Critical Appraisal of Two Clinical Epidemiology Studies in Detail
VerifiedAdded on 2023/06/09
|13
|3293
|357
Homework Assignment
AI Summary
This assignment presents a critical appraisal of two clinical epidemiology studies. The first study examines the efficacy of neurokinin-1 receptor (NK1R) antagonists in treating chemotherapy-induced nausea and vomiting (CINV), evaluating their impact on complete response rates and potential side effects. The study design is a systematic review and meta-analysis. The second study focuses on tamoxifen for breast cancer prevention, analyzing its effects on breast cancer rates, including invasive and noninvasive forms, and assessing its performance based on patient demographics and risk factors. This study is a randomized controlled trial. Both studies are evaluated for internal and external validity, and the findings are interpreted in terms of their clinical significance and applicability to patient care. The assignment includes detailed analyses of study methodologies, results, and the level of evidence presented.

Last Name1
Name:
Student id:
Instructor:
Course: Clinical Epidemiology
Date: 7/18/2018
Critical Appraisal Clinical Epidemiology
Study 1
dos Santos LV, Souza FH, Brunetto AT, Sasse AD, da Silveira Nogueira Lima JP. Neurokinin-1
receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl
Cancer Inst. 2012 Sep 5;104(17):1280-92. doi:10.1093/jnci/djs335. Epub 2012 Aug 21. Review.
PubMed PMID: 22911671.”
Step 1
• What was the exposure or intervention?
Some patients were subjected to neurokinin-1 receptor (NK1R) antagonists while on
antiemetic regimes in the treatment of chemotherapy-induced nausea and vomiting (CINV. the
outcome was then compared to the previous reports on effects of NK1R antagonists.
• What was the outcome variable(s) (endpoint(s)?
The primary outcome of the analysis was that CR and nausea were enhanced across
the phases together with sub-groups. The endpoint; anticipated side effects from NK1R antagonists
and those from previous reports had a very small difference. There was a rise from 2% to 6%
critical infection cases in the NK1R antagonist category as revealed by the study.
Name:
Student id:
Instructor:
Course: Clinical Epidemiology
Date: 7/18/2018
Critical Appraisal Clinical Epidemiology
Study 1
dos Santos LV, Souza FH, Brunetto AT, Sasse AD, da Silveira Nogueira Lima JP. Neurokinin-1
receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl
Cancer Inst. 2012 Sep 5;104(17):1280-92. doi:10.1093/jnci/djs335. Epub 2012 Aug 21. Review.
PubMed PMID: 22911671.”
Step 1
• What was the exposure or intervention?
Some patients were subjected to neurokinin-1 receptor (NK1R) antagonists while on
antiemetic regimes in the treatment of chemotherapy-induced nausea and vomiting (CINV. the
outcome was then compared to the previous reports on effects of NK1R antagonists.
• What was the outcome variable(s) (endpoint(s)?
The primary outcome of the analysis was that CR and nausea were enhanced across
the phases together with sub-groups. The endpoint; anticipated side effects from NK1R antagonists
and those from previous reports had a very small difference. There was a rise from 2% to 6%
critical infection cases in the NK1R antagonist category as revealed by the study.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Last Name2
• What was the study question(s) / aims?
The questions of the analysis included; CR and nausea improvement using NK1R.Effects
of NK1R antagonists on CINV in the overall phase, acute phase, and the delayed phase. The
effectiveness of sNK1R at both moderately and extremely emetogenic chemotherapy regimens and
lastly, NK1R use in relation to escalated rates of infection.
• What was the study about?
The study was about treatment efficacy of NK1R antagonists on CINV regimens. The
results were positive since NK1R improved nausea and Complete Response treatment.
• Determine the type of the study
The type of study was a systemic review. The study design was a meta-analysis one. the
study was aimed at assessing the capability of NK1R antagonists in improving nausea and
complete response treatment and its impacts on the different regime phases.
• Determine the Study Design
CENTRAL. MEDLINE as well as EMBASE were used as sources for randomized trials .
these experiments enabled examination of the NK1R antagonists together with the standard
antiemetic cure to prevent CINV infection. The absence of emesis and presence of rescue
treatments was explained by a full reaction to CR treatment.
• What was the study population
• What was the study question(s) / aims?
The questions of the analysis included; CR and nausea improvement using NK1R.Effects
of NK1R antagonists on CINV in the overall phase, acute phase, and the delayed phase. The
effectiveness of sNK1R at both moderately and extremely emetogenic chemotherapy regimens and
lastly, NK1R use in relation to escalated rates of infection.
• What was the study about?
The study was about treatment efficacy of NK1R antagonists on CINV regimens. The
results were positive since NK1R improved nausea and Complete Response treatment.
• Determine the type of the study
The type of study was a systemic review. The study design was a meta-analysis one. the
study was aimed at assessing the capability of NK1R antagonists in improving nausea and
complete response treatment and its impacts on the different regime phases.
• Determine the Study Design
CENTRAL. MEDLINE as well as EMBASE were used as sources for randomized trials .
these experiments enabled examination of the NK1R antagonists together with the standard
antiemetic cure to prevent CINV infection. The absence of emesis and presence of rescue
treatments was explained by a full reaction to CR treatment.
• What was the study population
Last Name3
Study evaluation population involved 8740 patients on whom, seventeen trials were
performed on. The whole period rate of the complete response was escalated from 54% to 72% ,
0.51, 95%CI=0.46 to 0.57,P<.001. Nausea and complete response enhancement was recorded at all
stages and subgroups. NK1R side effects anticipated almost matched the previous results.
Step 2
THERAPY STUDY: Are the results of the trial valid? (Internal Validity)
What question did the study ask?
