Chemistry for Applied Biology: Detailed Homework Assignment
VerifiedAdded on 2020/06/04
|20
|3522
|284
Homework Assignment
AI Summary
This chemistry assignment for applied biology delves into several key concepts. Task 1 explores the physical properties of various substances including water, methane, and pyruvic acid, along with enthalpy changes such as enthalpy of dissociation and hydration. Task 2 examines enthalpy changes in the oxidation of ethanol, calculating enthalpy of formation, entropy changes, and Gibbs free energy, along with an analysis of redox reactions and the feasibility of the process. Task 3 focuses on equilibrium systems, including factors affecting sucrose breakdown and the behavior of monoprotic weak acids. Finally, Task 4 investigates the relationship between molecular shapes and bonding, including structural formulae, systematic names, and isomerism. The assignment provides detailed explanations, calculations, and analyses of chemical reactions and processes relevant to applied biology.
Chemistry for applied biology
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
TASK 1 (AC: 1.1, 1.2, 1.3)..............................................................................................................1
A) Physical properties of substances...........................................................................................1
B) Enthalpy changes .................................................................................................................5
C) Factors affecting the rate of sucrose break down reaction ....................................................6
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4).......................................................................................................6
1) ................................................................................................................................................6
a) Enthalpy of formation and enthalpy change for the reaction..................................................6
b) Values of ΔSƟ at 298K..........................................................................................................7
c) Values of SƟ for all other substances and ΔSƟ for the system............................................7
d) ΔSƟ and ΔGƟ ......................................................................................................................7
e) Feasibility of the process and the assumptions.......................................................................7
2) Redox Reactions.....................................................................................................................7
3) Using oxidation numbers for deciding the redox nature of the reaction or process...............8
4) Feasibility of the process........................................................................................................9
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4).......................................................................................................9
A) Experimental work on equilibrium system............................................................................9
B) Shift in equilibrium position for favouring the formation of products................................10
C) Monoprotic weak acid..........................................................................................................10
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)...............................................................................................11
A) Relation of shapes and bonding in molecules .....................................................................11
B) Structures and systematic names .........................................................................................11
C) Reactions and their relation to structural formulae .............................................................12
D) Isomerism displayed ...........................................................................................................13
TASK 1 (AC: 1.1, 1.2, 1.3)..............................................................................................................1
A) Physical properties of substances...........................................................................................1
B) Enthalpy changes .................................................................................................................5
C) Factors affecting the rate of sucrose break down reaction ....................................................6
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4).......................................................................................................6
1) ................................................................................................................................................6
a) Enthalpy of formation and enthalpy change for the reaction..................................................6
b) Values of ΔSƟ at 298K..........................................................................................................7
c) Values of SƟ for all other substances and ΔSƟ for the system............................................7
d) ΔSƟ and ΔGƟ ......................................................................................................................7
e) Feasibility of the process and the assumptions.......................................................................7
2) Redox Reactions.....................................................................................................................7
3) Using oxidation numbers for deciding the redox nature of the reaction or process...............8
4) Feasibility of the process........................................................................................................9
TASK 3 (AC: 3.1, 3.2, 3.3, 3.4).......................................................................................................9
A) Experimental work on equilibrium system............................................................................9
B) Shift in equilibrium position for favouring the formation of products................................10
C) Monoprotic weak acid..........................................................................................................10
TASK 4 (AC: 4.1,4.2, 4.3, 4.4, 4.5)...............................................................................................11
A) Relation of shapes and bonding in molecules .....................................................................11
B) Structures and systematic names .........................................................................................11
C) Reactions and their relation to structural formulae .............................................................12
D) Isomerism displayed ...........................................................................................................13

⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

TASK 1 (AC: 1.1, 1.2, 1.3)
A) Physical properties of substances
a) Water
The molecular formula for water is H2O and the following is the structural formula
depicting the bonds and molecular arrangement of the molecules of water.
The shape of this molecular is contemplated as bent shape and bonds formed in this
structure are covalent bonds. These are quite strong but have weak force of attraction. This
property relates to the flexibility of water in having no specific shape. The fluidity is acquired
due to this nature of the bonds. There are two lone pairs denoted over oxygen. Water is found in
liquid form. Its gaseous form is known as water vapour while the solid version is ice. Water has
three forms which includes liquid, solid (ice) and gaseous as water vapour. The boiling point of
water is 100°C and freezing point is 0°C. The cohesiveness for water is very high while
viscosity is low because it has high flowing capacity.
