Chemistry Lab Report: Analysis of Chemical Reactions and Observations
VerifiedAdded on 2022/09/15
|11
|1739
|17
Report
AI Summary
This chemistry lab report details the experimental procedures, observations, and analysis of thirteen different chemical reactions. The report covers a range of reaction types, including single and double displacement, decomposition, combustion, and acid-base reactions. Each experiment outlines the apparatus and chemicals used, followed by a step-by-step procedure. The analysis section provides balanced chemical equations, observations of physical changes, and the type of reaction occurring. Discussions elaborate on the underlying chemical principles and mechanisms, with explanations for observed phenomena. The report concludes with the results of each reaction and a final conclusion summarizing the findings. Precautions taken during the experiments, such as the use of fume hoods and protective gear, are also highlighted. The report aims to determine the presence of chemical reactions and identify the products formed while balancing the chemical equations and determining the reaction types.

CHEMISTRY LAB REPORT
[DATE]
[DATE]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
Aim........................................................................................................................................................2
Apparatus required...............................................................................................................................2
Chemical required.................................................................................................................................2
Procedure..............................................................................................................................................3
Analysis & Observation..........................................................................................................................4
Discussion..............................................................................................................................................6
Results & Conclusion...........................................................................................................................10
Precaution...........................................................................................................................................10
1
Aim........................................................................................................................................................2
Apparatus required...............................................................................................................................2
Chemical required.................................................................................................................................2
Procedure..............................................................................................................................................3
Analysis & Observation..........................................................................................................................4
Discussion..............................................................................................................................................6
Results & Conclusion...........................................................................................................................10
Precaution...........................................................................................................................................10
1
Aim
The aim is to determine the existing of a chemical reaction and the formed products if the
evidences are present for the reaction. The next objective is to balance the chemical reaction
and determination of the type of reaction and various imperative observation and brief
discussion about results.
Apparatus required
Test tubes Test tube holder Graduated cylinder
Test tube rack Test tube clamps Tongs
Stirring rods Bunsen burner Spatula, dropper
Wooden splints Matchstick Fume hood
Chemical required
3 M HCl ( aq ) Na2 C O3 ( s )
0.1 MC aCl2 0.1 M Na3 P O4 ( aq )
Cu ¿ Distilled water
Zn solid metal piece 1 M CuSO4 ( aq )
Cu ( s ) wire 1M ZnSO4 ( aq )
Mg ( s ) O2 ( g )
6 M HCl ( aq ) 3 M NaOH ( aq )
0.1M Fe Cl3 ( aq ) 0.1 M KSCN (aq)
2
The aim is to determine the existing of a chemical reaction and the formed products if the
evidences are present for the reaction. The next objective is to balance the chemical reaction
and determination of the type of reaction and various imperative observation and brief
discussion about results.
Apparatus required
Test tubes Test tube holder Graduated cylinder
Test tube rack Test tube clamps Tongs
Stirring rods Bunsen burner Spatula, dropper
Wooden splints Matchstick Fume hood
Chemical required
3 M HCl ( aq ) Na2 C O3 ( s )
0.1 MC aCl2 0.1 M Na3 P O4 ( aq )
Cu ¿ Distilled water
Zn solid metal piece 1 M CuSO4 ( aq )
Cu ( s ) wire 1M ZnSO4 ( aq )
Mg ( s ) O2 ( g )
6 M HCl ( aq ) 3 M NaOH ( aq )
0.1M Fe Cl3 ( aq ) 0.1 M KSCN (aq)
2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3% H2 O2 ( aq ) MnO2 pinch sized
H2 O ( l ) Anhydrous CuSO4
1 M NaCl ( aq ) 1M KN O3 ( aq )
Procedure
13 tests have been performed in lab and major aspects of the test is highlighted below.
1) Place 3ml of hydrochloric acid of 3M concentration in a clean and airdry test tube and
after that place a small amount i.e. pea sized amount of Na2 C O3 and after that directly
placed a glowing splint in the test tube’s mouth.
2) Mix 2ml of each of the two compounds i.e. CaCl2 and Na3 P O4 ( aq ) in an empty test
tube.
