CO2 Miscibility and its Application in Oil Recovery: A Detailed Report
VerifiedAdded on 2023/01/18
|13
|2392
|67
Report
AI Summary
This report examines the miscibility of carbon dioxide (CO2) and its application in oil recovery processes. It explains how CO2, when injected into oil reservoirs, can form a uniform solution with oil, a process crucial for enhanced oil recovery. The report highlights the advantages of CO2, such as its lower minimum miscibility pressure compared to other gases, and illustrates the relationship between CO2 pressure and oil recovery through tables and graphical analyses. It details the concepts of miscible and immiscible displacement, emphasizing the preference for miscible displacement. The discussion covers factors affecting CO2 miscibility, methods for calculating minimum miscibility pressure, and the dynamic miscibility process. The report concludes by summarizing the conditions under which CO2 miscibility occurs and its effectiveness in oil recovery, emphasizing that miscible displacement is generally preferred. The report also provides relevant references. This report is a comprehensive overview of CO2 miscibility and its significance in the oil and gas industry.

CO2 MISCIBILITY
By (Name)
Course
Tutor
Learning Institution
Date
By (Name)
Course
Tutor
Learning Institution
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Content
Introduction……………………………………………………………………………………..2
Discussion………………………………………………………………………………………...3
Result…………….………………………………………………………………………………..4
1.0: Table of results for oil recovery.............................................................................…...4
1.1: Graphical analysis oil
recovery…………………………………............................5
1.2: Carbon dioxide miscibility process……………………………………….….6
Conclusion……………………………………………………………………………………..…7
References………………………………………………………………………………………..9
Introduction……………………………………………………………………………………..2
Discussion………………………………………………………………………………………...3
Result…………….………………………………………………………………………………..4
1.0: Table of results for oil recovery.............................................................................…...4
1.1: Graphical analysis oil
recovery…………………………………............................5
1.2: Carbon dioxide miscibility process……………………………………….….6
Conclusion……………………………………………………………………………………..…7
References………………………………………………………………………………………..9

Executive Summery
In a nutshell the report is all about the miscibility of carbon dioxide and its applications in
the oil recovery process. Carbon dioxide form a uniform solution with oil when pumped at a
minimum miscibility pressure into oil reservoir .Minimum miscibility pressure is the lowest
pressure at which oil mixes completely with oil. This make carbon dioxide be used in the oil
recovery process. It has several advantages such as lower minimum miscibility pressure that
makes it preferred to other gases.
A table of pressure and the corresponding recovery factors was obtained which was used to
illustrate the variation of oil recovery at different carbon dioxide pressure, refer to figure 1.1
above. Oil recovery occur through oil displacement by the carbon dioxide a process called
dynamic displacement as shown in figure 1.2 above
In a nutshell the report is all about the miscibility of carbon dioxide and its applications in
the oil recovery process. Carbon dioxide form a uniform solution with oil when pumped at a
minimum miscibility pressure into oil reservoir .Minimum miscibility pressure is the lowest
pressure at which oil mixes completely with oil. This make carbon dioxide be used in the oil
recovery process. It has several advantages such as lower minimum miscibility pressure that
makes it preferred to other gases.
A table of pressure and the corresponding recovery factors was obtained which was used to
illustrate the variation of oil recovery at different carbon dioxide pressure, refer to figure 1.1
above. Oil recovery occur through oil displacement by the carbon dioxide a process called
dynamic displacement as shown in figure 1.2 above
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Introduction
Carbon dioxide gas at atmospheric pressure and temperatures is colorless, odorless and is
about 1.5 times heavier than air. It has got a critical pressure and temperature of about 1,070.6
psia and 73.82kPa respectively. Critical pressure is that pressure at which no more amount of
CO2 can be liquefied further, while critical temperature is that temperature at which no more
amount of the gas can be liquefied .At these critical conditions, carbon dioxide gas and carbon
dioxide liquid coexist .Above the critical temperature and pressure, the two form a phase with a
density close to that of a liquid. This is the most preferred state to be used in the oil recovery
process due to its lowered viscosity (Haghtalab, Hasannataj, and Panah, 2018, p. 114).