Patients –
Intervention -
Comparison -
Outcome(s) –
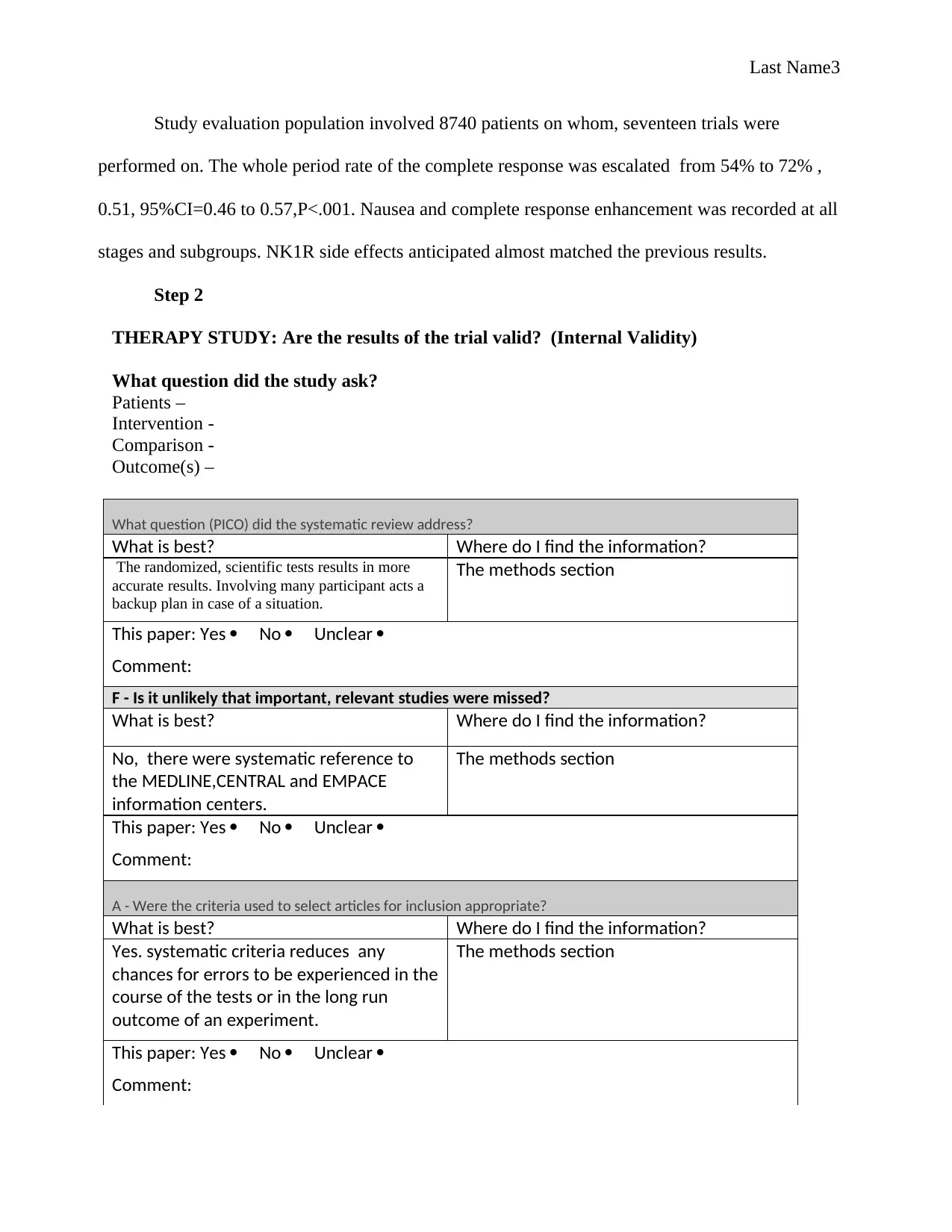
What question (PICO) did the systematic review address?
What is best? Where do I find the information?
The randomized, scientific tests results in more
accurate results. Involving many participant acts a
backup plan in case of a situation.
The methods section
This paper: Yes No Unclear
Comment:
F - Is it unlikely that important, relevant studies were missed?
What is best? Where do I find the information?
No, there were systematic reference to
the MEDLINE,CENTRAL and EMPACE
information centers.
The methods section
This paper: Yes No Unclear
Comment:
A - Were the criteria used to select articles for inclusion appropriate?
What is best? Where do I find the information?
Yes. systematic criteria reduces any
chances for errors to be experienced in the
course of the tests or in the long run
outcome of an experiment.
The methods section
This paper: Yes No Unclear
Comment:
Study evaluation population involved 8740 patients on whom, seventeen trials were
performed on. The whole period rate of the complete response was escalated from 54% to 72% ,
0.51, 95%CI=0.46 to 0.57,P<.001. Nausea and complete response enhancement was recorded at all
stages and subgroups. NK1R side effects anticipated almost matched the previous results.
Step 2
THERAPY STUDY: Are the results of the trial valid? (Internal Validity)
What question did the study ask?
Patients –
Intervention -
Comparison -
Outcome(s) –
What question (PICO) did the systematic review address?
What is best? Where do I find the information?
The randomized, scientific tests results in more
accurate results. Involving many participant acts a
backup plan in case of a situation.
The methods section
This paper: Yes No Unclear
Comment:
F - Is it unlikely that important, relevant studies were missed?
What is best? Where do I find the information?
No, there were systematic reference to
the MEDLINE,CENTRAL and EMPACE
information centers.
The methods section
This paper: Yes No Unclear
Comment:
A - Were the criteria used to select articles for inclusion appropriate?
What is best? Where do I find the information?