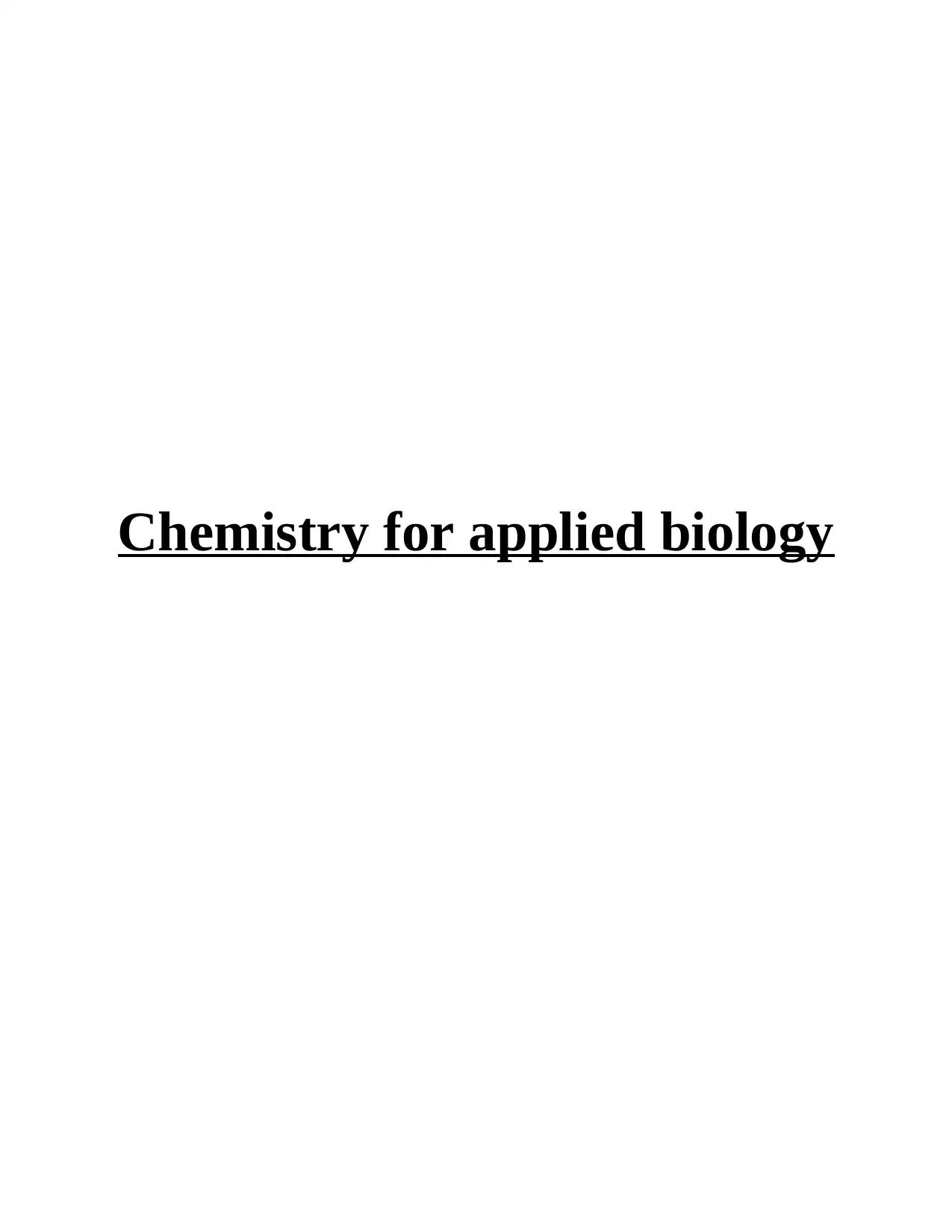
b) Methane
The chemical formula of methane is CH4 and the following is the structural formula:
1
Illustration 1: Structural formula of
water
A) Physical properties of substances
a) Water
The molecular formula for water is H2O and the following is the structural formula
depicting the bonds and molecular arrangement of the molecules of water.
The shape of this molecular is contemplated as bent shape and bonds formed in this
structure are covalent bonds. These are quite strong but have weak force of attraction. This
property relates to the flexibility of water in having no specific shape. The fluidity is acquired
due to this nature of the bonds. There are two lone pairs denoted over oxygen. Water is found in
liquid form. Its gaseous form is known as water vapour while the solid version is ice. Water has
three forms which includes liquid, solid (ice) and gaseous as water vapour. The boiling point of
water is 100°C and freezing point is 0°C. The cohesiveness for water is very high while
viscosity is low because it has high flowing capacity.
b) Methane
The chemical formula of methane is CH4 and the following is the structural formula:
1
Illustration 1: Structural formula of
water
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Methane is also known as methyl hydride. This compound is present in gaseous forms
and is also known as marsh gas. This compound is present in gaseous and liquid form. Carbon
can form four covalent bonds because of its four valence electrons while hydrogen can form only
one covalent bond. Methane has four covalent bonds formed between one carbon and 4 hydrogen
atoms. The structural formula depicts two types of representations i.e. solid lines and solid
wedges depicts a bond going back in the paper i.e. away from the viewer in a 3-D perspective.
Boiling point of methane is -258.7°F and the melting point -296.5°F. This compound is highly
inflammable and used as primary element of the fuel natural gas.