Carbon dioxide is a greenhouse gas. Its continuous increase into the atmosphere poses a
great threat to the environment as it raises the temperatures. Therefore, there is need to reduce
the concentration of Carbon (IV) Oxide in the atmosphere. To achieve this, the ability of CO2 to
form miscible solutions has been greatly exploited in the oil recovery process by injecting a
given quantity of the gas into the oil reserves. Carbon dioxide will then either form miscible or
immiscible solution with oil reserves hence displacing the oil (Li, Qin, & Yang, 2014, p. 3520).
Displacement taking place at pressures below minimum miscibility pressure is termed as
immiscible displacement, while those taking place at pressures above the minimum miscibility
pressure are referred to as miscible displacement. Miscible displacement is mostly preferred to
the immiscible displacement. This essay discusses on the miscibility of CO2, which makes it be a
suitable reagent for use in the oil recovery process.
2
Carbon dioxide gas at atmospheric pressure and temperatures is colorless, odorless and is
about 1.5 times heavier than air. It has got a critical pressure and temperature of about 1,070.6
psia and 73.82kPa respectively. Critical pressure is that pressure at which no more amount of
CO2 can be liquefied further, while critical temperature is that temperature at which no more
amount of the gas can be liquefied .At these critical conditions, carbon dioxide gas and carbon
dioxide liquid coexist .Above the critical temperature and pressure, the two form a phase with a
density close to that of a liquid. This is the most preferred state to be used in the oil recovery
process due to its lowered viscosity (Haghtalab, Hasannataj, and Panah, 2018, p. 114).
Carbon dioxide is a greenhouse gas. Its continuous increase into the atmosphere poses a
great threat to the environment as it raises the temperatures. Therefore, there is need to reduce
the concentration of Carbon (IV) Oxide in the atmosphere. To achieve this, the ability of CO2 to
form miscible solutions has been greatly exploited in the oil recovery process by injecting a
given quantity of the gas into the oil reserves. Carbon dioxide will then either form miscible or
immiscible solution with oil reserves hence displacing the oil (Li, Qin, & Yang, 2014, p. 3520).
Displacement taking place at pressures below minimum miscibility pressure is termed as
immiscible displacement, while those taking place at pressures above the minimum miscibility
pressure are referred to as miscible displacement. Miscible displacement is mostly preferred to
the immiscible displacement. This essay discusses on the miscibility of CO2, which makes it be a
suitable reagent for use in the oil recovery process.
2

Discussion
CO2 miscibility is the process where carbon dioxide pumped into the oil reservoir mixes
with the large volume of oil to form a single uniform layer a process that aid in oil recovery
(Haghtalab, Hasannataj, and Panah, 2018, p. 114). One main advantage using carbon dioxide
compared to other types of oil enhancing gas such as methane and nitrogen gas is its significantly
lower minimum miscibility pressure (Haghtalab, Hasannataj, and Panah, 2018, p. 114). In
addition to the lower miscibility pressure, carbon dioxide readily mixes with the crude oil hence
very suitable for the oil recovery process.
The uses of carbon dioxide in the oil recovery process again reduces the emission of the
gas into the atmosphere reducing the greenhouse effect of carbon dioxide. However its use in the
oil recovery process is very slow, time consuming as well as very expensive compared to other
oil enhancing gases such as methane.
Minimum miscibility pressure is a major factor in determining the miscibility of carbon dioxide.
