A Comprehensive Report on Applications of Combinatorial Chemistry
VerifiedAdded on 2023/06/03
|17
|2623
|439
Report
AI Summary
This report provides a detailed overview of combinatorial chemistry, a method used in the pharmaceutical sector for the rapid creation of chemical libraries for drug discovery. It discusses various methods for producing combinatorial libraries, including biological and spatially addressable parallel solid-phase library approaches. The report also covers encoding techniques used to identify active compounds within these libraries. Furthermore, it explores specific applications such as the synthesis of peptoids, combinatorial lead optimization of neuropeptide-FF antagonists, generation of benzodiazepine libraries, histamine H3 receptor antagonists, and dihydrofolate reductase inhibitors. The conclusion emphasizes the importance of combinatorial chemistry in reducing drug discovery costs and increasing the probability of finding novel lead molecules, making it a crucial tool for the pharmaceutical industry. Desklib provides access to this and other solved assignments.
Applications of combinatorial chemistry1
APPLICATIONS OF COMBINATORIAL CHEMISTRY
Name:
Department:
Course
Date:
APPLICATIONS OF COMBINATORIAL CHEMISTRY
Name:
Department:
Course
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Applications of combinatorial chemistry 2
Applications of combinatorial chemistry
Introduction
Combinatorial chemistry is a novel method created in the pharmaceutical sector, which
comprises the production of substances in bulk instead of a lone substance, which is selected as a
complete blend for specific biological action. Due to quick creation of compound, the
technique saves cost and time connected with the drug discovery. In this novel period of
medicinal chemistry, the focus is concentrated on the provision of chemical collections for the
creation of novel direction for drug finding. Chemical libraries are deliberately building
collection of various particles, which can be generated unnaturally or biosynthetically and
examined for biological action in a range of setups. For instance; recombinant peptide libraries,
peptides libraries on bacteriophage, soluble fragments libraries, and compounds tied to resin lead
libraries, and solid support or silica chips. Combinatorial chemistry is utilised to build a large
populace of fundamentally diverse bits referred to as chemical libraries in a short period that can
be scrutinized at once against a range of targets. In 1962 and 1963, growth of ugi-
multicomponent reaction and Merrifield solid phase synthesis respectively, presented important
techniques to synthesise libraries of trivial organic mixtures, however, the initial combinatorial
production did not effect until 20 years (Fujita 2012, pp. 14). From 1990 onwards, there has been
an increase in combinatorial production and small particles are created as a multicomponent
blend. Since then, combinatorial chemistry has extended from peptides to organic, inorganic,
organometallic and polymer chemistry (West 2014, pp. 22).
Combinatorial chemistry may also be well-defined as repetitive and methodical, a
covalent link of a set of various building masses of different assemblies to each other to produce
Applications of combinatorial chemistry
Introduction
Combinatorial chemistry is a novel method created in the pharmaceutical sector, which
comprises the production of substances in bulk instead of a lone substance, which is selected as a
complete blend for specific biological action. Due to quick creation of compound, the
technique saves cost and time connected with the drug discovery. In this novel period of
medicinal chemistry, the focus is concentrated on the provision of chemical collections for the
creation of novel direction for drug finding. Chemical libraries are deliberately building
collection of various particles, which can be generated unnaturally or biosynthetically and
examined for biological action in a range of setups. For instance; recombinant peptide libraries,
peptides libraries on bacteriophage, soluble fragments libraries, and compounds tied to resin lead
libraries, and solid support or silica chips. Combinatorial chemistry is utilised to build a large
populace of fundamentally diverse bits referred to as chemical libraries in a short period that can
be scrutinized at once against a range of targets. In 1962 and 1963, growth of ugi-
multicomponent reaction and Merrifield solid phase synthesis respectively, presented important
techniques to synthesise libraries of trivial organic mixtures, however, the initial combinatorial
production did not effect until 20 years (Fujita 2012, pp. 14). From 1990 onwards, there has been
an increase in combinatorial production and small particles are created as a multicomponent
blend. Since then, combinatorial chemistry has extended from peptides to organic, inorganic,
organometallic and polymer chemistry (West 2014, pp. 22).