Yes. systematic criteria reduces any
chances for errors to be experienced in the
course of the tests or in the long run
outcome of an experiment.
The methods section
This paper: Yes No Unclear
Comment:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Last Name4
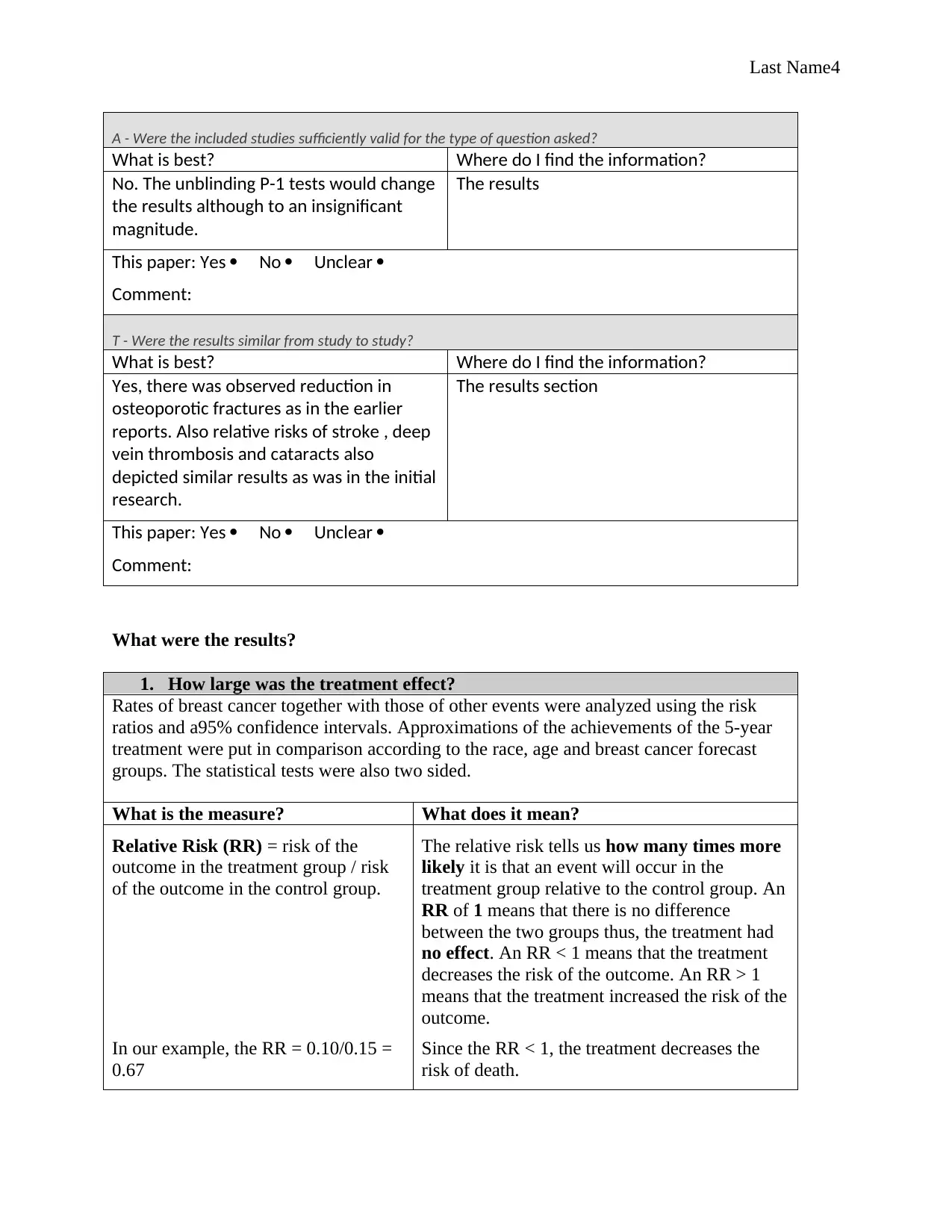
A - Were the included studies sufficiently valid for the type of question asked?
What is best? Where do I find the information?
No. The unblinding P-1 tests would change
the results although to an insignificant
magnitude.
The results
This paper: Yes No Unclear
Comment:
T - Were the results similar from study to study?
What is best? Where do I find the information?
Yes, there was observed reduction in
osteoporotic fractures as in the earlier
reports. Also relative risks of stroke , deep
vein thrombosis and cataracts also
depicted similar results as was in the initial
research.
The results section
This paper: Yes No Unclear
Comment:
What were the results?
1. How large was the treatment effect?
Rates of breast cancer together with those of other events were analyzed using the risk
ratios and a95% confidence intervals. Approximations of the achievements of the 5-year
treatment were put in comparison according to the race, age and breast cancer forecast
groups. The statistical tests were also two sided.
What is the measure? What does it mean?
Relative Risk (RR) = risk of the
outcome in the treatment group / risk
of the outcome in the control group.
The relative risk tells us how many times more
likely it is that an event will occur in the
treatment group relative to the control group. An
RR of 1 means that there is no difference
between the two groups thus, the treatment had
no effect. An RR < 1 means that the treatment
decreases the risk of the outcome. An RR > 1
means that the treatment increased the risk of the
outcome.
In our example, the RR = 0.10/0.15 =
0.67
Since the RR < 1, the treatment decreases the
risk of death.
A - Were the included studies sufficiently valid for the type of question asked?
What is best? Where do I find the information?
No. The unblinding P-1 tests would change
the results although to an insignificant
magnitude.
The results
This paper: Yes No Unclear
Comment:
T - Were the results similar from study to study?
What is best? Where do I find the information?