It is a key factor in the recovery process of oil. Displacement efficiency is the ease of which oil is
displaced by either immiscible or miscible CO2.Several factors such as; the reservoir’s fluid
composition, the injection gas composition and the reservoir’s temperature and pressure affect
the miscibility of CO2. Minimum miscibility pressure of CO2 can be calculated using three
methods including; the use of rising bubble equipment, the narrow tube apparatus and finally by
the type of correction involved (John, & Michael, 2016, p.8).. In the determination of pressure
for CO2 in the reservoir, simulation was done for the slim tube model .The length of the model
was first taken to be 100m, with 600 grids and a porosity of about 0.15. The model permeability
was taken to be 200mcd.The length of 100m together with the model height and width ensures
that the miscibility is fully developed and the result of the transition region minimized to a value
3
CO2 miscibility is the process where carbon dioxide pumped into the oil reservoir mixes
with the large volume of oil to form a single uniform layer a process that aid in oil recovery
(Haghtalab, Hasannataj, and Panah, 2018, p. 114). One main advantage using carbon dioxide
compared to other types of oil enhancing gas such as methane and nitrogen gas is its significantly
lower minimum miscibility pressure (Haghtalab, Hasannataj, and Panah, 2018, p. 114). In
addition to the lower miscibility pressure, carbon dioxide readily mixes with the crude oil hence
very suitable for the oil recovery process.
The uses of carbon dioxide in the oil recovery process again reduces the emission of the
gas into the atmosphere reducing the greenhouse effect of carbon dioxide. However its use in the
oil recovery process is very slow, time consuming as well as very expensive compared to other
oil enhancing gases such as methane.
Minimum miscibility pressure is a major factor in determining the miscibility of carbon dioxide.
It is a key factor in the recovery process of oil. Displacement efficiency is the ease of which oil is
displaced by either immiscible or miscible CO2.Several factors such as; the reservoir’s fluid
composition, the injection gas composition and the reservoir’s temperature and pressure affect
the miscibility of CO2. Minimum miscibility pressure of CO2 can be calculated using three
methods including; the use of rising bubble equipment, the narrow tube apparatus and finally by
the type of correction involved (John, & Michael, 2016, p.8).. In the determination of pressure
for CO2 in the reservoir, simulation was done for the slim tube model .The length of the model
was first taken to be 100m, with 600 grids and a porosity of about 0.15. The model permeability
was taken to be 200mcd.The length of 100m together with the model height and width ensures
that the miscibility is fully developed and the result of the transition region minimized to a value
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
as much as possible. To prevent viscous fingering which may occur during the simulation
process, a smaller diameter for the model was chosen. A number of the narrow –tubes
simulations were then run at different pressures and a grid of about 200 blocks. This helps to find
the lowest pressure at which CO2 forms a single solution with oil.
Results
Table 1.0: Oil recovery
The table below shows how the oil recovery process was done at different pressure giving
different recovery factors .At a pressure of 1900, 55.36 recovery factor was achieved.
Pressure Recovery
Factor
1900 55.36
2650 66.00
3500 70.70
4500 84.86
5100 86.70
5700 87.19
6130 89.93
6670 90.13
7000 92.79
Adapted from (John, & Michael, 2016, p.8).