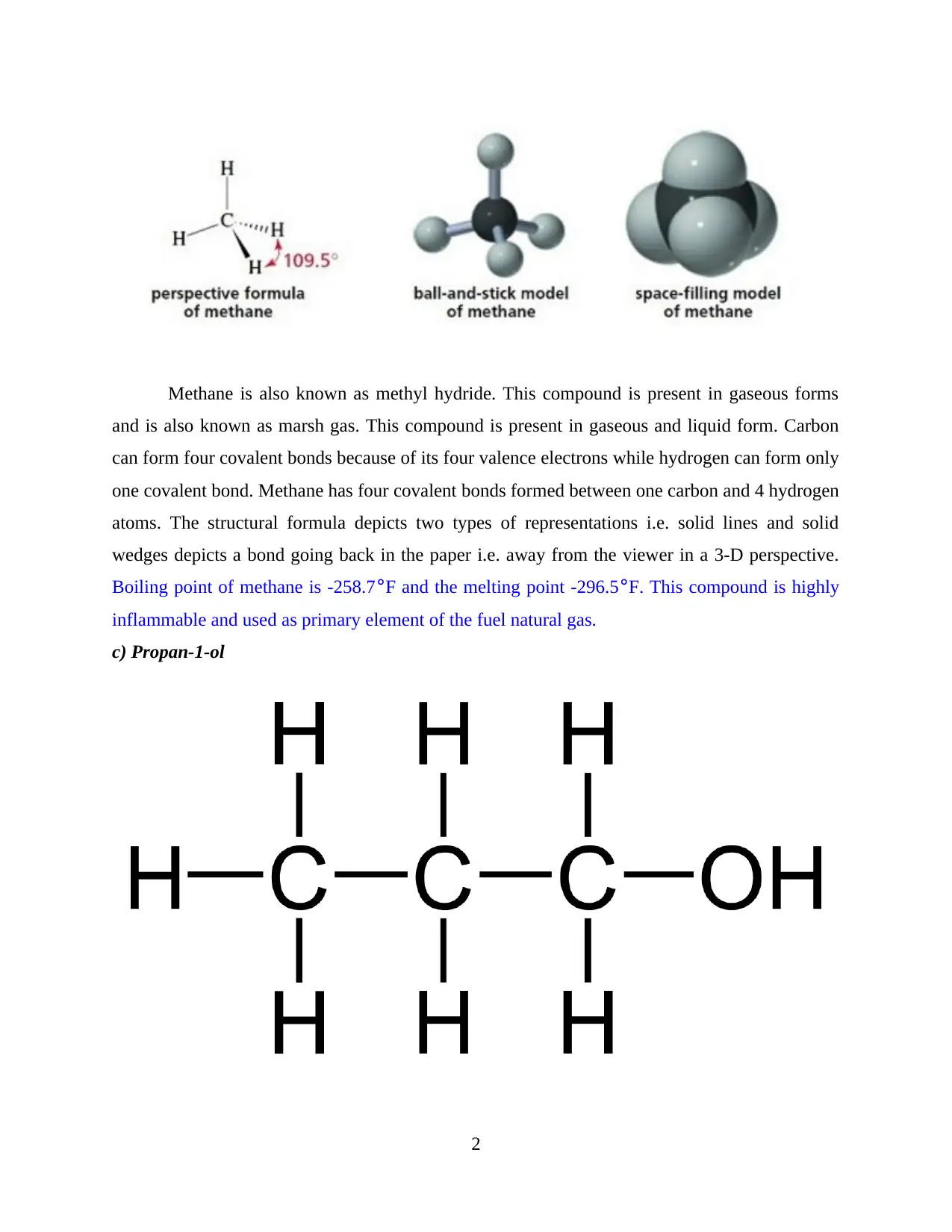
c) Propan-1-ol
2
and is also known as marsh gas. This compound is present in gaseous and liquid form. Carbon
can form four covalent bonds because of its four valence electrons while hydrogen can form only
one covalent bond. Methane has four covalent bonds formed between one carbon and 4 hydrogen
atoms. The structural formula depicts two types of representations i.e. solid lines and solid
wedges depicts a bond going back in the paper i.e. away from the viewer in a 3-D perspective.
Boiling point of methane is -258.7°F and the melting point -296.5°F. This compound is highly
inflammable and used as primary element of the fuel natural gas.
c) Propan-1-ol
2
Propan-1-ol is also known as 1-propanol or propyl alcohol. The chemical formula of this
compound is C3H8O. Belonging to the alcohol group, this compound occurs in colourless form
and liquid state. The bonds are covalent in nature. This compound is used in resins and
formulation of cellulose esters. The odour of this compound is very much similar to ethanol and
tastes like ripe fruits. However, there is a burning taste experienced after consumption. The
boiling point of this Propan-1-ol is 207° F while melting point is -195.2° F.
