 ml beaker
Method:
The required amount of hypo solution was put inside a 250 ml beaker, a 100 ml measuring
cylinder was used to measure 50 ml of hypo solution. A cross was drawn on a white piece of
paper. The 50 ml of hypo solution were transferred to a 100 ml flask, the flask was positioned so
it’s center was directly above the cross. 5 ml of hydrochloric acid were added to the hypo
solution and swirled twice to mix it’s contents at the same time starting the stopwatch. When
viewed down the mouth of the flask. The stopwatch was stopped when the cross disappeared.
The process was repeated and the concentration of the hypo solution (sodium thiosulphate) was
changed by adding water to it. The hypo solution and water were added of equal amount to 50
ml. For instance, if 30 ml of hypo solution were in the cylinder, 20 ml of water should be added
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 5
so they equal 50. The data was recorded and the observations looked at how the concentration of
hypo solution affects its reaction with the hydrochloric acid and effects the results3.
RESULTS
Volume (m) Time (s)
19.91
0.2 25.27
0.15 32.43
0.1 45.15
0.5 75.55
0.025 192.66
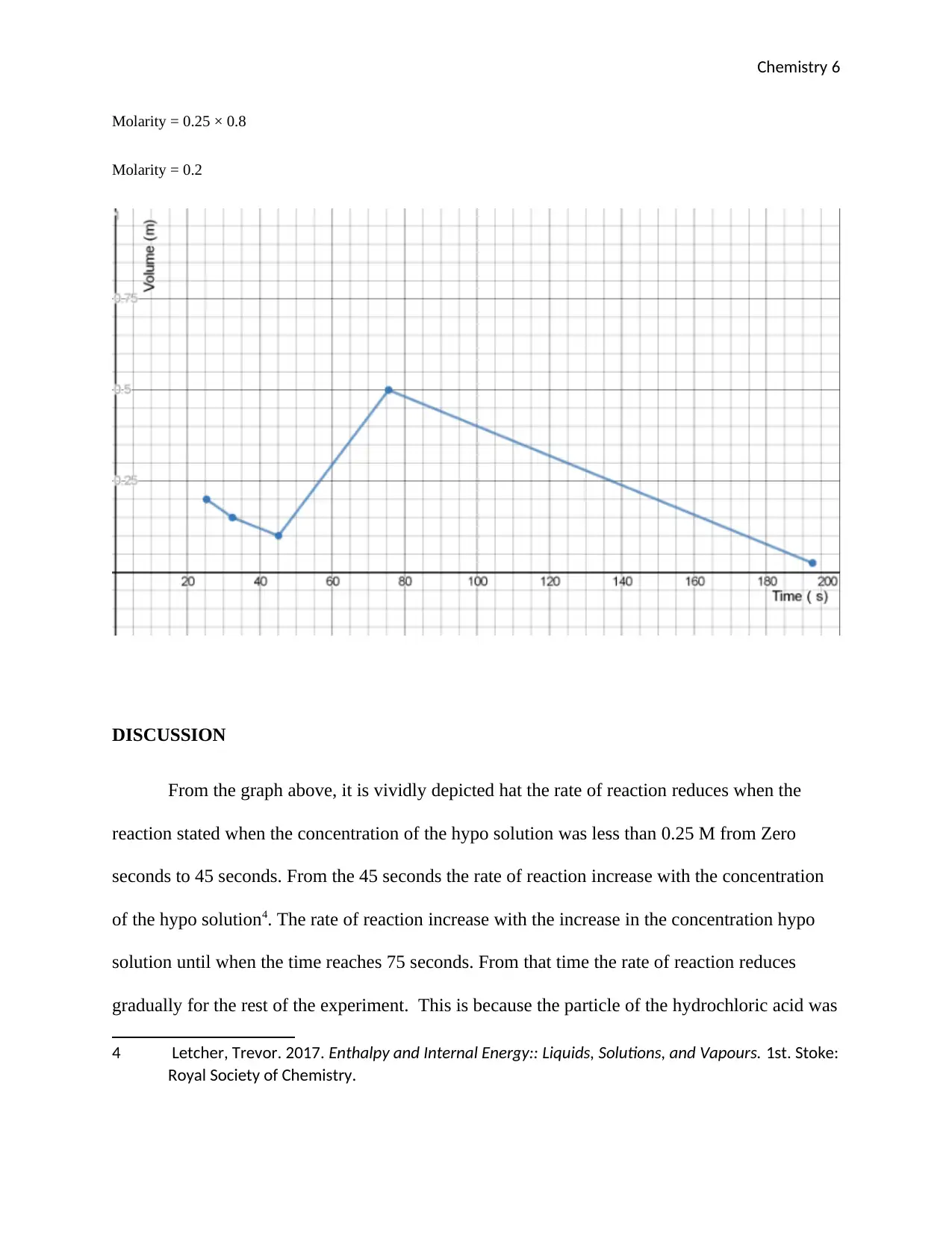
The values obtained from the experiment which are in the results above were then plotted as seen
in the graph below;
Calculation of concentration of hypo solution
The volume of hypo solution is 40 ml.