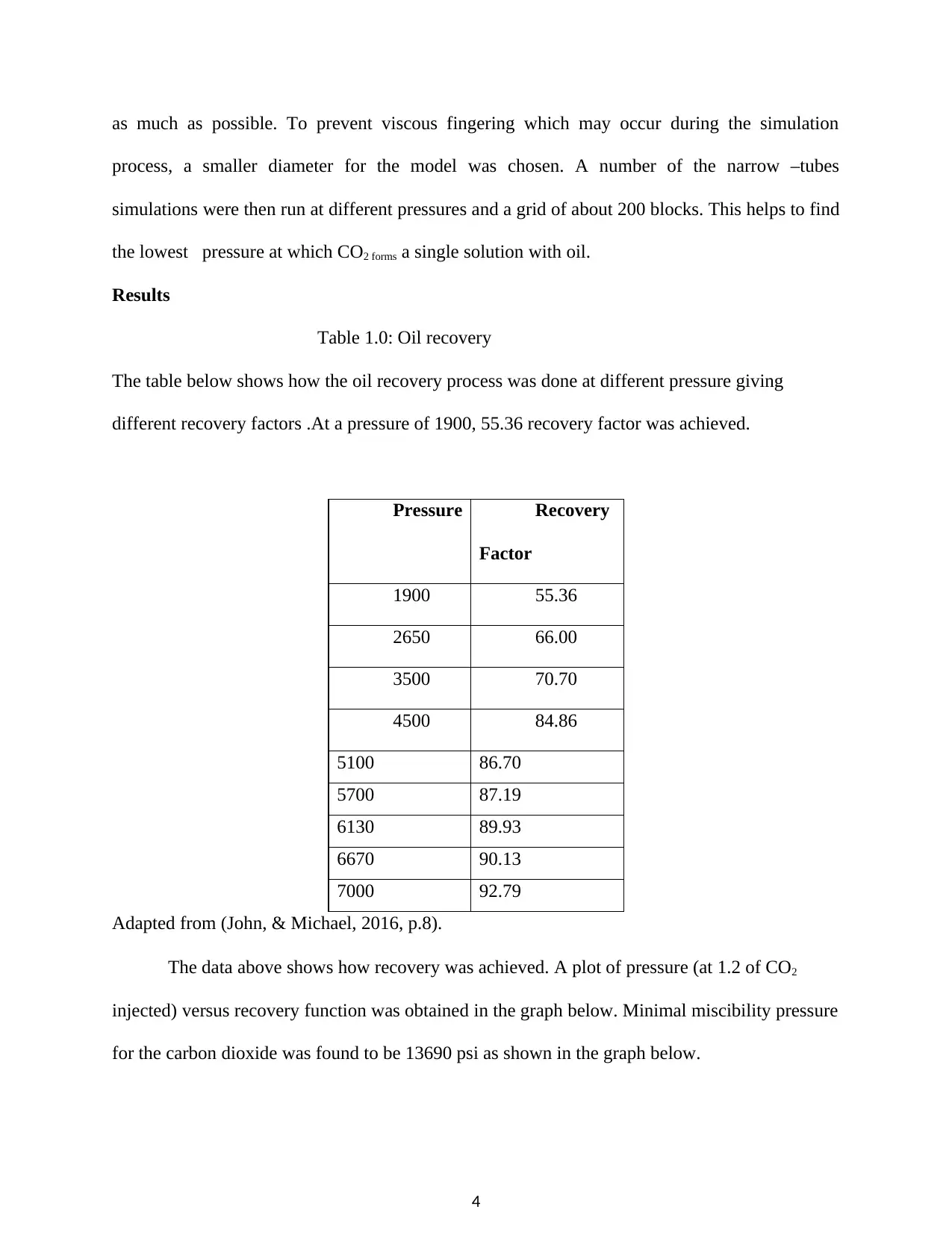
The data above shows how recovery was achieved. A plot of pressure (at 1.2 of CO2
injected) versus recovery function was obtained in the graph below. Minimal miscibility pressure
for the carbon dioxide was found to be 13690 psi as shown in the graph below.
4
process, a smaller diameter for the model was chosen. A number of the narrow –tubes
simulations were then run at different pressures and a grid of about 200 blocks. This helps to find
the lowest pressure at which CO2 forms a single solution with oil.
Results
Table 1.0: Oil recovery
The table below shows how the oil recovery process was done at different pressure giving
different recovery factors .At a pressure of 1900, 55.36 recovery factor was achieved.
Pressure Recovery
Factor
1900 55.36
2650 66.00
3500 70.70
4500 84.86
5100 86.70
5700 87.19
6130 89.93
6670 90.13
7000 92.79
Adapted from (John, & Michael, 2016, p.8).