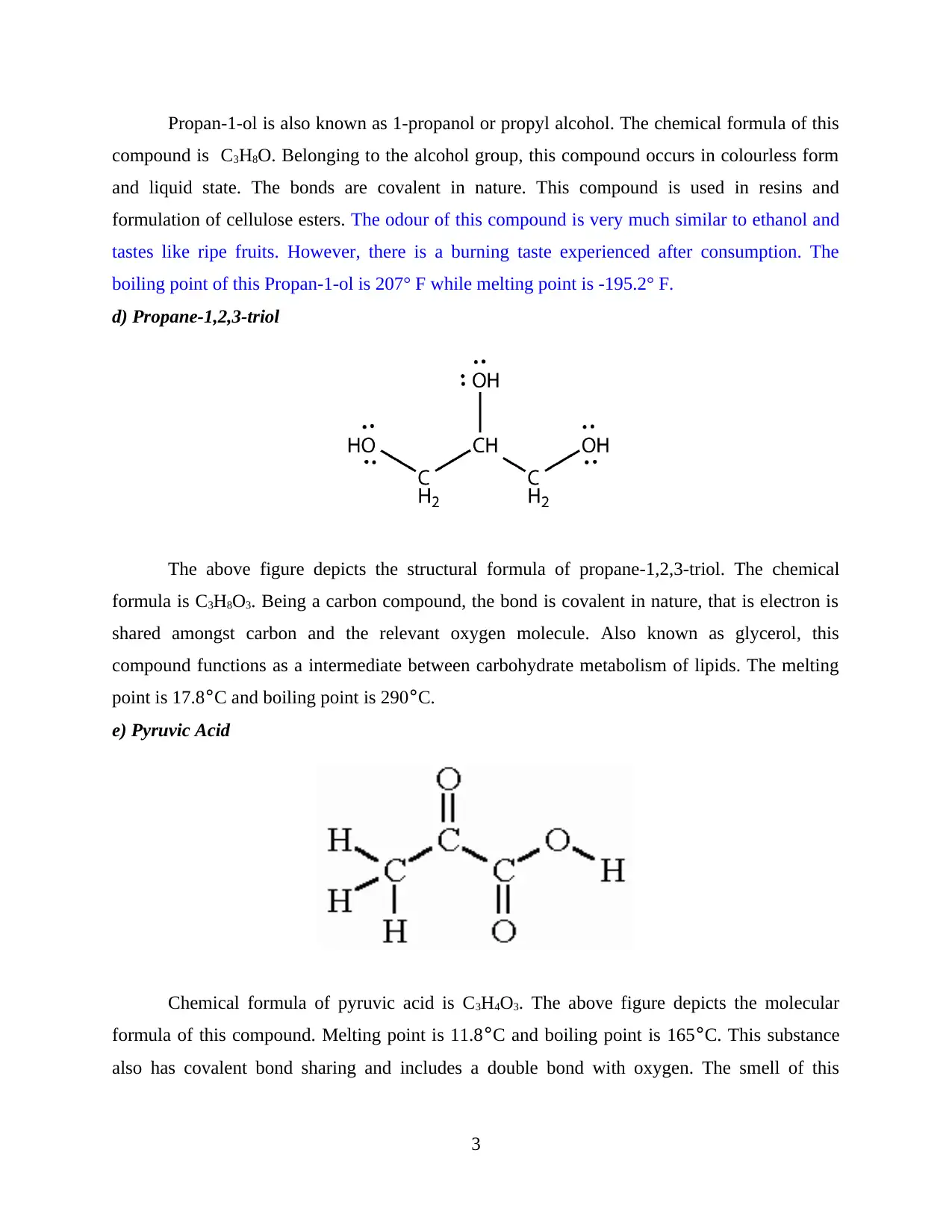
d) Propane-1,2,3-triol
The above figure depicts the structural formula of propane-1,2,3-triol. The chemical
formula is C3H8O3. Being a carbon compound, the bond is covalent in nature, that is electron is
shared amongst carbon and the relevant oxygen molecule. Also known as glycerol, this
compound functions as a intermediate between carbohydrate metabolism of lipids. The melting
point is 17.8°C and boiling point is 290°C.
e) Pyruvic Acid
Chemical formula of pyruvic acid is C3H4O3. The above figure depicts the molecular
formula of this compound. Melting point is 11.8°C and boiling point is 165°C. This substance
also has covalent bond sharing and includes a double bond with oxygen. The smell of this
3
compound is C3H8O. Belonging to the alcohol group, this compound occurs in colourless form
and liquid state. The bonds are covalent in nature. This compound is used in resins and
formulation of cellulose esters. The odour of this compound is very much similar to ethanol and
tastes like ripe fruits. However, there is a burning taste experienced after consumption. The
boiling point of this Propan-1-ol is 207° F while melting point is -195.2° F.
d) Propane-1,2,3-triol
The above figure depicts the structural formula of propane-1,2,3-triol. The chemical
formula is C3H8O3. Being a carbon compound, the bond is covalent in nature, that is electron is
shared amongst carbon and the relevant oxygen molecule. Also known as glycerol, this
compound functions as a intermediate between carbohydrate metabolism of lipids. The melting
point is 17.8°C and boiling point is 290°C.
e) Pyruvic Acid
Chemical formula of pyruvic acid is C3H4O3. The above figure depicts the molecular
formula of this compound. Melting point is 11.8°C and boiling point is 165°C. This substance
also has covalent bond sharing and includes a double bond with oxygen. The smell of this
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
substance is quite similar to acetic acid and is colourless. The production of compound is
initiated through metabolism of glucose.