Yes, there was observed reduction in
osteoporotic fractures as in the earlier
reports. Also relative risks of stroke , deep
vein thrombosis and cataracts also
depicted similar results as was in the initial
research.
The results section
This paper: Yes No Unclear
Comment:
What were the results?
1. How large was the treatment effect?
Rates of breast cancer together with those of other events were analyzed using the risk
ratios and a95% confidence intervals. Approximations of the achievements of the 5-year
treatment were put in comparison according to the race, age and breast cancer forecast
groups. The statistical tests were also two sided.
What is the measure? What does it mean?
Relative Risk (RR) = risk of the
outcome in the treatment group / risk
of the outcome in the control group.
The relative risk tells us how many times more
likely it is that an event will occur in the
treatment group relative to the control group. An
RR of 1 means that there is no difference
between the two groups thus, the treatment had
no effect. An RR < 1 means that the treatment
decreases the risk of the outcome. An RR > 1
means that the treatment increased the risk of the
outcome.
In our example, the RR = 0.10/0.15 =
0.67
Since the RR < 1, the treatment decreases the
risk of death.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Last Name5
Absolute Risk Reduction (ARR) =
risk of the outcome in the control
group - risk of the outcome in the
treatment group. This is also known as
the absolute risk difference.
The absolute risk reduction tells us the absolute
difference in the rates of events between the two
groups and gives an indication of the baseline
risk and treatment effect. An ARR of 0 means
that there is no difference between the two
groups thus, the treatment had no effect.
In our example, the ARR = 0.15 - 0.10
= 0.05 or 5%
The absolute benefit of treatment is a 5%
reduction in the death rate.
Relative Risk Reduction (RRR) =
absolute risk reduction / risk of the
outcome in the control group. An
alternative way to calculate the RRR is
to subtract the RR from 1 (eg. RRR =
1 - RR)
The relative risk reduction is the complement of
the RR and is probably the most commonly
reported measure of treatment effects. It tells us
the reduction in the rate of the outcome in the
treatment group relative to that in the control
group.
In our example, the RRR = 0.05/0.15
= 0.33 or 33%
Or RRR = 1 - 0.67 = 0.33
or 33%
The treatment reduced the risk of death by 33%
relative to that occurring in the control group.
Number Needed to Treat (NNT) =
inverse of the ARR and is calculated
as 1 / ARR.
The number needed to treat represents the
number of patients we need to treat with the
experimental therapy in order to prevent 1 bad
outcome and incorporates the duration of
treatment. Clinical significance can be
determined to some extent by looking at the
NNTs, but also by weighing the NNTs against
any harms or adverse effects (NNHs) of therapy.
In our example, the NNT = 1/ 0.04 =
25
We would need to treat 20 people for 2 years in
order to prevent 1 death.
2. How precise was the estimate of the treatment effect?
The precision of risk assessment was 95%, which means that the results obtained after the
therapy, was systematic and effective like in the placebo group (RR = 0.63, 95% CI = 0.45 to
0.89) and (RR = 0.57, 95% CI = 0.46 to 0.70) in the tamoxifen group
Will the results help me in caring for my patient? (ExternalValidity/Applicability)
Yes. The results will help me in taking care of my clients because they were
effective having produced similar results as those produced from initial therapies.
My clients being no different from those involved in carrying out research will
benefit from the study too.
Absolute Risk Reduction (ARR) =
risk of the outcome in the control
group - risk of the outcome in the
treatment group. This is also known as
the absolute risk difference.
The absolute risk reduction tells us the absolute
difference in the rates of events between the two
groups and gives an indication of the baseline
risk and treatment effect. An ARR of 0 means
that there is no difference between the two
groups thus, the treatment had no effect.
In our example, the ARR = 0.15 - 0.10
= 0.05 or 5%
The absolute benefit of treatment is a 5%
reduction in the death rate.
Relative Risk Reduction (RRR) =
absolute risk reduction / risk of the
outcome in the control group. An
alternative way to calculate the RRR is
to subtract the RR from 1 (eg. RRR =
1 - RR)
The relative risk reduction is the complement of
the RR and is probably the most commonly
reported measure of treatment effects. It tells us
the reduction in the rate of the outcome in the
treatment group relative to that in the control
group.
In our example, the RRR = 0.05/0.15
= 0.33 or 33%
Or RRR = 1 - 0.67 = 0.33
or 33%
The treatment reduced the risk of death by 33%
relative to that occurring in the control group.
Number Needed to Treat (NNT) =
inverse of the ARR and is calculated
as 1 / ARR.
The number needed to treat represents the
number of patients we need to treat with the
experimental therapy in order to prevent 1 bad
outcome and incorporates the duration of
treatment. Clinical significance can be
determined to some extent by looking at the
NNTs, but also by weighing the NNTs against
any harms or adverse effects (NNHs) of therapy.
In our example, the NNT = 1/ 0.04 =
25
We would need to treat 20 people for 2 years in
order to prevent 1 death.
2. How precise was the estimate of the treatment effect?
The precision of risk assessment was 95%, which means that the results obtained after the
therapy, was systematic and effective like in the placebo group (RR = 0.63, 95% CI = 0.45 to
0.89) and (RR = 0.57, 95% CI = 0.46 to 0.70) in the tamoxifen group
Will the results help me in caring for my patient? (ExternalValidity/Applicability)
Yes. The results will help me in taking care of my clients because they were
effective having produced similar results as those produced from initial therapies.
My clients being no different from those involved in carrying out research will
benefit from the study too.