The data above shows how recovery was achieved. A plot of pressure (at 1.2 of CO2
injected) versus recovery function was obtained in the graph below. Minimal miscibility pressure
for the carbon dioxide was found to be 13690 psi as shown in the graph below.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Adapted from (John & Michael, 2016, p. 8)
The circles on the graph indicate the multiple hydrocarbon miscible mechanisms. They
are around three in number; the first contact the vaporizing gas drive (also known high-pressure
gas drive) and lastly on the far end is the condensing gas drive. The rectangular shapes on the
graph, on the other hand, indicate the points that are immiscible. At the first contact, carbon
dioxide does not form a uniform solution with the oil reservoir; however, it begins to form a
uniform solution with the series of contacts which improves oil recovery. Dynamic miscibility is
achieved at the vaporizing –drive point where the intermediate molecular weight hydrocarbon is
injected into the CO2 gas. CO2 gas is then transferred into the oil reservoir in the condensing gas-
drive process, a process that perfects the dynamic miscibility in the vaporizing gas drive phase
(John & Michael, 2016, p. 8).
Miscibility between the gas and oil reservoir is attained when the oil pressure is just above the
minimal oil CO2 solution pressure. Displacement of the oil by the CO2 gas occurs with time and
this process is referred to as dynamic miscibility. Vaporization of both the intermediate and the
5
The circles on the graph indicate the multiple hydrocarbon miscible mechanisms. They
are around three in number; the first contact the vaporizing gas drive (also known high-pressure
gas drive) and lastly on the far end is the condensing gas drive. The rectangular shapes on the
graph, on the other hand, indicate the points that are immiscible. At the first contact, carbon
dioxide does not form a uniform solution with the oil reservoir; however, it begins to form a
uniform solution with the series of contacts which improves oil recovery. Dynamic miscibility is
achieved at the vaporizing –drive point where the intermediate molecular weight hydrocarbon is