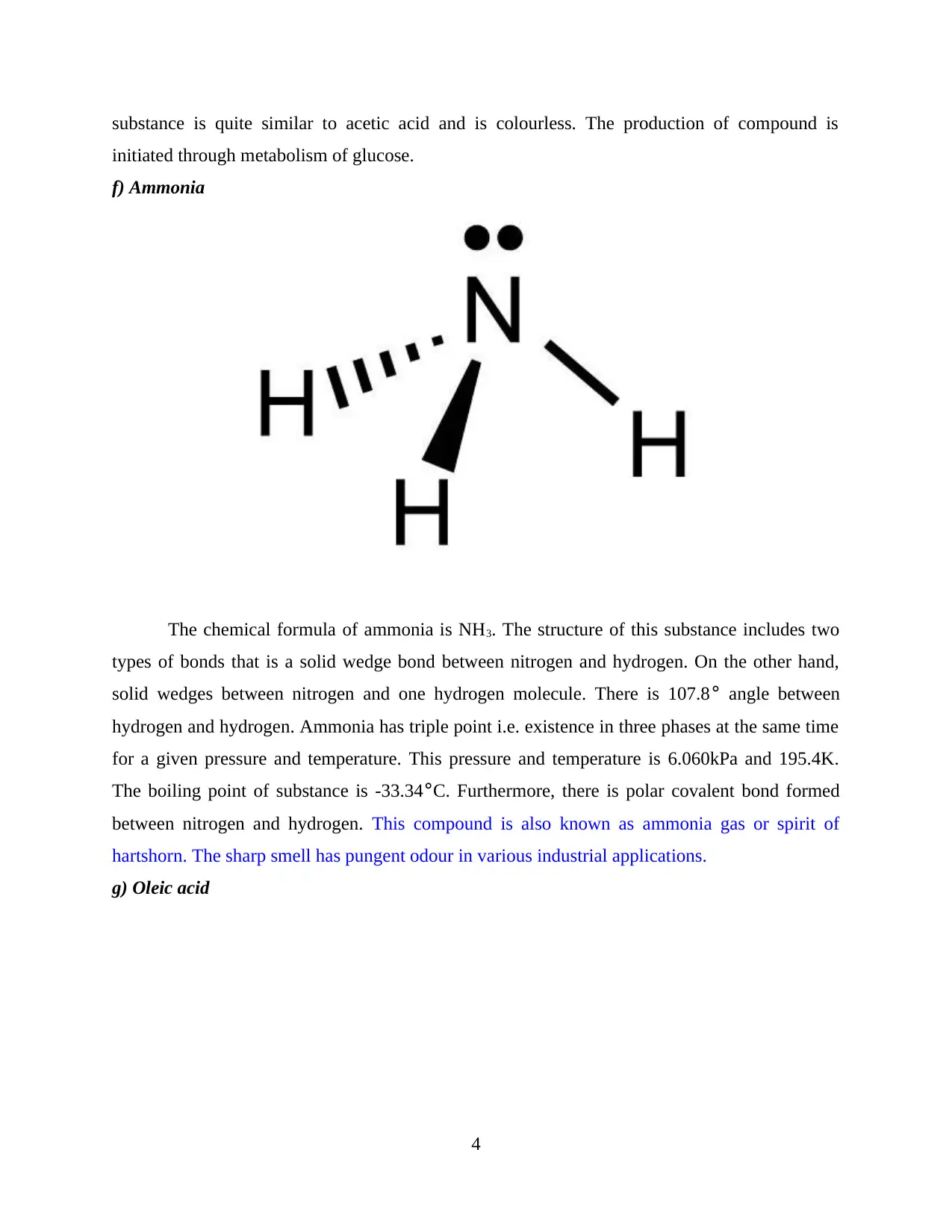
f) Ammonia
The chemical formula of ammonia is NH3. The structure of this substance includes two
types of bonds that is a solid wedge bond between nitrogen and hydrogen. On the other hand,
solid wedges between nitrogen and one hydrogen molecule. There is 107.8° angle between
hydrogen and hydrogen. Ammonia has triple point i.e. existence in three phases at the same time
for a given pressure and temperature. This pressure and temperature is 6.060kPa and 195.4K.
The boiling point of substance is -33.34°C. Furthermore, there is polar covalent bond formed
between nitrogen and hydrogen. This compound is also known as ammonia gas or spirit of
hartshorn. The sharp smell has pungent odour in various industrial applications.
g) Oleic acid
4
initiated through metabolism of glucose.
f) Ammonia
The chemical formula of ammonia is NH3. The structure of this substance includes two
types of bonds that is a solid wedge bond between nitrogen and hydrogen. On the other hand,
solid wedges between nitrogen and one hydrogen molecule. There is 107.8° angle between
hydrogen and hydrogen. Ammonia has triple point i.e. existence in three phases at the same time
for a given pressure and temperature. This pressure and temperature is 6.060kPa and 195.4K.