Combinatorial chemistry may also be well-defined as repetitive and methodical, a
covalent link of a set of various building masses of different assemblies to each other to produce
Applications of combinatorial chemistry 3
a big collection of different molecular units. In this process, a huge number of compounds are
synthesized directly through organizing many lone elements in parallel or numerous compounds
concurrently in blends. The procedure is efficient, quicker, and inexpensive and provides rise in
millions of mixtures in the same interval, as it takes to create one compound. To upsurge the
likelihoods of discovering an achievement to increase the quantity and variety of compounds
generated, combinatorial synthesis is done in such a way that combinations of compounds are
built in each reaction container, permitting a single chemist to create thousands of new
assemblies (Huang and Leung 2016, pp. 910).
Methods
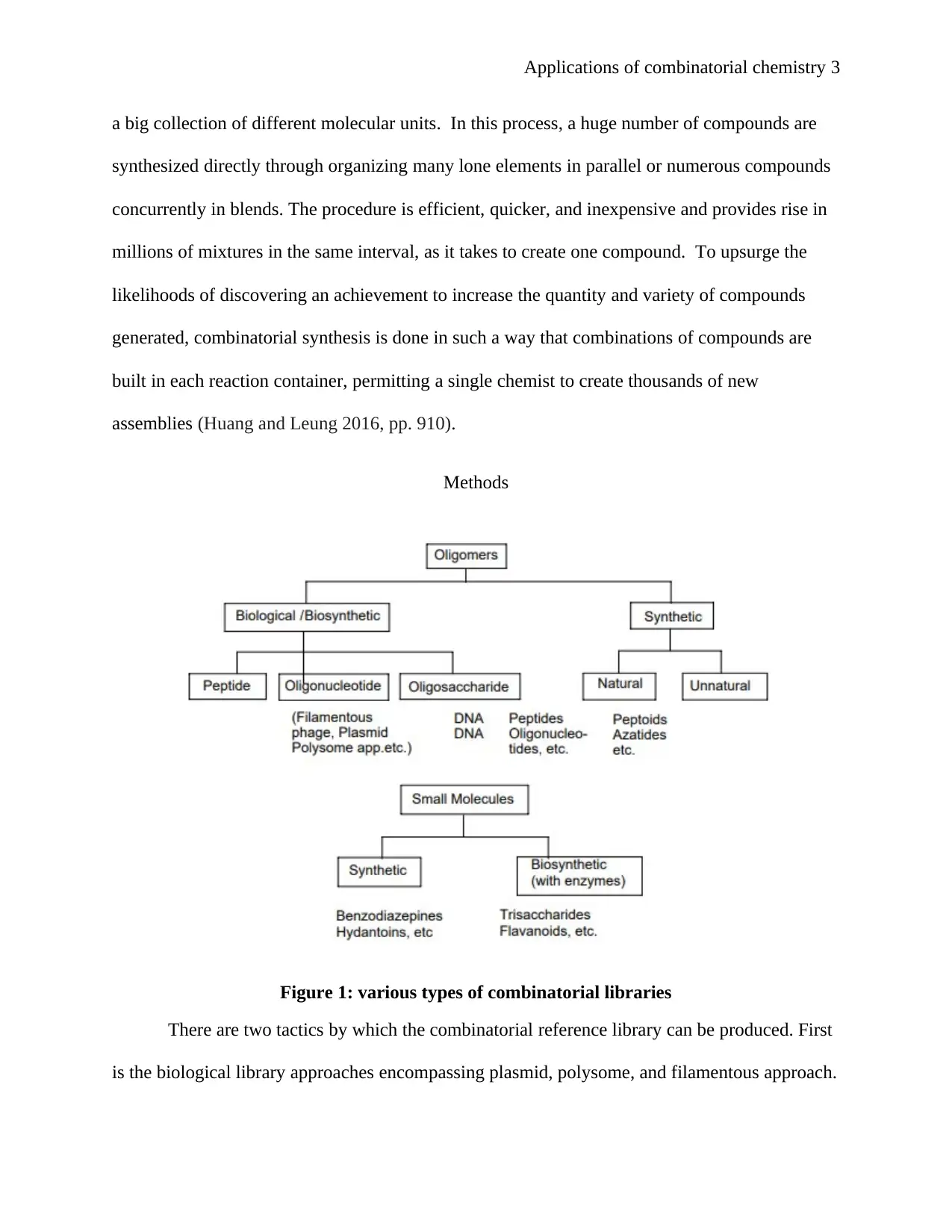
Figure 1: various types of combinatorial libraries
There are two tactics by which the combinatorial reference library can be produced. First
is the biological library approaches encompassing plasmid, polysome, and filamentous approach.
a big collection of different molecular units. In this process, a huge number of compounds are
synthesized directly through organizing many lone elements in parallel or numerous compounds
concurrently in blends. The procedure is efficient, quicker, and inexpensive and provides rise in
millions of mixtures in the same interval, as it takes to create one compound. To upsurge the
likelihoods of discovering an achievement to increase the quantity and variety of compounds
generated, combinatorial synthesis is done in such a way that combinations of compounds are
built in each reaction container, permitting a single chemist to create thousands of new
assemblies (Huang and Leung 2016, pp. 910).