3) Put in a clean test tube app. 0.5 grams of Cu ¿and then heat the test tube very carefully in
the flame of Bunsen burner and then keeps on moving the test tube in the direct flame.
4) Put in a clean test tube 3ml of the CuSO4 and then slowly place a small sized metal piece
made up of zinc and then let the tube at rest in the test tube stand for minimum period of 5
minutes.
5) Put in a clean test tube 3ml of the ZnSO4 and then slowly place a small piece of wire
made up of copper and then let the tube at rest in the test tube stand for minimum period
of 5 minutes.
6) Just ignite a magnesium ribbon in Bunsen burner’s flame directly and be careful to not
look into the ignited ribbon directly from the naked eye. (Used fume hood while
conducting the test).
7) Put in a clean test tube 2ml of the 6M HCl and then slowly place a 3ml of NaOH and
thoroughly mix the two chemicals with the help of stirring rod.
3
H2 O ( l ) Anhydrous CuSO4
1 M NaCl ( aq ) 1M KN O3 ( aq )
Procedure
13 tests have been performed in lab and major aspects of the test is highlighted below.
1) Place 3ml of hydrochloric acid of 3M concentration in a clean and airdry test tube and
after that place a small amount i.e. pea sized amount of Na2 C O3 and after that directly
placed a glowing splint in the test tube’s mouth.
2) Mix 2ml of each of the two compounds i.e. CaCl2 and Na3 P O4 ( aq ) in an empty test
tube.
3) Put in a clean test tube app. 0.5 grams of Cu ¿and then heat the test tube very carefully in
the flame of Bunsen burner and then keeps on moving the test tube in the direct flame.
4) Put in a clean test tube 3ml of the CuSO4 and then slowly place a small sized metal piece
made up of zinc and then let the tube at rest in the test tube stand for minimum period of 5
minutes.
5) Put in a clean test tube 3ml of the ZnSO4 and then slowly place a small piece of wire
made up of copper and then let the tube at rest in the test tube stand for minimum period
of 5 minutes.
6) Just ignite a magnesium ribbon in Bunsen burner’s flame directly and be careful to not
look into the ignited ribbon directly from the naked eye. (Used fume hood while
conducting the test).
7) Put in a clean test tube 2ml of the 6M HCl and then slowly place a 3ml of NaOH and
thoroughly mix the two chemicals with the help of stirring rod.
3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8) Put in a clean test tube 2ml of the 0.1M Fe Cl3 ( aq )and then slowly place a 2ml of 0.1 M
KSCN and thoroughly mix the two chemicals with the help of stirring rod.
9) Put in a clean test tube 2ml of the 3M HCland then slowly place a small piece of zinc and
after that directly placed a glowing splint in the test tube’s mouth.
10) Put in a clean test tube 2ml of the 3M HCland then slowly place a small piece (about 1
inch in length) wire of copper.
11) Put in a clean test tube 2ml of the 3% H2 O2 ( aq )and then slowly place a small amount i.e.
pinch sized manganese di oxide and after that directly placed a glowing splint in the test
tube’s mouth.
12) Put anhydrous cupper around half full spatula in a dry test tube and then after add slowly
3 drops of distilled water through a dropper.
13) Put in a clean test tube 2ml of the 1M NaCl and then slowly place a 2ml of 1M potassium
nitrate and thoroughly mix the two chemicals with the help of stirring rod.
Analysis & Observation
Balanced chemical reaction of the test and observation along with the type of reaction
1 2 HCl ( aq ) + Na2 C O3 ( s ) →2 NaCl(aq)+ H2 O(l)+CO2 ( g)
Double displacement reaction
Ember from splint
2 3 CaCl2 ( aq ) +2 Na3 P O4 ( aq ) →C a3 ¿
Double displacement reaction
Cloudy white solution
4
KSCN and thoroughly mix the two chemicals with the help of stirring rod.
9) Put in a clean test tube 2ml of the 3M HCland then slowly place a small piece of zinc and
after that directly placed a glowing splint in the test tube’s mouth.