Volume of water is 50 ml
Therefore the concentration can be calculated as below
Molarity= 0.25 × 40
50
3 Tsao, Jeffrey. 2012. Materials Fundamentals of Molecular Beam Epitaxy. 2nd. Manchester: Academic
Press.
so they equal 50. The data was recorded and the observations looked at how the concentration of
hypo solution affects its reaction with the hydrochloric acid and effects the results3.
RESULTS
Volume (m) Time (s)
19.91
0.2 25.27
0.15 32.43
0.1 45.15
0.5 75.55
0.025 192.66
The values obtained from the experiment which are in the results above were then plotted as seen
in the graph below;
Calculation of concentration of hypo solution
The volume of hypo solution is 40 ml.
Volume of water is 50 ml
Therefore the concentration can be calculated as below
Molarity= 0.25 × 40
50
3 Tsao, Jeffrey. 2012. Materials Fundamentals of Molecular Beam Epitaxy. 2nd. Manchester: Academic
Press.
Chemistry 6
Molarity = 0.25 × 0.8
Molarity = 0.2
DISCUSSION
From the graph above, it is vividly depicted hat the rate of reaction reduces when the
reaction stated when the concentration of the hypo solution was less than 0.25 M from Zero
seconds to 45 seconds. From the 45 seconds the rate of reaction increase with the concentration
of the hypo solution4. The rate of reaction increase with the increase in the concentration hypo
solution until when the time reaches 75 seconds. From that time the rate of reaction reduces
gradually for the rest of the experiment. This is because the particle of the hydrochloric acid was
4 Letcher, Trevor. 2017. Enthalpy and Internal Energy:: Liquids, Solutions, and Vapours. 1st. Stoke:
Royal Society of Chemistry.
Molarity = 0.25 × 0.8
Molarity = 0.2
DISCUSSION
From the graph above, it is vividly depicted hat the rate of reaction reduces when the
reaction stated when the concentration of the hypo solution was less than 0.25 M from Zero
seconds to 45 seconds. From the 45 seconds the rate of reaction increase with the concentration
of the hypo solution4. The rate of reaction increase with the increase in the concentration hypo
solution until when the time reaches 75 seconds. From that time the rate of reaction reduces
gradually for the rest of the experiment. This is because the particle of the hydrochloric acid was
4 Letcher, Trevor. 2017. Enthalpy and Internal Energy:: Liquids, Solutions, and Vapours. 1st. Stoke:
Royal Society of Chemistry.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry 7
being used up gradually until when the amount of the hydrochloric acid was fully used up and
the reaction hence stops at that point ( End of the experiment). From the shape obtained in the
graph, it is true to say results obtained are very reliable as the reaction will reduce as the time
increase (the point when the particles of the reactants are being used up). For a good and very
clear results, it is advisable to use relatively more volume of hypo solution and more volume of
the hydrochloric acid. This will enable the reaction to take more time hence more values can be
obtained which help in obtaining reliable results.
CONCLUSION
After undertaking a successful experiment, it was possible to meet the objective of the
experiment which was to examine how the concentration of hypo solution affects the rate of
reaction time with hydrochloric acid. From the graph above it is true that the hypo solution truly
affects the rate of reaction between hypo solution and the hydrochloric acid. Therefore it is true
that the concentration of hypo solution increases with the increase in the rate of chemical
reaction.
Bibliography
Ahmed, Mohamed. 2011. Chemistry of enthalpy. 3rd. London: Springer.
Mwala, Angela. 2010. Chemistry of the chemical reactions. Manchester: Springer.
Ladislav, Comic. 2015. Thermodynamics in Mineral Sciences: An Introduction. 2nd. Leicester: Springer
Science & Business Media.
Kevin, Dahm. 2014. Fundamentals of Chemical Engineering Thermodynamics. 3rd. Chicago: Cengage
Learning.
being used up gradually until when the amount of the hydrochloric acid was fully used up and
the reaction hence stops at that point ( End of the experiment). From the shape obtained in the
graph, it is true to say results obtained are very reliable as the reaction will reduce as the time
increase (the point when the particles of the reactants are being used up). For a good and very
clear results, it is advisable to use relatively more volume of hypo solution and more volume of
the hydrochloric acid. This will enable the reaction to take more time hence more values can be
obtained which help in obtaining reliable results.