injected into the CO2 gas. CO2 gas is then transferred into the oil reservoir in the condensing gas-
drive process, a process that perfects the dynamic miscibility in the vaporizing gas drive phase
(John & Michael, 2016, p. 8).
Miscibility between the gas and oil reservoir is attained when the oil pressure is just above the
minimal oil CO2 solution pressure. Displacement of the oil by the CO2 gas occurs with time and
this process is referred to as dynamic miscibility. Vaporization of both the intermediate and the
5
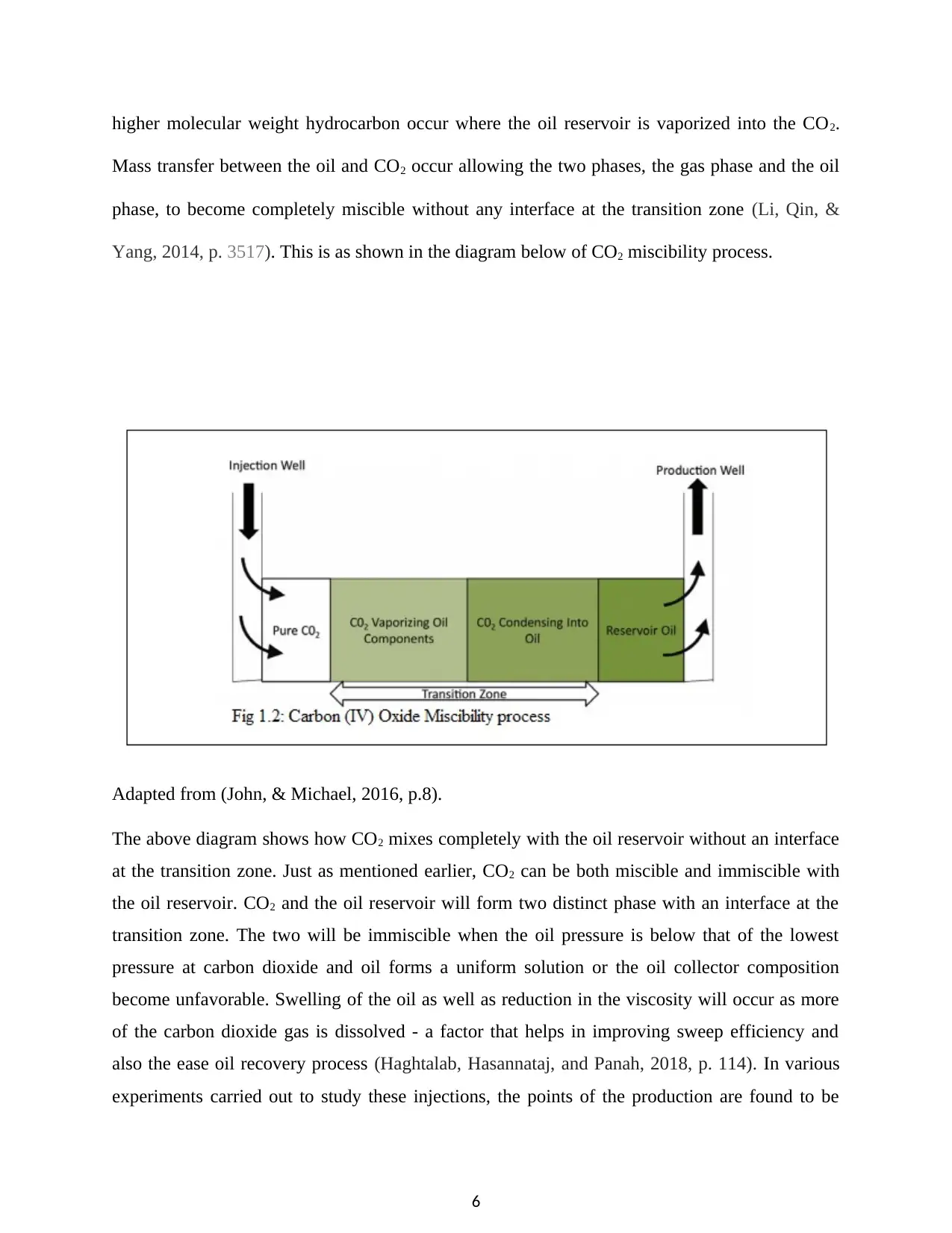
higher molecular weight hydrocarbon occur where the oil reservoir is vaporized into the CO2.
Mass transfer between the oil and CO2 occur allowing the two phases, the gas phase and the oil
phase, to become completely miscible without any interface at the transition zone (Li, Qin, &
Yang, 2014, p. 3517). This is as shown in the diagram below of CO2 miscibility process.
Adapted from (John, & Michael, 2016, p.8).
The above diagram shows how CO2 mixes completely with the oil reservoir without an interface
at the transition zone. Just as mentioned earlier, CO2 can be both miscible and immiscible with
the oil reservoir. CO2 and the oil reservoir will form two distinct phase with an interface at the
transition zone. The two will be immiscible when the oil pressure is below that of the lowest
pressure at carbon dioxide and oil forms a uniform solution or the oil collector composition
become unfavorable. Swelling of the oil as well as reduction in the viscosity will occur as more
of the carbon dioxide gas is dissolved - a factor that helps in improving sweep efficiency and
also the ease oil recovery process (Haghtalab, Hasannataj, and Panah, 2018, p. 114). In various
experiments carried out to study these injections, the points of the production are found to be
6
Mass transfer between the oil and CO2 occur allowing the two phases, the gas phase and the oil
phase, to become completely miscible without any interface at the transition zone (Li, Qin, &
Yang, 2014, p. 3517). This is as shown in the diagram below of CO2 miscibility process.
Adapted from (John, & Michael, 2016, p.8).