10) Put in a clean test tube 2ml of the 3M HCland then slowly place a small piece (about 1
inch in length) wire of copper.
11) Put in a clean test tube 2ml of the 3% H2 O2 ( aq )and then slowly place a small amount i.e.
pinch sized manganese di oxide and after that directly placed a glowing splint in the test
tube’s mouth.
12) Put anhydrous cupper around half full spatula in a dry test tube and then after add slowly
3 drops of distilled water through a dropper.
13) Put in a clean test tube 2ml of the 1M NaCl and then slowly place a 2ml of 1M potassium
nitrate and thoroughly mix the two chemicals with the help of stirring rod.
Analysis & Observation
Balanced chemical reaction of the test and observation along with the type of reaction
1 2 HCl ( aq ) + Na2 C O3 ( s ) →2 NaCl(aq)+ H2 O(l)+CO2 ( g)
Double displacement reaction
Ember from splint
2 3 CaCl2 ( aq ) +2 Na3 P O4 ( aq ) →C a3 ¿
Double displacement reaction
Cloudy white solution
4

3 Cu ¿
Decomposition reaction (D)
Blue coloured becomes black along with a liquid
4 Zn ( s ) +CuSO4 ( aq ) → ZnSO4 ( aq ) +Cu ( s )
Single displacement reaction
5 Cu ( s ) + ZnSO4 ( aq ) → NAR
No evidence of a reaction
6 2 Mg ( s ) +O2 ( g ) → 2 MgO ( s ) +energy
Combustion (CU) reaction Combined reaction
Bright light produced and whitish powdered formed later on
7 HCl ( aq )+ NaOH ( aq ) → NaCl ( aq ) + H2 O (l )
Acid-base reaction (Double displacement reaction)
Heat generated and sodium chloride solution formed
8 Fe Cl3 ( aq ) + 3 KSCN (aq) → Fe ¿
Double displacement reaction
Dard red coloured formed in test tube
9 Zn ( s ) +2 HCl ( aq ) → H 2(g) ↑+Zn Cl2( aq)
Single replacement reaction
Zinc dissolved totally in acid and splint glows
10 Cu ( s ) + HCl ( aq ) → NAR
No evidence of a reaction
11 H2 O2 ( aq )⃗ Mn O2 H2 O ( aq )+ 1
2 O2 ( g)↑
Decomposition (D) reaction
Splint glows
12 CuSO4 ( s ) +5 H 2 O ( l ) → CuSO4 . 5 H2 O
Synthesis reaction: Combination reaction (C)
Test tube become warm and coloured turned blue
13 NaCl ( aq ) +KN O3 ( aq ) → KCl ( aq ) + NaN O3 ( aq )
Double displacement reaction also known as Metathesis
5
Decomposition reaction (D)
Blue coloured becomes black along with a liquid
4 Zn ( s ) +CuSO4 ( aq ) → ZnSO4 ( aq ) +Cu ( s )
Single displacement reaction
5 Cu ( s ) + ZnSO4 ( aq ) → NAR
No evidence of a reaction
6 2 Mg ( s ) +O2 ( g ) → 2 MgO ( s ) +energy
Combustion (CU) reaction Combined reaction
Bright light produced and whitish powdered formed later on
7 HCl ( aq )+ NaOH ( aq ) → NaCl ( aq ) + H2 O (l )
Acid-base reaction (Double displacement reaction)
Heat generated and sodium chloride solution formed
8 Fe Cl3 ( aq ) + 3 KSCN (aq) → Fe ¿
Double displacement reaction
Dard red coloured formed in test tube
9 Zn ( s ) +2 HCl ( aq ) → H 2(g) ↑+Zn Cl2( aq)
Single replacement reaction
Zinc dissolved totally in acid and splint glows
10 Cu ( s ) + HCl ( aq ) → NAR
No evidence of a reaction
11 H2 O2 ( aq )⃗ Mn O2 H2 O ( aq )+ 1
2 O2 ( g)↑
Decomposition (D) reaction
Splint glows
12 CuSO4 ( s ) +5 H 2 O ( l ) → CuSO4 . 5 H2 O
Synthesis reaction: Combination reaction (C)
Test tube become warm and coloured turned blue
13 NaCl ( aq ) +KN O3 ( aq ) → KCl ( aq ) + NaN O3 ( aq )
Double displacement reaction also known as Metathesis
5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Reality no product formed
Discussion
A chemical reaction happened when two or more reactants interact with each other in certain
condition then formation of products occurred which are totally different than the reactants in
terms of properties and basic characteristics.
