Organic Chemistry Assignment: Isomers, Conformations, and Reactions
VerifiedAdded on 2021/04/24
|12
|697
|308
Homework Assignment
AI Summary
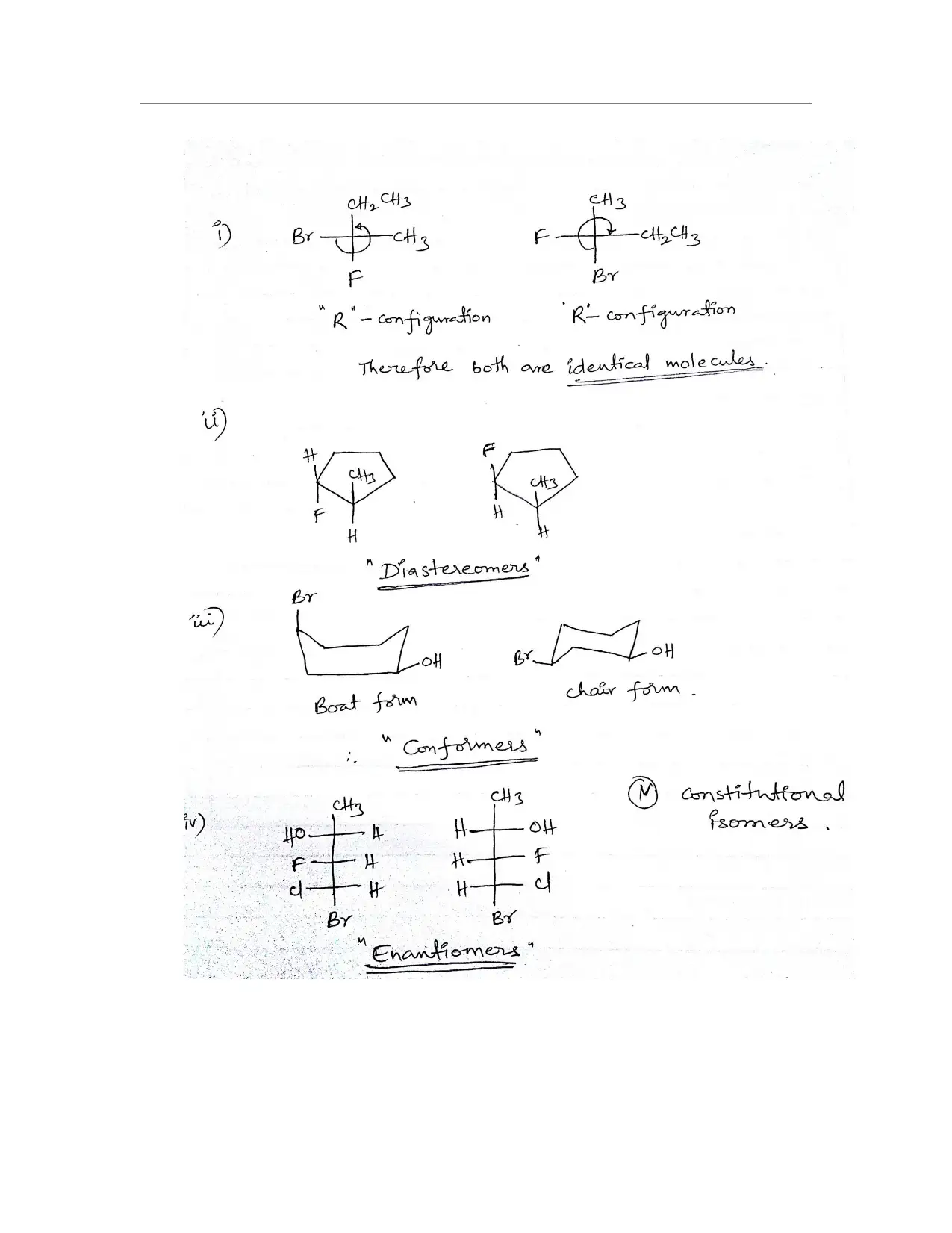
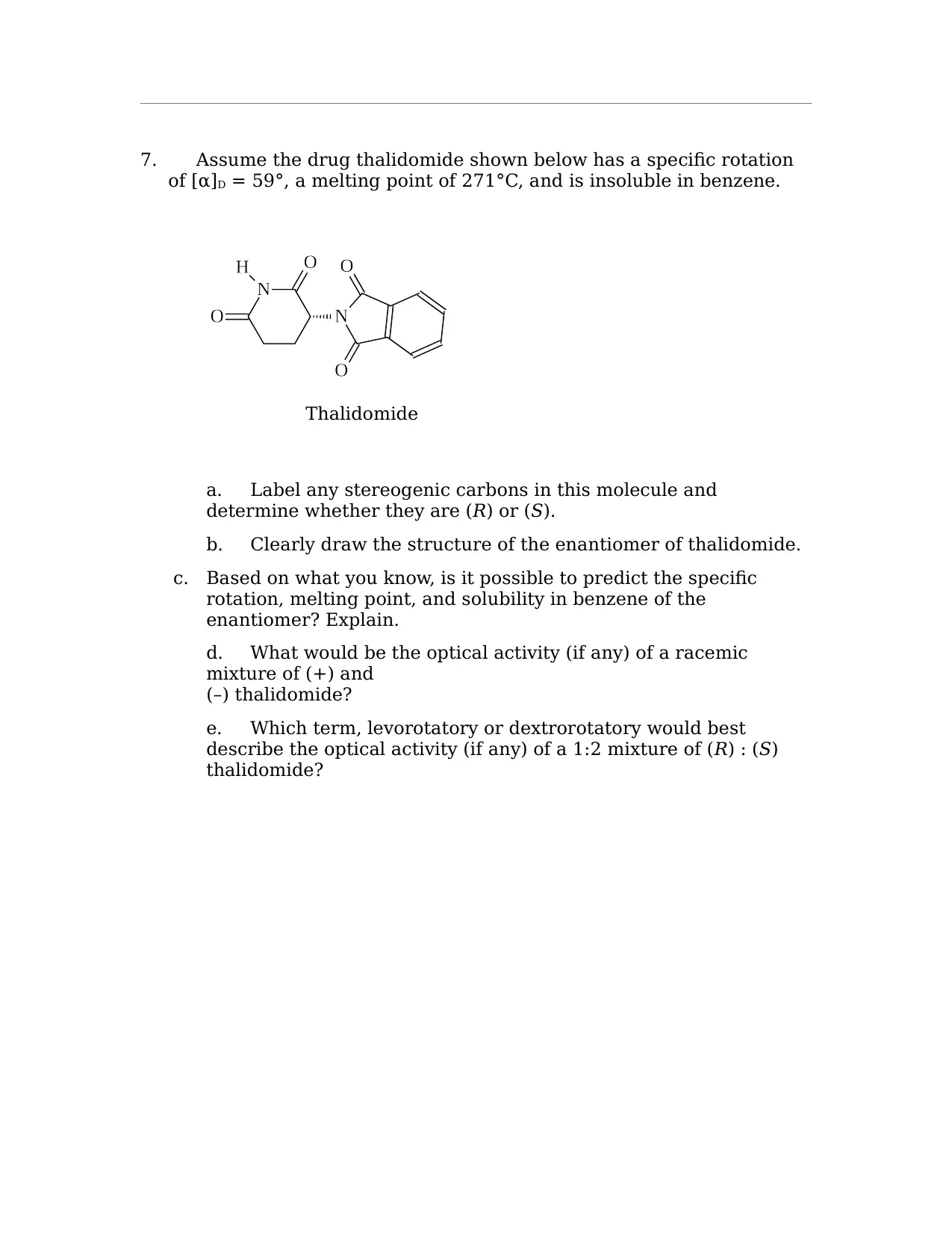
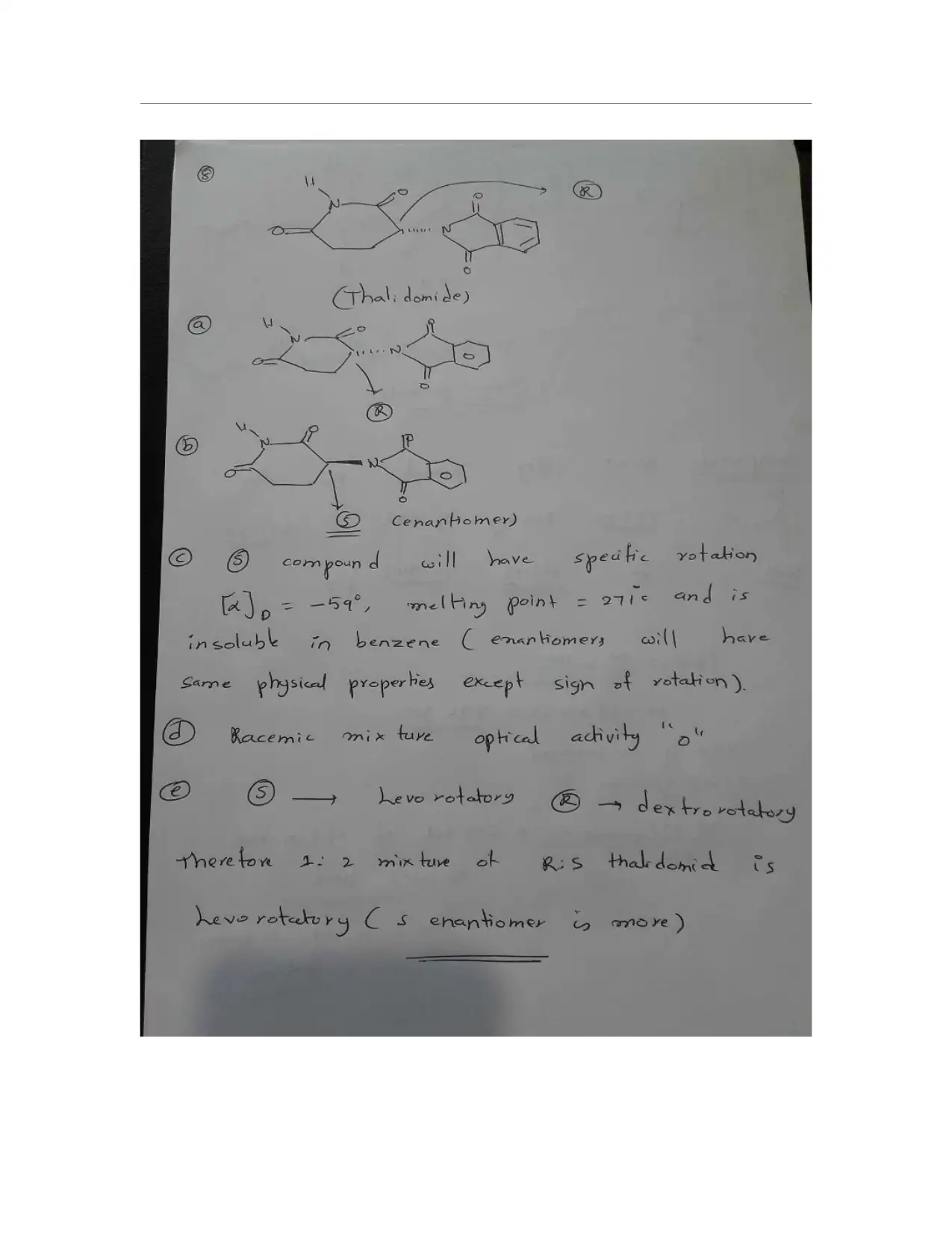
This organic chemistry assignment solution addresses a variety of key concepts including IUPAC nomenclature, identification of 1°, 2°, and 3° hydrogen atoms, and the naming of organic compounds. It covers the drawing and identification of cis and trans isomers in cyclohexane derivatives, including their most stable configurations. The solution also includes the construction of potential energy diagrams for conformational analysis, specifically for 1,2-dichloroethane, and the identification of chiral compounds, assignment of (R) and (S) designations, and drawing stereoisomers. Additionally, the assignment covers Fischer projections, the designation of (E) and (Z) isomers, the identification of stereochemical relationships, and the analysis of thalidomide's stereogenic carbons, enantiomer, and optical activity. The assignment provides comprehensive solutions to problems related to isomers, conformations, and stereochemistry.
1 out of 12


![[object Object]](/_next/static/media/star-bottom.7253800d.svg)