Methods
Figure 1: various types of combinatorial libraries
There are two tactics by which the combinatorial reference library can be produced. First
is the biological library approaches encompassing plasmid, polysome, and filamentous approach.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Applications of combinatorial chemistry 4
Second is the spatially addressable parallel solid phase library method which comprises the
multi-pin, tea bag methodology and light directed peptide synthesis on resin support. Apart
from biological library method, which is restricted to peptide libraries with eukaryotic amino
acids, another artificial method is functional to a peptide, non-peptide oligomers or small
molecule libraries (Janson 2012, pp.45).
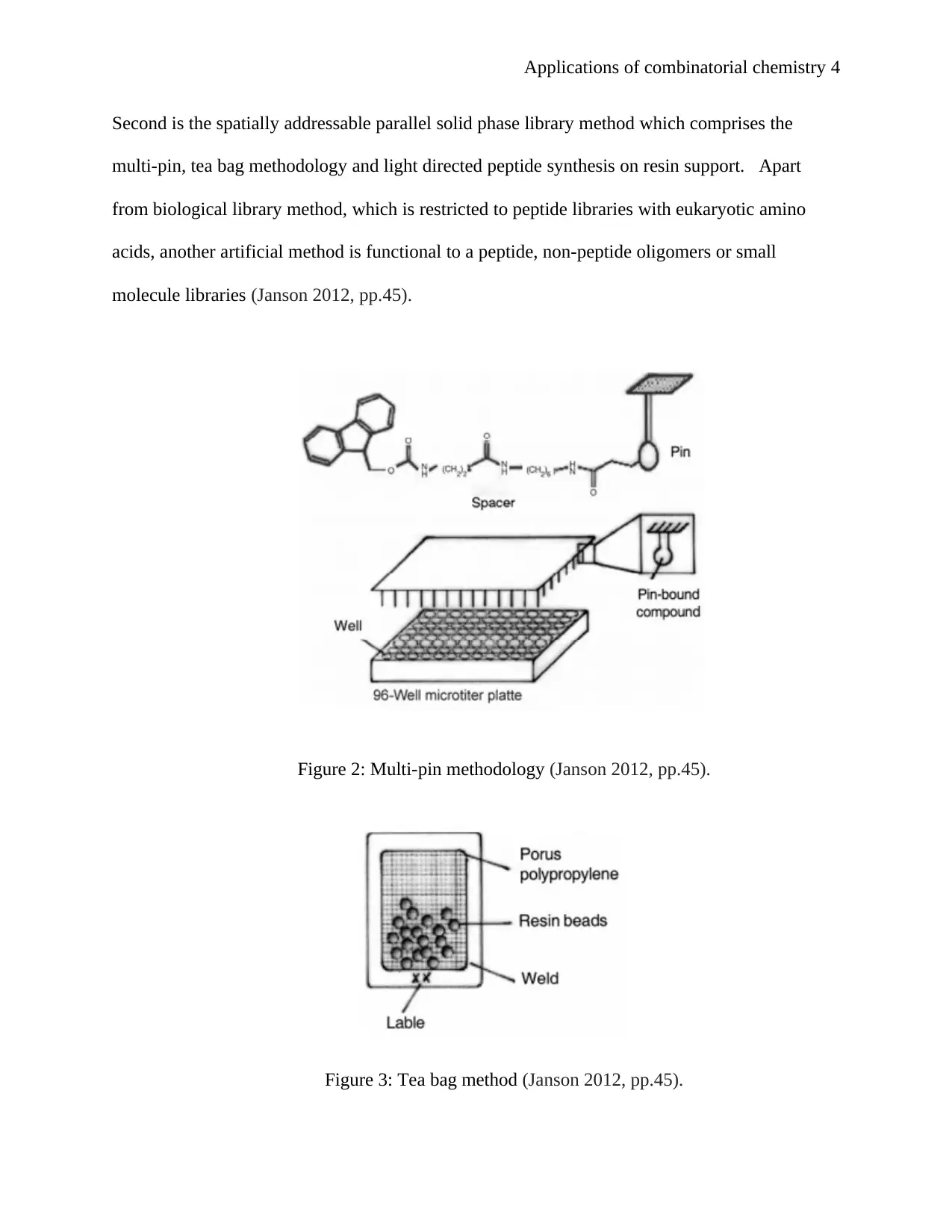
Figure 2: Multi-pin methodology (Janson 2012, pp.45).