Combination reaction: when two or higher than two substances combined together to form a
new product.
Decomposition reaction: When one substance takes part in a reaction and at certain
conditions, the reactant decompose in the reaction and formed products.
Combustion reaction: It occurs when any substance exposed into fire in the presence of
oxygen.
Single displacement reaction: When a more active element substitutes the less active
element from the compound to make another compound.
Double displacement reaction: When ions of two reactants exchanges their respective
partners to make another compound.
1) Reaction
2 HCl ( aq ) +Na2 C O3 ( s ) →2 NaCl(aq)+ H2 O(l)+CO2 ( g)
Double displacement reaction
It is because sodium combines with the chlorine and displace the hydrogen and on the other
hand, the oxygen from Na2 C O3 combined with the hydrogen and displace the chlorine and
hence, it is a double displacement reaction. The product contains water and carbon dioxide
and dissolved sodium chloride.
2) Reaction
3 CaCl2 ( aq ) +2 Na3 P O4 ( aq ) →C a3 ¿
Double displacement reaction
6
Discussion
A chemical reaction happened when two or more reactants interact with each other in certain
condition then formation of products occurred which are totally different than the reactants in
terms of properties and basic characteristics.
Combination reaction: when two or higher than two substances combined together to form a
new product.
Decomposition reaction: When one substance takes part in a reaction and at certain
conditions, the reactant decompose in the reaction and formed products.
Combustion reaction: It occurs when any substance exposed into fire in the presence of
oxygen.
Single displacement reaction: When a more active element substitutes the less active
element from the compound to make another compound.
Double displacement reaction: When ions of two reactants exchanges their respective
partners to make another compound.
1) Reaction
2 HCl ( aq ) +Na2 C O3 ( s ) →2 NaCl(aq)+ H2 O(l)+CO2 ( g)
Double displacement reaction
It is because sodium combines with the chlorine and displace the hydrogen and on the other
hand, the oxygen from Na2 C O3 combined with the hydrogen and displace the chlorine and
hence, it is a double displacement reaction. The product contains water and carbon dioxide
and dissolved sodium chloride.
2) Reaction
3 CaCl2 ( aq ) +2 Na3 P O4 ( aq ) →C a3 ¿
Double displacement reaction
6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Here, an insoluble white solid is formed because calcium phosphate is precipitate in the
aqueous solution of solidum chloride formed.
3) Reaction
Cu ¿
Decomposition reaction (D)
This is a decomposition reaction as it breaks Cu ¿ into two different molecules. The blue
coloured cupric hydroxide sold compound decomposed while heating into water and black
coloured cupric oxide.
4) Reaction
Zn ( s ) +CuSO4 ( aq ) → ZnSO4 ( aq ) +Cu ( s )
Single displacement reaction
This is a replacement reaction in which a less active metal i.e. copper is replaced by a more
active metal i.e. zinc. As a result of this, zinc has replaced the Cu from cupric sulfate and
formed zinc sulfate and solid copper. The solution initially in blue colour but after
replacement reaction the thick coat of copper metal powder is appeared on the zinc piece and
the blue colour of the solution becomes significantly lightened.
5) Reaction
Cu ( s ) + ZnSO4 ( aq ) → NAR
No evidence of a reaction
As copper is considered to be highly unreactive metal to react with others and it does not
form any product while in the contact with zinc sulfate because it does not have reactivity to
replace zinc from zinc sulfate.
7
aqueous solution of solidum chloride formed.