The boiling point of substance is -33.34°C. Furthermore, there is polar covalent bond formed
between nitrogen and hydrogen. This compound is also known as ammonia gas or spirit of
hartshorn. The sharp smell has pungent odour in various industrial applications.
g) Oleic acid
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
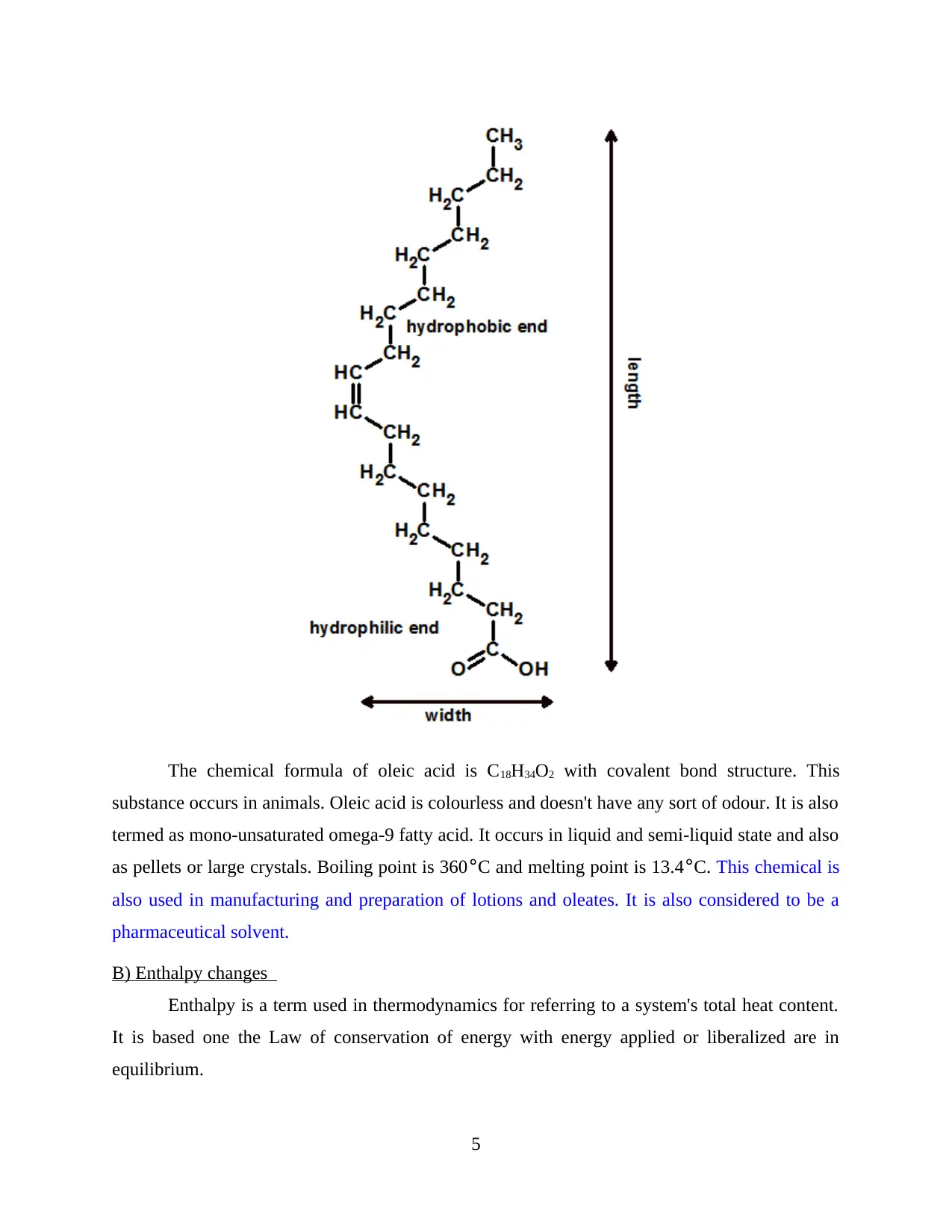
The chemical formula of oleic acid is C18H34O2 with covalent bond structure. This
substance occurs in animals. Oleic acid is colourless and doesn't have any sort of odour. It is also
termed as mono-unsaturated omega-9 fatty acid. It occurs in liquid and semi-liquid state and also
as pellets or large crystals. Boiling point is 360°C and melting point is 13.4°C. This chemical is
also used in manufacturing and preparation of lotions and oleates. It is also considered to be a
pharmaceutical solvent.
B) Enthalpy changes
Enthalpy is a term used in thermodynamics for referring to a system's total heat content.
It is based one the Law of conservation of energy with energy applied or liberalized are in
equilibrium.
5
substance occurs in animals. Oleic acid is colourless and doesn't have any sort of odour. It is also
termed as mono-unsaturated omega-9 fatty acid. It occurs in liquid and semi-liquid state and also
as pellets or large crystals. Boiling point is 360°C and melting point is 13.4°C. This chemical is
also used in manufacturing and preparation of lotions and oleates. It is also considered to be a
pharmaceutical solvent.
B) Enthalpy changes
Enthalpy is a term used in thermodynamics for referring to a system's total heat content.
It is based one the Law of conservation of energy with energy applied or liberalized are in
equilibrium.
5

Enthalpy of dissociation: The change in enthalpy which takes place during the
breakdown or cleavage of a bond in a homo-lytic reaction is known as enthalpy of dissociation.
The chemical reactions in which new compounds are formed or certain elements are displaced
have bond dissociation enthalpies. However, this type of enthalpy change doesn't take place in
diatomic molecules.
Enthalpy of solution: Also known as the heat of solution, this enthalpy is completely
linked with mixing or dissolution of a particular substance on application of constant pressure.