Last Name6
Results
An analysis involving 8740 patients, resulted in an outcome where NK1R competitor
heightened the complete response rate in the entire period from 54% to 72% (OR =0.51, 95%
CI=0.46 to 0.57,P<0.001). Nausea and complete response were all enhanced across the stages and
the sub-categories. The outcome from NK1R competitor revealed almost same results as those
from earlier researches. Nevertheless, this assessment proposed the capability of a rise in severe
infection from 2% to 4% in the NK1R competitor group involving RCTs with a figure of 1480
patients; OR=3.10; 95% CI=1.69 to 5.67, <0.01).
STEP 3
The level of evidence in this study was 1b because of having less than 80% follows up
and that it was a individual cohort research. Good follow up is also evident .Both treated and
untreated samples were used in the split confirmation.
Study 2
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A,
Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG,
Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the
National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer
Results
An analysis involving 8740 patients, resulted in an outcome where NK1R competitor
heightened the complete response rate in the entire period from 54% to 72% (OR =0.51, 95%
CI=0.46 to 0.57,P<0.001). Nausea and complete response were all enhanced across the stages and
the sub-categories. The outcome from NK1R competitor revealed almost same results as those
from earlier researches. Nevertheless, this assessment proposed the capability of a rise in severe
infection from 2% to 4% in the NK1R competitor group involving RCTs with a figure of 1480
patients; OR=3.10; 95% CI=1.69 to 5.67, <0.01).
STEP 3
The level of evidence in this study was 1b because of having less than 80% follows up
and that it was a individual cohort research. Good follow up is also evident .Both treated and
untreated samples were used in the split confirmation.
Study 2
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A,
Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG,
Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the
National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Last Name7
Inst. 2005 Nov 16;97(22):1652-62. PubMed PMID: 16288118.
STEP 1
• What was the exposure or intervention?”
A therapy was performed on 13388 women. The exposure was performed on randomly
assigned women to take Tamoxifen for a period of 5 years after which rates of breast cancer was
then correlated using the risk ratios and 95% CIs. Two-sided statistical experiments were
performed by comparing treatment effects 5-year Tamoxifen treatment basing on race, age and
other possible groups of breast cancer risk to those of placebo women.
What was the outcome variable(s) (endpoint(s)?
After the positive outcome in the tests where tamoxifen was found to decrease the danger of
estrogen receptor –positive tumors together with the revelation that even the osteoporotic risk
fractures in women were also lowered at increased threat of breast cancer, a follow up was then
initiated . The endpoint resulted in a decreased rate of combined aggressive breast cancer from
42.5 per 1000 placebo women to 24.8 per inactive drug women. The rate of noninvasive breast
cancer also went down from 15.8 per 1000 women in the nostrum category to 10.2 per 1000
women in the tamoxifen category. The outcomes were similar to the earlier reports.
What was the study question(s) / aims?
The aims included the analysis of the rate of breast cancer infection under various
conditions, effects of tamoxifen on the treatment of breast cancer, and performance of tamoxifen
based on age, race and other possible groupings.
Inst. 2005 Nov 16;97(22):1652-62. PubMed PMID: 16288118.
STEP 1
• What was the exposure or intervention?”
A therapy was performed on 13388 women. The exposure was performed on randomly
assigned women to take Tamoxifen for a period of 5 years after which rates of breast cancer was
then correlated using the risk ratios and 95% CIs. Two-sided statistical experiments were
performed by comparing treatment effects 5-year Tamoxifen treatment basing on race, age and
other possible groups of breast cancer risk to those of placebo women.
What was the outcome variable(s) (endpoint(s)?
After the positive outcome in the tests where tamoxifen was found to decrease the danger of
estrogen receptor –positive tumors together with the revelation that even the osteoporotic risk
fractures in women were also lowered at increased threat of breast cancer, a follow up was then
initiated . The endpoint resulted in a decreased rate of combined aggressive breast cancer from
42.5 per 1000 placebo women to 24.8 per inactive drug women. The rate of noninvasive breast
cancer also went down from 15.8 per 1000 women in the nostrum category to 10.2 per 1000
women in the tamoxifen category. The outcomes were similar to the earlier reports.
What was the study question(s) / aims?
The aims included the analysis of the rate of breast cancer infection under various
conditions, effects of tamoxifen on the treatment of breast cancer, and performance of tamoxifen
based on age, race and other possible groupings.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Last Name8
• What was the study about?
The research was about the efficacy of tamoxifen on breast cancer treatment.
• Determine the type of the study
The type of study is secondary
• Determine the Study Design
The study design is a randomized controlled trial since women were randomly assigned to
receive either placebo or tamoxifen for a period of 5 years.
• What was the study population
For a period of 5 years, a total of 98018 women were subjected to a risk evaluation .based
on their risks, 57641 (58.8 ) were given a go ahead to participate in the test while 14453 gave their
consent on being assessed on medical conditions to decide on their admissibility. In the end, only
13954 managed to get their names listed for the test. The research population involved 13,388
women who engaged in either taking the tamoxifen (6681 women) drugs or placebo (6707 women)
for testing of the breast cancer rates in women.
CAP analysis see attachment. The level of evidence is level 2b since its <80 follow up and
involves RCT.
STEP 2
• What was the study about?
The research was about the efficacy of tamoxifen on breast cancer treatment.
• Determine the type of the study
The type of study is secondary
• Determine the Study Design
The study design is a randomized controlled trial since women were randomly assigned to
receive either placebo or tamoxifen for a period of 5 years.
• What was the study population
For a period of 5 years, a total of 98018 women were subjected to a risk evaluation .based
on their risks, 57641 (58.8 ) were given a go ahead to participate in the test while 14453 gave their
consent on being assessed on medical conditions to decide on their admissibility. In the end, only
13954 managed to get their names listed for the test. The research population involved 13,388
women who engaged in either taking the tamoxifen (6681 women) drugs or placebo (6707 women)
for testing of the breast cancer rates in women.