3) Reaction
Cu ¿
Decomposition reaction (D)
This is a decomposition reaction as it breaks Cu ¿ into two different molecules. The blue
coloured cupric hydroxide sold compound decomposed while heating into water and black
coloured cupric oxide.
4) Reaction
Zn ( s ) +CuSO4 ( aq ) → ZnSO4 ( aq ) +Cu ( s )
Single displacement reaction
This is a replacement reaction in which a less active metal i.e. copper is replaced by a more
active metal i.e. zinc. As a result of this, zinc has replaced the Cu from cupric sulfate and
formed zinc sulfate and solid copper. The solution initially in blue colour but after
replacement reaction the thick coat of copper metal powder is appeared on the zinc piece and
the blue colour of the solution becomes significantly lightened.
5) Reaction
Cu ( s ) + ZnSO4 ( aq ) → NAR
No evidence of a reaction
As copper is considered to be highly unreactive metal to react with others and it does not
form any product while in the contact with zinc sulfate because it does not have reactivity to
replace zinc from zinc sulfate.
7

6) Reaction
2 Mg ( s ) +O2 ( g ) → 2 MgO ( s ) +energy
Combustion (CU) reaction Combined reaction
The main observation is that when the magnesium oxide formed from the reaction of
magnesium and oxygen then the heat of formation is produced which means it is an
exothermic reaction. Further, the energy of combustion occurred as light energy. Finally, it
can be said that through this reaction brilliant light as well as intense heat both are produced
along with the magnesium oxide solid. One precaution that was essential in conducting this
reaction in lab is that one must take care of eyes and other body parts because the burning of
magnesium produced significant light and heat to temporary loss of eye sight and burning
sensation.
7) Reaction
HCl ( aq ) + NaOH ( aq ) → NaCl ( aq ) + H2 O ( l )
Acid-base reaction (Double displacement reaction)
A slight colour changed occurred into a slightly yellowish liquid.
8) Reaction
Fe Cl3 ( aq ) +3 KSCN (aq)→ Fe ¿
Double displacement reaction
It is because Fe combines with the SCN and displace the and similarly, K combines with the
chlorine and displace the Fe and thus, it is a double displacement reaction. The formation of
products has been confirmed from the blood red colour of the test tube which is due to the
thiocyanatorium ion.
9) Reaction
Zn ( s ) +2 HCl ( aq ) → H 2(g)↑+Zn Cl2( aq)
8
2 Mg ( s ) +O2 ( g ) → 2 MgO ( s ) +energy
Combustion (CU) reaction Combined reaction
The main observation is that when the magnesium oxide formed from the reaction of
magnesium and oxygen then the heat of formation is produced which means it is an
exothermic reaction. Further, the energy of combustion occurred as light energy. Finally, it
can be said that through this reaction brilliant light as well as intense heat both are produced
along with the magnesium oxide solid. One precaution that was essential in conducting this
reaction in lab is that one must take care of eyes and other body parts because the burning of
magnesium produced significant light and heat to temporary loss of eye sight and burning
sensation.
7) Reaction
HCl ( aq ) + NaOH ( aq ) → NaCl ( aq ) + H2 O ( l )
Acid-base reaction (Double displacement reaction)
A slight colour changed occurred into a slightly yellowish liquid.
8) Reaction
Fe Cl3 ( aq ) +3 KSCN (aq)→ Fe ¿
Double displacement reaction
It is because Fe combines with the SCN and displace the and similarly, K combines with the
chlorine and displace the Fe and thus, it is a double displacement reaction. The formation of
products has been confirmed from the blood red colour of the test tube which is due to the
thiocyanatorium ion.
