CHEM101 Lab: Synthesis of Benzalacetophenone via Claisen-Schmidt
VerifiedAdded on 2021/04/24
|4
|755
|123
Practical Assignment
AI Summary
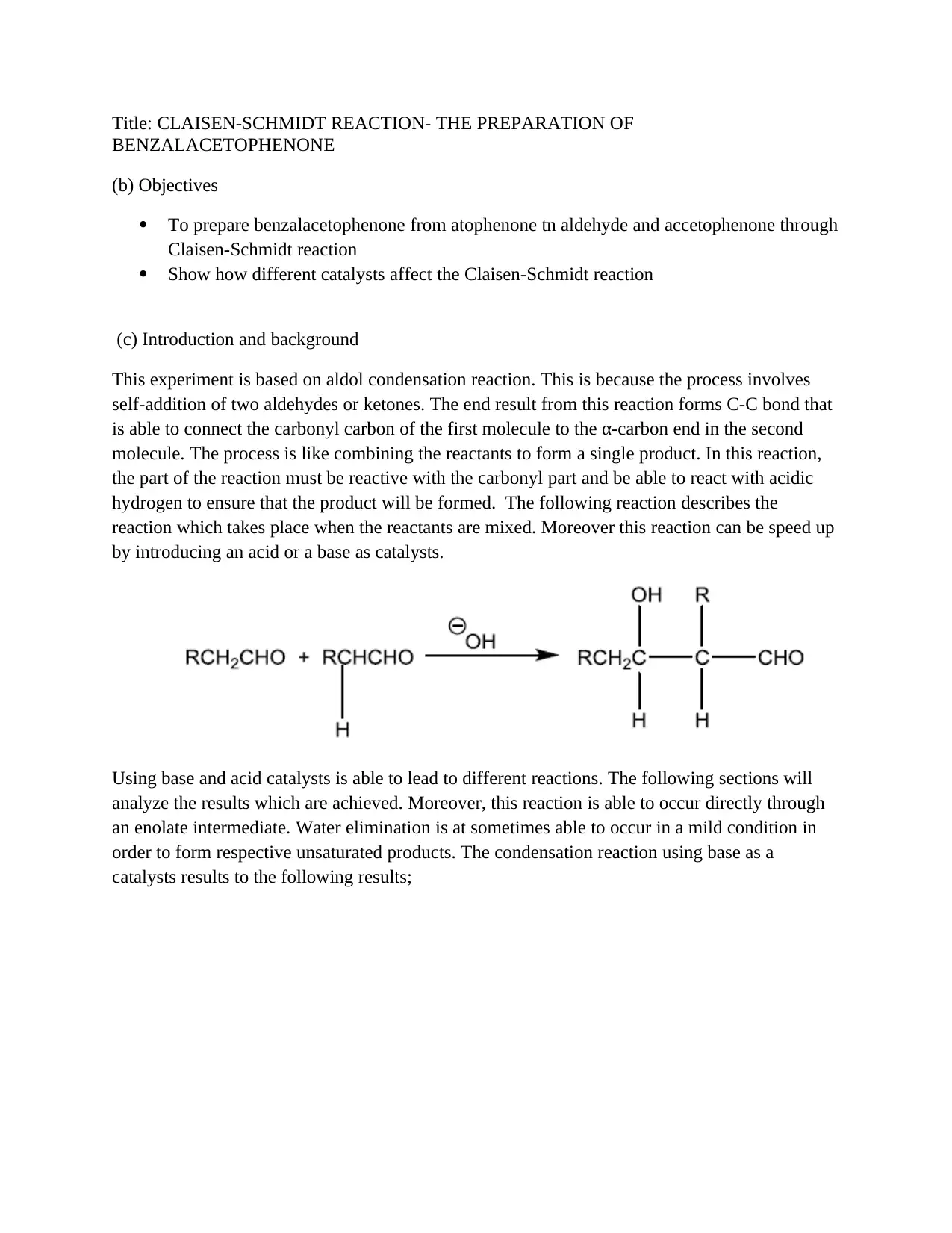
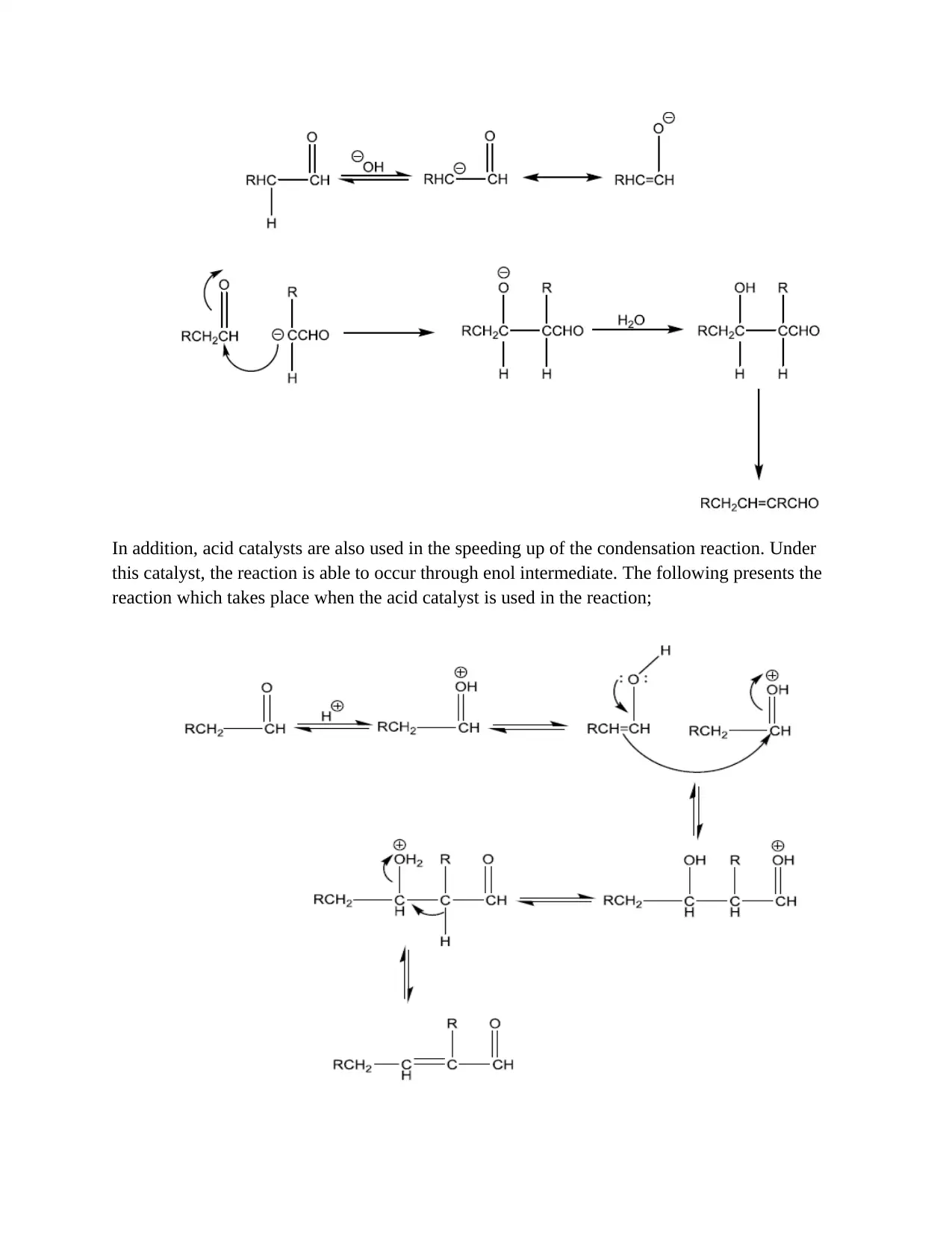
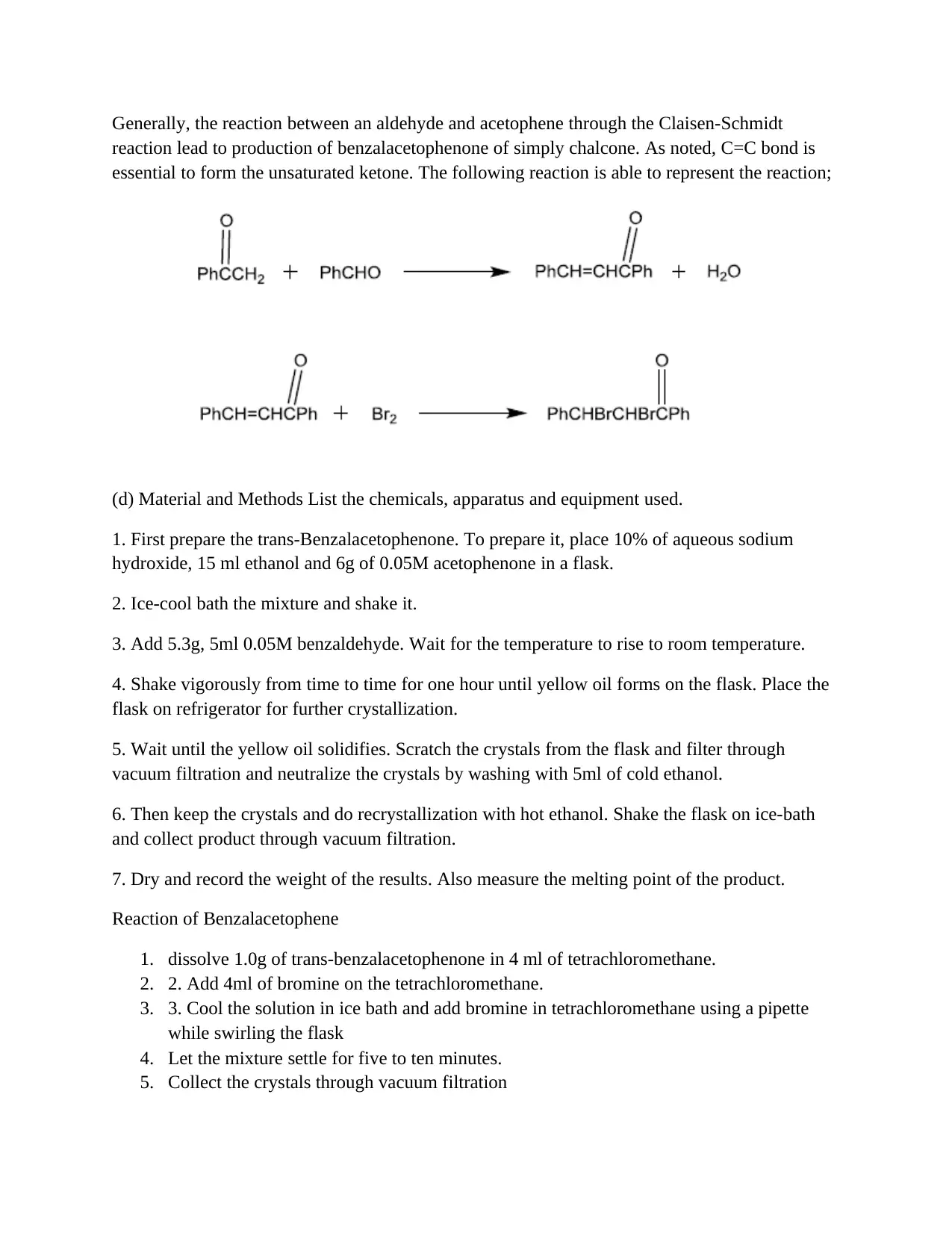
This assignment details the experimental procedure for synthesizing benzalacetophenone through the Claisen-Schmidt reaction, an aldol condensation. The report outlines the objectives, which include preparing benzalacetophenone from acetophenone and benzaldehyde, and observing the effects of different catalysts. The introduction explains the reaction's mechanism, emphasizing the importance of C-C bond formation and the role of acid or base catalysts. The materials and methods section describes the preparation of trans-benzalacetophenone using sodium hydroxide, ethanol, acetophenone, and benzaldehyde, including crystallization and recrystallization steps. The reaction of benzalacetophenone with bromine is also detailed. The results section presents the weight and melting point of the product, along with potential sources of error. The conclusion suggests that the base catalyst led to a higher yield and potentially lower melting point due to impurities. This assignment provides a comprehensive overview of the Claisen-Schmidt reaction, covering experimental procedures, results, and analysis.
1 out of 4






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)