CAP analysis see attachment. The level of evidence is level 2b since its <80 follow up and
involves RCT.
STEP 2
Last Name9
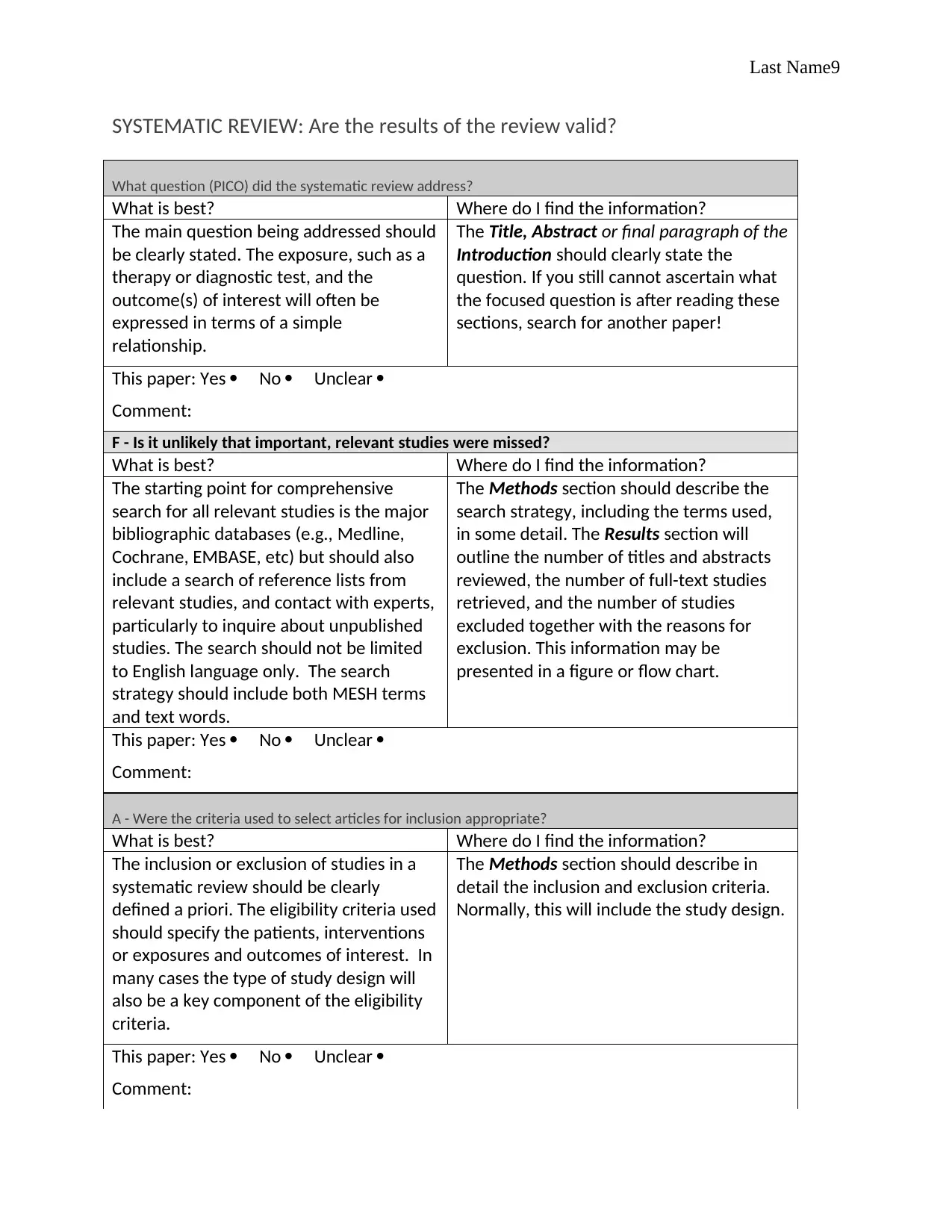
SYSTEMATIC REVIEW: Are the results of the review valid?
What question (PICO) did the systematic review address?
What is best? Where do I find the information?
The main question being addressed should
be clearly stated. The exposure, such as a
therapy or diagnostic test, and the
outcome(s) of interest will often be
expressed in terms of a simple
relationship.
The Title, Abstract or final paragraph of the
Introduction should clearly state the
question. If you still cannot ascertain what
the focused question is after reading these
sections, search for another paper!
This paper: Yes No Unclear
Comment:
F - Is it unlikely that important, relevant studies were missed?
What is best? Where do I find the information?
The starting point for comprehensive
search for all relevant studies is the major
bibliographic databases (e.g., Medline,
Cochrane, EMBASE, etc) but should also
include a search of reference lists from
relevant studies, and contact with experts,
particularly to inquire about unpublished
studies. The search should not be limited
to English language only. The search
strategy should include both MESH terms
and text words.
The Methods section should describe the
search strategy, including the terms used,
in some detail. The Results section will
outline the number of titles and abstracts
reviewed, the number of full-text studies
retrieved, and the number of studies
excluded together with the reasons for
exclusion. This information may be
presented in a figure or flow chart.
This paper: Yes No Unclear
Comment:
A - Were the criteria used to select articles for inclusion appropriate?
What is best? Where do I find the information?
The inclusion or exclusion of studies in a
systematic review should be clearly
defined a priori. The eligibility criteria used
should specify the patients, interventions
or exposures and outcomes of interest. In
many cases the type of study design will
also be a key component of the eligibility
criteria.
The Methods section should describe in
detail the inclusion and exclusion criteria.