9) Reaction
Zn ( s ) +2 HCl ( aq ) → H 2(g)↑+Zn Cl2( aq)
8
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
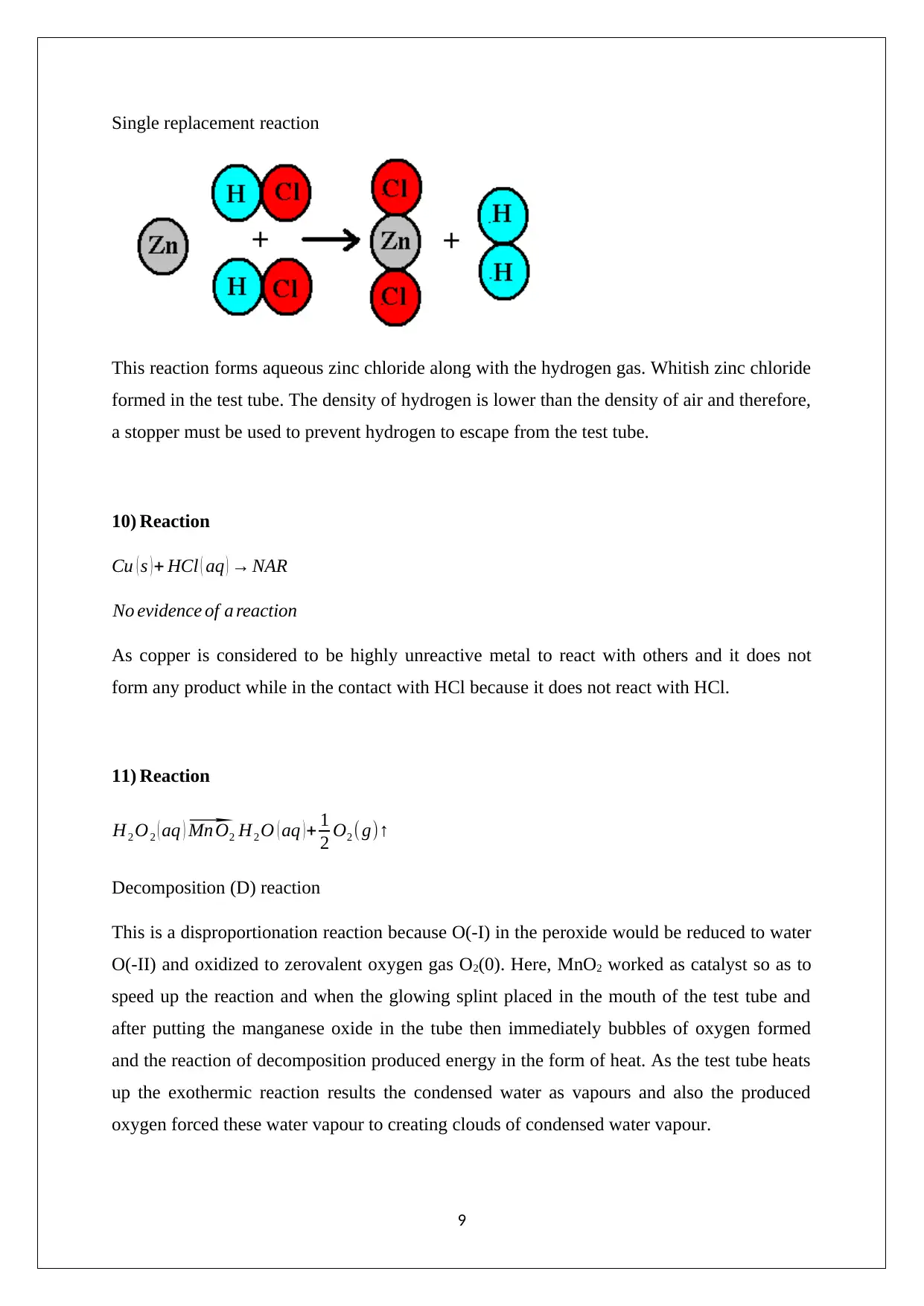
Single replacement reaction
This reaction forms aqueous zinc chloride along with the hydrogen gas. Whitish zinc chloride
formed in the test tube. The density of hydrogen is lower than the density of air and therefore,
a stopper must be used to prevent hydrogen to escape from the test tube.
10) Reaction
Cu ( s )+ HCl ( aq ) → NAR
No evidence of a reaction
As copper is considered to be highly unreactive metal to react with others and it does not
form any product while in the contact with HCl because it does not react with HCl.