The solvent and substance are constantly dissolving because of certain pressure. The heat
evolved or absorbed in this process is referred to as enthalpy of solution. The temperature is
mostly kept constant.
Lattice enthalpy: There are certain forces active in the lattice structure of a solid i.e.
between different ions. The strength of these forces is measured in the form of lattice enthalpy.
Ionic compounds are formed within standard atmospheric conditions and there is exchange of
charged ions which further helps in formation of the lattice.
Enthalpy of hydration: The energy released during the formation of ionic bonds and
water molecules is known as enthalpy of hydration. The term hydration depicts that a particular
compound or substance is introduced in water. For instance: dissolving copper sulphate in water
results in production of enthalpy of hydration.
Enthalpy profile for oxidation of ethanol is provided as follows:
6
breakdown or cleavage of a bond in a homo-lytic reaction is known as enthalpy of dissociation.
The chemical reactions in which new compounds are formed or certain elements are displaced
have bond dissociation enthalpies. However, this type of enthalpy change doesn't take place in
diatomic molecules.
Enthalpy of solution: Also known as the heat of solution, this enthalpy is completely
linked with mixing or dissolution of a particular substance on application of constant pressure.
The solvent and substance are constantly dissolving because of certain pressure. The heat
evolved or absorbed in this process is referred to as enthalpy of solution. The temperature is
mostly kept constant.
Lattice enthalpy: There are certain forces active in the lattice structure of a solid i.e.
between different ions. The strength of these forces is measured in the form of lattice enthalpy.
Ionic compounds are formed within standard atmospheric conditions and there is exchange of
charged ions which further helps in formation of the lattice.
Enthalpy of hydration: The energy released during the formation of ionic bonds and
water molecules is known as enthalpy of hydration. The term hydration depicts that a particular
compound or substance is introduced in water. For instance: dissolving copper sulphate in water
results in production of enthalpy of hydration.
Enthalpy profile for oxidation of ethanol is provided as follows:
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The above reaction depicts formation of ethanoic acid after oxidation of ethanol. The
entire process is exothermic and following is the chemical reaction
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
The production of acetic acid is a result of oxidation of alcohol. This reaction is a
exothermic reaction.
Enthalpy of formation is considered as the enthalpy change which occurs when a substance is
formed from certain components. In the oxidation of ethanol, a new substance is formed which is
ethanoic acid and water is released.
C) Factors affecting the rate of sucrose break down reaction
The breakdown of sucrose into glucose and fructose is also known as hydrolysis of
sucrose. The speed of this reaction is affected by following factors:
Concentration of the enzyme
Atmospheric conditions in which the reaction is taking place.
Supply of oxygen in the body.
If the enzyme is not produced in sufficient forms then the reaction rate will be
simultaneously affected. On the other hand, if there is no proper supply of oxygen in the body
7
entire process is exothermic and following is the chemical reaction
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
The production of acetic acid is a result of oxidation of alcohol. This reaction is a
exothermic reaction.
Enthalpy of formation is considered as the enthalpy change which occurs when a substance is
formed from certain components. In the oxidation of ethanol, a new substance is formed which is
ethanoic acid and water is released.
C) Factors affecting the rate of sucrose break down reaction
The breakdown of sucrose into glucose and fructose is also known as hydrolysis of
sucrose. The speed of this reaction is affected by following factors:
Concentration of the enzyme
Atmospheric conditions in which the reaction is taking place.
Supply of oxygen in the body.
If the enzyme is not produced in sufficient forms then the reaction rate will be
simultaneously affected. On the other hand, if there is no proper supply of oxygen in the body
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

then reaction rate is affected. The break down process always requires certain amount of energy
so that reaction can be facilitated. When body gets more oxygenated blood then significant
energy is supplied to such reactions of breakdown of sucrose. Furthermore, the concentration of
enzyme when present in excess amount results in slower reaction while of the concentration is
reduced then reaction will not be possible. This implies body will not gain any benefits from
sucrose breakdown which further interrupts other depending processes and reactions.
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4)
1)
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
a) Enthalpy of formation and enthalpy change for the reaction
The enthalpy of formation and enthalpy change for the reaction of oxidation of ethanol is
depicted as follows:
The enthalpy of solution = 17.87kJ mol-1
Enthalpy change ∆H = cm∆T;
∆T = change in temperature during the reaction
Moles of water molecules produced = 1 mole
Enthalpy change for one mole of water = -8.54 kJ
Neutralisation enthalpy = -56.9 kJ/mol
c = 4.18 kJ kg-1 °C-1
m = 1 kg
∆H = -627 kJ/mol
The enthalpy of formation when considering oxidation of ethanol is provided as follows:
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
∆fH = Sum (∆fH for products) – Sum (∆fH for reactants)
∆fH is the enthalpy as per the thermodynamic tables.
∆fH for ethanol = -1058 kJ/mol
∆fH for Oxygen = 0
∆fH for acetic acid = -483.5 kJ/mol
∆fH for water = -285.9 kJ/mol
Substituting the values as per the formula:
8
so that reaction can be facilitated. When body gets more oxygenated blood then significant
energy is supplied to such reactions of breakdown of sucrose. Furthermore, the concentration of
enzyme when present in excess amount results in slower reaction while of the concentration is
reduced then reaction will not be possible. This implies body will not gain any benefits from
sucrose breakdown which further interrupts other depending processes and reactions.