CONCLUSION
After undertaking a successful experiment, it was possible to meet the objective of the
experiment which was to examine how the concentration of hypo solution affects the rate of
reaction time with hydrochloric acid. From the graph above it is true that the hypo solution truly
affects the rate of reaction between hypo solution and the hydrochloric acid. Therefore it is true
that the concentration of hypo solution increases with the increase in the rate of chemical
reaction.
Bibliography
Ahmed, Mohamed. 2011. Chemistry of enthalpy. 3rd. London: Springer.
Mwala, Angela. 2010. Chemistry of the chemical reactions. Manchester: Springer.
Ladislav, Comic. 2015. Thermodynamics in Mineral Sciences: An Introduction. 2nd. Leicester: Springer
Science & Business Media.
Kevin, Dahm. 2014. Fundamentals of Chemical Engineering Thermodynamics. 3rd. Chicago: Cengage
Learning.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 8
Bavon, James. 2014. How concentration of sodium Thiosulphate affects the rate of reaction with HCl.
Florida: Willy and sons.
David, James. 2010. Sodium Thiosulphate reaction with HCl. Hull: CRC.
Rose, Kate. 2012. Factor affecting the rate of reaction between HCl and Hypo solution. Chicago:
Springer.
Carl, Knopf. 2011. Modeling, Analysis and Optimization of Process and Energy Systems for the chemical
reactions. 4th. Manchester: John Wiley & Sons.
Trevor, Letcher. 2017. Enthalpy and Internal Energy:: Liquids, Solutions, and Vapours. 1st. Stoke: Royal
Society of Chemistry.
Boris, Lvov. 2012. Thermal Decomposition of Solids and Melts: New Thermochemical Approach to the
Mechanism, Kinetics, and Methodology. 3rd. Hull: Springer Science & Business Media.
Krul, Max. 2013. The rate of reaction of sodium Thiosulphate. Chicago: Haiver.
John, Mbadi. 2010. Enthalpy of combustion: molar enthalpy of formation. 2nd. Hull: CRC.
Kennedy, Rao. 2010. Stoichiometry and Thermodynamics of Metallurgical Processes. 1st. Hull: CUP
Archive,
Norio, Sato. 2014. Chemical Energy and Exergy: An Introduction to Chemical Thermodynamics for
Engineers. 3rd. London: Elsevier.
Peter, Tremaine. 2012. Steam, Water, and Hydrothermal Systems: Physics and Chemistry Meeting the
Needs of Industry: Proceedings of the 13th International Conference on the Properties of Water
and Steam. 2nd. Hull: NRC Research Press.
Jeffrey, Tsao. 2012. Materials Fundamentals of Molecular Beam Epitaxy. 2nd. Manchester: Academic
Press.
Christian, Wohlfarth. 2016. CRC Handbook of Enthalpy Data of Polymer-Solvent Systems. 4th.
Manchester: CRC Press.
Bavon, James. 2014. How concentration of sodium Thiosulphate affects the rate of reaction with HCl.
Florida: Willy and sons.
David, James. 2010. Sodium Thiosulphate reaction with HCl. Hull: CRC.
Rose, Kate. 2012. Factor affecting the rate of reaction between HCl and Hypo solution. Chicago:
Springer.
Carl, Knopf. 2011. Modeling, Analysis and Optimization of Process and Energy Systems for the chemical
reactions. 4th. Manchester: John Wiley & Sons.
Trevor, Letcher. 2017. Enthalpy and Internal Energy:: Liquids, Solutions, and Vapours. 1st. Stoke: Royal
Society of Chemistry.
Boris, Lvov. 2012. Thermal Decomposition of Solids and Melts: New Thermochemical Approach to the
Mechanism, Kinetics, and Methodology. 3rd. Hull: Springer Science & Business Media.
Krul, Max. 2013. The rate of reaction of sodium Thiosulphate. Chicago: Haiver.
John, Mbadi. 2010. Enthalpy of combustion: molar enthalpy of formation. 2nd. Hull: CRC.
Kennedy, Rao. 2010. Stoichiometry and Thermodynamics of Metallurgical Processes. 1st. Hull: CUP
Archive,
Norio, Sato. 2014. Chemical Energy and Exergy: An Introduction to Chemical Thermodynamics for
Engineers. 3rd. London: Elsevier.
Peter, Tremaine. 2012. Steam, Water, and Hydrothermal Systems: Physics and Chemistry Meeting the
Needs of Industry: Proceedings of the 13th International Conference on the Properties of Water
and Steam. 2nd. Hull: NRC Research Press.
Jeffrey, Tsao. 2012. Materials Fundamentals of Molecular Beam Epitaxy. 2nd. Manchester: Academic
Press.
Christian, Wohlfarth. 2016. CRC Handbook of Enthalpy Data of Polymer-Solvent Systems. 4th.
Manchester: CRC Press.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