11) Reaction
H2 O2 ( aq )⃗ Mn O2 H2 O ( aq ) + 1
2 O2 ( g)↑
Decomposition (D) reaction
This is a disproportionation reaction because O(-I) in the peroxide would be reduced to water
O(-II) and oxidized to zerovalent oxygen gas O2(0). Here, MnO2 worked as catalyst so as to
speed up the reaction and when the glowing splint placed in the mouth of the test tube and
after putting the manganese oxide in the tube then immediately bubbles of oxygen formed
and the reaction of decomposition produced energy in the form of heat. As the test tube heats
up the exothermic reaction results the condensed water as vapours and also the produced
oxygen forced these water vapour to creating clouds of condensed water vapour.
9
This reaction forms aqueous zinc chloride along with the hydrogen gas. Whitish zinc chloride
formed in the test tube. The density of hydrogen is lower than the density of air and therefore,
a stopper must be used to prevent hydrogen to escape from the test tube.
10) Reaction
Cu ( s )+ HCl ( aq ) → NAR
No evidence of a reaction
As copper is considered to be highly unreactive metal to react with others and it does not
form any product while in the contact with HCl because it does not react with HCl.
11) Reaction
H2 O2 ( aq )⃗ Mn O2 H2 O ( aq ) + 1
2 O2 ( g)↑
Decomposition (D) reaction
This is a disproportionation reaction because O(-I) in the peroxide would be reduced to water
O(-II) and oxidized to zerovalent oxygen gas O2(0). Here, MnO2 worked as catalyst so as to
speed up the reaction and when the glowing splint placed in the mouth of the test tube and
after putting the manganese oxide in the tube then immediately bubbles of oxygen formed
and the reaction of decomposition produced energy in the form of heat. As the test tube heats
up the exothermic reaction results the condensed water as vapours and also the produced
oxygen forced these water vapour to creating clouds of condensed water vapour.
9
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

12) Reaction
CuSO4 ( s )+5 H 2 O ( l ) → CuSO4 . 5 H2 O
Synthesis reaction: Combination reaction (C)
Solid copper sulfate absorbed liquid i.e. water and then colour changes into a turquoise
colour.
13) Reaction
NaCl ( aq ) + KN O3 ( aq ) → KCl ( aq ) + NaN O3 ( aq )
Double displacement reaction also known as Metathesis
Both sodium nitrate and potassium chloride are soluble salts. It means in reality no reaction is
occurred because both reactant yields KCl + NaN O3 both of which are soluble and no
precipitates are formed thus, no reaction.
Results & Conclusion
The final outcomes of the reactions have been observed and discussed in the analysis part and
the residuals/ products have been dumped into wastage carefully. Each test shows the results
and physically visible changed which are reported and discussed.
Precaution
Glassware must be washed with distilled water and air dried before use.
All the test in which flame or burner is used must be taken place with the help of safety
gloves, goggles and stand, tong.
Products of the chemical reaction carefully placed into wastage.
Glassware must be washed with the distilled water and/or with soap before putting it back
to rack.
10
CuSO4 ( s )+5 H 2 O ( l ) → CuSO4 . 5 H2 O
Synthesis reaction: Combination reaction (C)
Solid copper sulfate absorbed liquid i.e. water and then colour changes into a turquoise
colour.
13) Reaction
NaCl ( aq ) + KN O3 ( aq ) → KCl ( aq ) + NaN O3 ( aq )
Double displacement reaction also known as Metathesis
Both sodium nitrate and potassium chloride are soluble salts. It means in reality no reaction is
occurred because both reactant yields KCl + NaN O3 both of which are soluble and no
precipitates are formed thus, no reaction.
Results & Conclusion
The final outcomes of the reactions have been observed and discussed in the analysis part and
the residuals/ products have been dumped into wastage carefully. Each test shows the results
and physically visible changed which are reported and discussed.
Precaution
Glassware must be washed with distilled water and air dried before use.
All the test in which flame or burner is used must be taken place with the help of safety
gloves, goggles and stand, tong.
Products of the chemical reaction carefully placed into wastage.
Glassware must be washed with the distilled water and/or with soap before putting it back
to rack.
10
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