The above diagram shows how CO2 mixes completely with the oil reservoir without an interface
at the transition zone. Just as mentioned earlier, CO2 can be both miscible and immiscible with
the oil reservoir. CO2 and the oil reservoir will form two distinct phase with an interface at the
transition zone. The two will be immiscible when the oil pressure is below that of the lowest
pressure at carbon dioxide and oil forms a uniform solution or the oil collector composition
become unfavorable. Swelling of the oil as well as reduction in the viscosity will occur as more
of the carbon dioxide gas is dissolved - a factor that helps in improving sweep efficiency and
also the ease oil recovery process (Haghtalab, Hasannataj, and Panah, 2018, p. 114). In various
experiments carried out to study these injections, the points of the production are found to be
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

similar to those of the immiscible scenario. When carbon dioxide is introduced into the model, it
moves randomly making no visible contact with oil reserves. This is referred to as fingering in
the fractured reserves - a scenario that causes carbon dioxide gas and the oil reservoir ratio to
increase in the production wells. Generally, miscible carbon dioxide scenario chosen as the best
injection in the oil recovery as it increases the reservoir pressure, which suits the oil recovery
process (Haghtalab, Hasannataj, and Panah, 2018, p. 114).
.
Conclusion
In conclusion, the above discussion indeed justifies that Carbon dioxide gas can be both
miscible and immiscible. When the lowest pressure at which the two solution mixes is above that
of the reservoir, miscibility is achieved while when it is below the lowest pressure at which the
two solution mixes then the two becomes immiscible. At critical temperature and pressure,
carbon dioxide gas coexist with the liquid and gas in phases and as the pressure of the oil reserve
goes below the minimum miscibility pressure, the two phases forms a single phase with no
interface at the transition zone. However when then the two are immiscible, carbon dioxide and
the oil reservoir forms two separate phase separated by an interface at the transition zone. The
lowest pressure at which the two solutions mixes or form a uniform solution is the pressure at
which series of contacts can be attained. It plays a critical role in the determination of the
miscibility of the carbon dioxide and can be calculated and determined through various methods
such as; the slim tube method, the rising bubble and the use of correlation. Oil displacement by
the carbon dioxide gas can either be miscible or immiscible. Miscible displacement is usually
preferred to the immiscible displacement. For the minimum miscibility pressure calculation
using the slim – tube for carbon dioxide and the oil reservoir, the value was found to be 13690
(as obtained from the graph of figure 1.1).
7
moves randomly making no visible contact with oil reserves. This is referred to as fingering in
the fractured reserves - a scenario that causes carbon dioxide gas and the oil reservoir ratio to
increase in the production wells. Generally, miscible carbon dioxide scenario chosen as the best
injection in the oil recovery as it increases the reservoir pressure, which suits the oil recovery
process (Haghtalab, Hasannataj, and Panah, 2018, p. 114).
.
Conclusion
In conclusion, the above discussion indeed justifies that Carbon dioxide gas can be both
miscible and immiscible. When the lowest pressure at which the two solution mixes is above that
of the reservoir, miscibility is achieved while when it is below the lowest pressure at which the
two solution mixes then the two becomes immiscible. At critical temperature and pressure,
carbon dioxide gas coexist with the liquid and gas in phases and as the pressure of the oil reserve
goes below the minimum miscibility pressure, the two phases forms a single phase with no
interface at the transition zone. However when then the two are immiscible, carbon dioxide and
the oil reservoir forms two separate phase separated by an interface at the transition zone. The
lowest pressure at which the two solutions mixes or form a uniform solution is the pressure at
which series of contacts can be attained. It plays a critical role in the determination of the
miscibility of the carbon dioxide and can be calculated and determined through various methods
such as; the slim tube method, the rising bubble and the use of correlation. Oil displacement by
the carbon dioxide gas can either be miscible or immiscible. Miscible displacement is usually
preferred to the immiscible displacement. For the minimum miscibility pressure calculation
using the slim – tube for carbon dioxide and the oil reservoir, the value was found to be 13690
(as obtained from the graph of figure 1.1).