TASK 2 (AC: 2.1, 2.2, 2.3, 2.4)
1)
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
a) Enthalpy of formation and enthalpy change for the reaction
The enthalpy of formation and enthalpy change for the reaction of oxidation of ethanol is
depicted as follows:
The enthalpy of solution = 17.87kJ mol-1
Enthalpy change ∆H = cm∆T;
∆T = change in temperature during the reaction
Moles of water molecules produced = 1 mole
Enthalpy change for one mole of water = -8.54 kJ
Neutralisation enthalpy = -56.9 kJ/mol
c = 4.18 kJ kg-1 °C-1
m = 1 kg
∆H = -627 kJ/mol
The enthalpy of formation when considering oxidation of ethanol is provided as follows:
C2H5OH (l) + O2 (g) → CH3COOH (aq) + H2O (l)
∆fH = Sum (∆fH for products) – Sum (∆fH for reactants)
∆fH is the enthalpy as per the thermodynamic tables.
∆fH for ethanol = -1058 kJ/mol
∆fH for Oxygen = 0
∆fH for acetic acid = -483.5 kJ/mol
∆fH for water = -285.9 kJ/mol
Substituting the values as per the formula:
8

∆fH = (-285.9 – 483.5 ) - (-1058)
∆fH = 1058 – 769.4
∆fH = 288.6 kJ/mol is the enthalpy of formation
b) Values of ΔSƟ at 298K
The molecular entropy of the initial reaction that is at the reactant end is 365.7 J/(mol K)
and the final value of S is 229.9 J/(mol K).
c) Values of SƟ for all other substances and ΔSƟ for the system
Molecular and total entropy for ethanol is 160.7 J/(mol K) and oxygen is 205 J/(mol K).
This can also be considered as initial values and the following are final values.
Acetic acid has molecular entropy as 160 J/(mol K) and water has molecular entropy as
69.91 J/(mol K)
Hence, SƟ for C2H4O2 is 160 J/(mol K) and for H2O is 69.91 J/(mol K)
ΔSƟ = 229.9-365.7
= -135.8 J/(mol K)
The value of ΔSƟ at 298 K is -135.8 J/(mol K) for the entire system
d) ΔSƟ and ΔGƟ
ΔGƟ = ΔHƟ – T ΔSƟsystem
Here, delta G is known as Gibbs energy or Gibbs free energy
ΔGƟ = -627- 56.9
ΔGƟ = -683.9 kJ/mol (exergonic)
e) Feasibility of the process and the assumptions
The assumptions for this reaction include standard values of specific heats and the
standard temperature at which reaction takes place. Further, the molecular entropies for all the
substances have been taken as per the standards. The feasibility of process is quite large and
there is sufficient production of energy which means the reaction is exothermic.
2) Redox Reactions
Following are the displacement reactions between chlorine and bromine
Cl2(aq) + 2KBr(aq) ==> 2KCl(aq) + Br2(aq)__________(i)
Cl2(aq) + 2Br–(aq) ==> 2Cl–(aq) + Br2(aq)_________________(1)
The below produced reactions are the half reactions for displacement of ions:
9
∆fH = 1058 – 769.4
∆fH = 288.6 kJ/mol is the enthalpy of formation
b) Values of ΔSƟ at 298K
The molecular entropy of the initial reaction that is at the reactant end is 365.7 J/(mol K)
and the final value of S is 229.9 J/(mol K).
c) Values of SƟ for all other substances and ΔSƟ for the system
Molecular and total entropy for ethanol is 160.7 J/(mol K) and oxygen is 205 J/(mol K).
This can also be considered as initial values and the following are final values.
Acetic acid has molecular entropy as 160 J/(mol K) and water has molecular entropy as
69.91 J/(mol K)
Hence, SƟ for C2H4O2 is 160 J/(mol K) and for H2O is 69.91 J/(mol K)
ΔSƟ = 229.9-365.7
= -135.8 J/(mol K)
The value of ΔSƟ at 298 K is -135.8 J/(mol K) for the entire system
d) ΔSƟ and ΔGƟ
ΔGƟ = ΔHƟ – T ΔSƟsystem
Here, delta G is known as Gibbs energy or Gibbs free energy
ΔGƟ = -627- 56.9
ΔGƟ = -683.9 kJ/mol (exergonic)
e) Feasibility of the process and the assumptions
The assumptions for this reaction include standard values of specific heats and the
standard temperature at which reaction takes place. Further, the molecular entropies for all the
substances have been taken as per the standards. The feasibility of process is quite large and
there is sufficient production of energy which means the reaction is exothermic.
2) Redox Reactions
Following are the displacement reactions between chlorine and bromine
Cl2(aq) + 2KBr(aq) ==> 2KCl(aq) + Br2(aq)__________(i)
Cl2(aq) + 2Br–(aq) ==> 2Cl–(aq) + Br2(aq)_________________(1)
The below produced reactions are the half reactions for displacement of ions:
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 20
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