Normally, this will include the study design.
This paper: Yes No Unclear
Comment:
SYSTEMATIC REVIEW: Are the results of the review valid?
What question (PICO) did the systematic review address?
What is best? Where do I find the information?
The main question being addressed should
be clearly stated. The exposure, such as a
therapy or diagnostic test, and the
outcome(s) of interest will often be
expressed in terms of a simple
relationship.
The Title, Abstract or final paragraph of the
Introduction should clearly state the
question. If you still cannot ascertain what
the focused question is after reading these
sections, search for another paper!
This paper: Yes No Unclear
Comment:
F - Is it unlikely that important, relevant studies were missed?
What is best? Where do I find the information?
The starting point for comprehensive
search for all relevant studies is the major
bibliographic databases (e.g., Medline,
Cochrane, EMBASE, etc) but should also
include a search of reference lists from
relevant studies, and contact with experts,
particularly to inquire about unpublished
studies. The search should not be limited
to English language only. The search
strategy should include both MESH terms
and text words.
The Methods section should describe the
search strategy, including the terms used,
in some detail. The Results section will
outline the number of titles and abstracts
reviewed, the number of full-text studies
retrieved, and the number of studies
excluded together with the reasons for
exclusion. This information may be
presented in a figure or flow chart.
This paper: Yes No Unclear
Comment:
A - Were the criteria used to select articles for inclusion appropriate?
What is best? Where do I find the information?
The inclusion or exclusion of studies in a
systematic review should be clearly
defined a priori. The eligibility criteria used
should specify the patients, interventions
or exposures and outcomes of interest. In
many cases the type of study design will
also be a key component of the eligibility
criteria.
The Methods section should describe in
detail the inclusion and exclusion criteria.
Normally, this will include the study design.
This paper: Yes No Unclear
Comment:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Last Name10
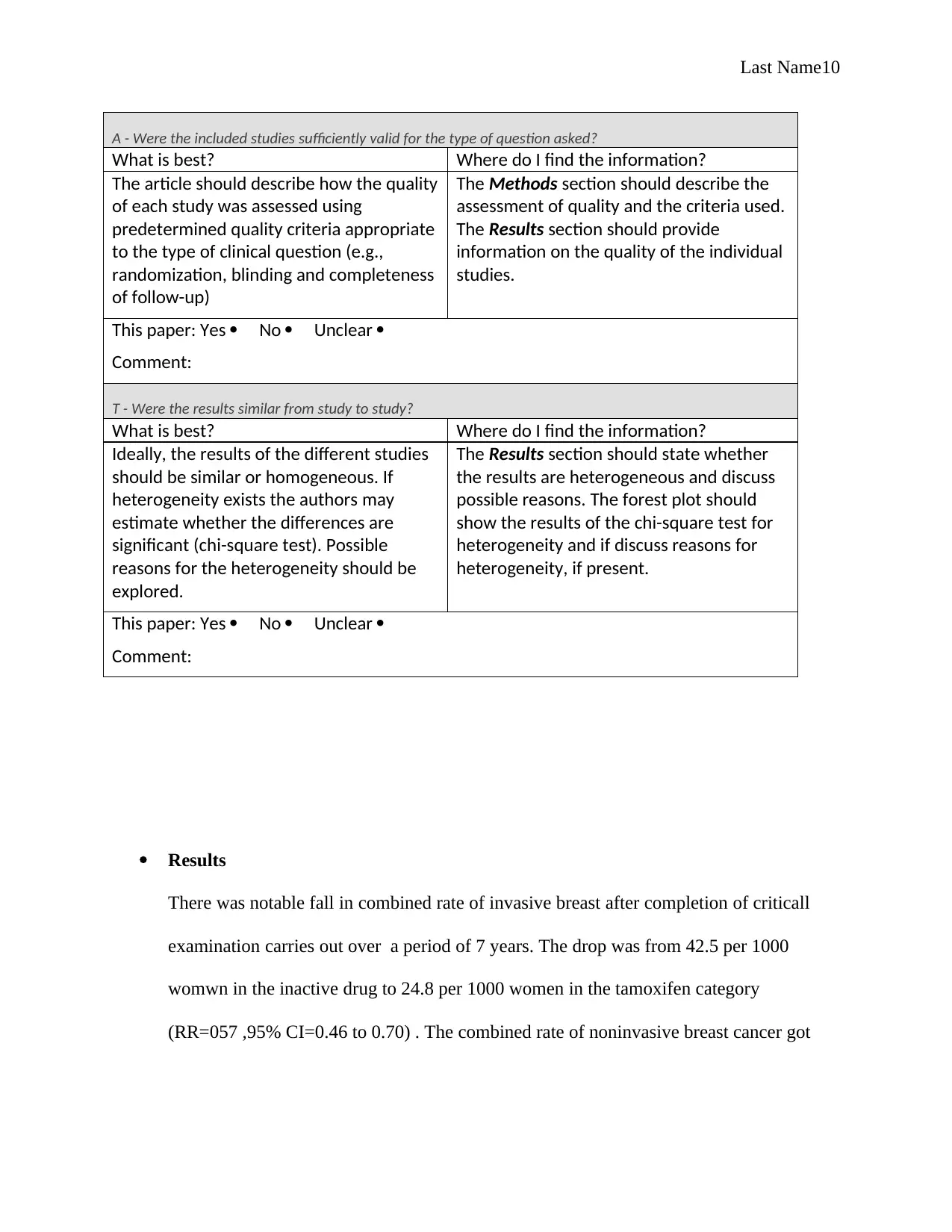
A - Were the included studies sufficiently valid for the type of question asked?
What is best? Where do I find the information?