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8

References
Abramson, E.H., Bollengier, O. and Brown, J.M., 2017. The water-carbon dioxide miscibility
surface to 450° C and 7 GPa. American Journal of Science, 317(9), pp.967-989.
Budiaman, I.G.S., Suhadi, T.R., Winata, D.T., Sitompul, V., Buhari, A. and Satya, S.A., 2018,
July. Swelling/Extraction Test of Carbon Dioxide Injection on Dead Oil Field Structure
X for Phase Behavior Study. In Seminar Nasional Teknik Kimia Kejuangan (p. 11)..
Higgins, D., Landers, A.T., Ji, Y., Nitopi, S., Morales-Guio, C.G., Wang, L., Chan, K., Hahn, C.
and Jaramillo, T.F., 2018. Guiding Electrochemical Carbon Dioxide Reduction toward
Carbonyls Using Copper Silver Thin Films with Interphase Miscibility. ACS Energy
Letters, 3(12), pp.2947-2955.
Haghtalab, A., Hasannataj, H. and Panah, H.S., 2018. Prediction of minimum miscibility
pressure of pure CO2, carbon dioxide gas mixtures and polymer-supercritical CO2 in oil
using modified quadrupole Cubic Plus Association Equation of State (mqCPA EoS).
Fluid Phase Equilibria, 478, pp.114-128.
John, K. M., & Michael, A. T. (2016). A New Simple CO2 Minimum Miscibility Pressure
Correlation. Oil & Gas Research, 02(03), 2-14.
Lashgari, H.R., Sun, A., Zhang, T., Pope, G.A. and Lake, L.W., 2019. Evaluation of carbon
dioxide storage and miscible gas EOR in shale oil reservoirs. Fuel, 241, pp.1223-1235.
Li, H., Qin, J., & Yang, D. (2014). An Improved CO2–Oil Minimum Miscibility Pressure
Correlation for Live and Dead Crude Oils. Industrial & Engineering Chemistry Research,
51(8), 3516-3523.
9
Abramson, E.H., Bollengier, O. and Brown, J.M., 2017. The water-carbon dioxide miscibility
surface to 450° C and 7 GPa. American Journal of Science, 317(9), pp.967-989.
Budiaman, I.G.S., Suhadi, T.R., Winata, D.T., Sitompul, V., Buhari, A. and Satya, S.A., 2018,
July. Swelling/Extraction Test of Carbon Dioxide Injection on Dead Oil Field Structure
X for Phase Behavior Study. In Seminar Nasional Teknik Kimia Kejuangan (p. 11)..
Higgins, D., Landers, A.T., Ji, Y., Nitopi, S., Morales-Guio, C.G., Wang, L., Chan, K., Hahn, C.
and Jaramillo, T.F., 2018. Guiding Electrochemical Carbon Dioxide Reduction toward
Carbonyls Using Copper Silver Thin Films with Interphase Miscibility. ACS Energy
Letters, 3(12), pp.2947-2955.
Haghtalab, A., Hasannataj, H. and Panah, H.S., 2018. Prediction of minimum miscibility
pressure of pure CO2, carbon dioxide gas mixtures and polymer-supercritical CO2 in oil
using modified quadrupole Cubic Plus Association Equation of State (mqCPA EoS).
Fluid Phase Equilibria, 478, pp.114-128.
John, K. M., & Michael, A. T. (2016). A New Simple CO2 Minimum Miscibility Pressure
Correlation. Oil & Gas Research, 02(03), 2-14.
Lashgari, H.R., Sun, A., Zhang, T., Pope, G.A. and Lake, L.W., 2019. Evaluation of carbon
dioxide storage and miscible gas EOR in shale oil reservoirs. Fuel, 241, pp.1223-1235.
Li, H., Qin, J., & Yang, D. (2014). An Improved CO2–Oil Minimum Miscibility Pressure
Correlation for Live and Dead Crude Oils. Industrial & Engineering Chemistry Research,
51(8), 3516-3523.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