The article should describe how the quality
of each study was assessed using
predetermined quality criteria appropriate
to the type of clinical question (e.g.,
randomization, blinding and completeness
of follow-up)
The Methods section should describe the
assessment of quality and the criteria used.
The Results section should provide
information on the quality of the individual
studies.
This paper: Yes No Unclear
Comment:
T - Were the results similar from study to study?
What is best? Where do I find the information?
Ideally, the results of the different studies
should be similar or homogeneous. If
heterogeneity exists the authors may
estimate whether the differences are
significant (chi-square test). Possible
reasons for the heterogeneity should be
explored.
The Results section should state whether
the results are heterogeneous and discuss
possible reasons. The forest plot should
show the results of the chi-square test for
heterogeneity and if discuss reasons for
heterogeneity, if present.
This paper: Yes No Unclear
Comment:
Results
There was notable fall in combined rate of invasive breast after completion of criticall
examination carries out over a period of 7 years. The drop was from 42.5 per 1000
womwn in the inactive drug to 24.8 per 1000 women in the tamoxifen category
(RR=057 ,95% CI=0.46 to 0.70) . The combined rate of noninvasive breast cancer got
A - Were the included studies sufficiently valid for the type of question asked?
What is best? Where do I find the information?
The article should describe how the quality
of each study was assessed using
predetermined quality criteria appropriate
to the type of clinical question (e.g.,
randomization, blinding and completeness
of follow-up)
The Methods section should describe the
assessment of quality and the criteria used.
The Results section should provide
information on the quality of the individual
studies.
This paper: Yes No Unclear
Comment:
T - Were the results similar from study to study?
What is best? Where do I find the information?
Ideally, the results of the different studies
should be similar or homogeneous. If
heterogeneity exists the authors may
estimate whether the differences are
significant (chi-square test). Possible
reasons for the heterogeneity should be
explored.
The Results section should state whether
the results are heterogeneous and discuss
possible reasons. The forest plot should
show the results of the chi-square test for
heterogeneity and if discuss reasons for
heterogeneity, if present.
This paper: Yes No Unclear
Comment:
Results
There was notable fall in combined rate of invasive breast after completion of criticall
examination carries out over a period of 7 years. The drop was from 42.5 per 1000
womwn in the inactive drug to 24.8 per 1000 women in the tamoxifen category
(RR=057 ,95% CI=0.46 to 0.70) . The combined rate of noninvasive breast cancer got
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Last Name11
lowered from15.8 per 1000 women in the inactive drug category to that of 10.2 per 1000
women in the counter category of tamoxifen (RR= 0.63,95% CI=0.45 to .0
89). These results matched those of the study documented in the earlier study. A 32%
drop in the osteoporotic fractures ( RR.068.95% CI=0.51 to 0.92). In addition,
comparative risks of deep-vein thrombosis, stroke, ischemic heart ailments and cataracts
also resulted in matching outcome as those carried out in the previous research.
However , dangers of pulmonary embolism was estimated to be at 11% lower than the
initial report while dangers of endometrial cancer rose by approximately 29% although
the two results were not beneficial to use for statistics .Finally ,there was variance based
on race, age together with the danger of breast cancer level on the net achievement of
tamoxifen.
STEP 3
The level of evidence in this study was 2b because of having less than 80% follows up
and that it was a personal cohort research. Both treated and untreated samples was used in the
split validation .
lowered from15.8 per 1000 women in the inactive drug category to that of 10.2 per 1000
women in the counter category of tamoxifen (RR= 0.63,95% CI=0.45 to .0
89). These results matched those of the study documented in the earlier study. A 32%
drop in the osteoporotic fractures ( RR.068.95% CI=0.51 to 0.92). In addition,
comparative risks of deep-vein thrombosis, stroke, ischemic heart ailments and cataracts
also resulted in matching outcome as those carried out in the previous research.
However , dangers of pulmonary embolism was estimated to be at 11% lower than the
initial report while dangers of endometrial cancer rose by approximately 29% although
the two results were not beneficial to use for statistics .Finally ,there was variance based
on race, age together with the danger of breast cancer level on the net achievement of
tamoxifen.
STEP 3
The level of evidence in this study was 2b because of having less than 80% follows up
and that it was a personal cohort research. Both treated and untreated samples was used in the
split validation .

Last Name12
Work cited
Crowe, Michael, and Lorraine Sheppard. "A review of critical appraisal tools show they
lack rigor: alternative tool structure is proposed." Journal of clinical epidemiology 64.1 (2011): 79-
89.
dos Santos, Lucas Vieira, et al. "Neurokinin-1 receptor antagonists for chemotherapy-
induced nausea and vomiting: a systematic review." Journal of the National Cancer
Institute104.17 (2012): 1280-1292.
Fisher, Bernard, et al. "Tamoxifen for the prevention of breast cancer: current status of the
National Surgical Adjuvant Breast and Bowel Project P-1 study." Journal of the national cancer
institute 97.22 (2005): 1652-1662.
Work cited
Crowe, Michael, and Lorraine Sheppard. "A review of critical appraisal tools show they
lack rigor: alternative tool structure is proposed." Journal of clinical epidemiology 64.1 (2011): 79-
89.
dos Santos, Lucas Vieira, et al. "Neurokinin-1 receptor antagonists for chemotherapy-
induced nausea and vomiting: a systematic review." Journal of the National Cancer
Institute104.17 (2012): 1280-1292.
Fisher, Bernard, et al. "Tamoxifen for the prevention of breast cancer: current status of the
National Surgical Adjuvant Breast and Bowel Project P-1 study." Journal of the national cancer
institute 97.22 (2005): 1652-1662.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.