Figure 3: Tea bag method (Janson 2012, pp.45).
Second is the spatially addressable parallel solid phase library method which comprises the
multi-pin, tea bag methodology and light directed peptide synthesis on resin support. Apart
from biological library method, which is restricted to peptide libraries with eukaryotic amino
acids, another artificial method is functional to a peptide, non-peptide oligomers or small
molecule libraries (Janson 2012, pp.45).
Figure 2: Multi-pin methodology (Janson 2012, pp.45).
Figure 3: Tea bag method (Janson 2012, pp.45).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Applications of combinatorial chemistry 5
A biological method to produce molecular range
The biological structure used for the creation of peptide diversity simulates an evolution
generation of protein multiplicity. Synthetic evolution is greatly developed by the diversity
introduction into the arrangement at a much high scope than that happen naturally. The source
of the diversity in combinatorial synthesis is the structure of oligonucleotides. Oligonucleotides
production allow constricted regulation of the composition of the mixture made and the
degenerated sequence produced are then cloned and denoted as peptides. The biological tactic
assists one to take the advantage of well-known protein folds for instance immunoglobulin fold
by joining arbitrary oligopeptides on such as tertiary folding. But, there are also some of the
limitation like the combination of unnatural amino acid to other carbon-based moieties into this
library is not practicable. Also, the biological approach is generally limited to 20 eukaryotic
amino acids (Georgakilas et al. 2012, pp. 6157).
Spatially addressable parallel solid phase library approach: the wish to advance and
discover SAR around peptide lead complex has positioned incredible weights on the yield of
peptide chemistry. Brief methods of main methods are; First, a multi-pin methodology is where
the synthesis takes place on polyethylene pin functionalized with acrylic acid organized in 96
well setups. A screen is prepared by way of enzyme connected immunosorbent assay (ELISA) to
examine the binding ability of the covalently bound peptide to antibodies. Tea bag method: the
peptide synthesis happens on the resin that is closed inside polypropylene bags. Amino acids are
joined to the resin by putting the bag in the solution of suitable discrete activated monomers. All
the common stages such as resin washing and amino group Deprotonation are done concurrently.
A biological method to produce molecular range
The biological structure used for the creation of peptide diversity simulates an evolution
generation of protein multiplicity. Synthetic evolution is greatly developed by the diversity
introduction into the arrangement at a much high scope than that happen naturally. The source
of the diversity in combinatorial synthesis is the structure of oligonucleotides. Oligonucleotides
production allow constricted regulation of the composition of the mixture made and the
degenerated sequence produced are then cloned and denoted as peptides. The biological tactic
assists one to take the advantage of well-known protein folds for instance immunoglobulin fold
by joining arbitrary oligopeptides on such as tertiary folding. But, there are also some of the
limitation like the combination of unnatural amino acid to other carbon-based moieties into this
library is not practicable. Also, the biological approach is generally limited to 20 eukaryotic
amino acids (Georgakilas et al. 2012, pp. 6157).
Spatially addressable parallel solid phase library approach: the wish to advance and
discover SAR around peptide lead complex has positioned incredible weights on the yield of
peptide chemistry. Brief methods of main methods are; First, a multi-pin methodology is where
the synthesis takes place on polyethylene pin functionalized with acrylic acid organized in 96
well setups. A screen is prepared by way of enzyme connected immunosorbent assay (ELISA) to
examine the binding ability of the covalently bound peptide to antibodies. Tea bag method: the
peptide synthesis happens on the resin that is closed inside polypropylene bags. Amino acids are
joined to the resin by putting the bag in the solution of suitable discrete activated monomers. All
the common stages such as resin washing and amino group Deprotonation are done concurrently.
Applications of combinatorial chemistry 6
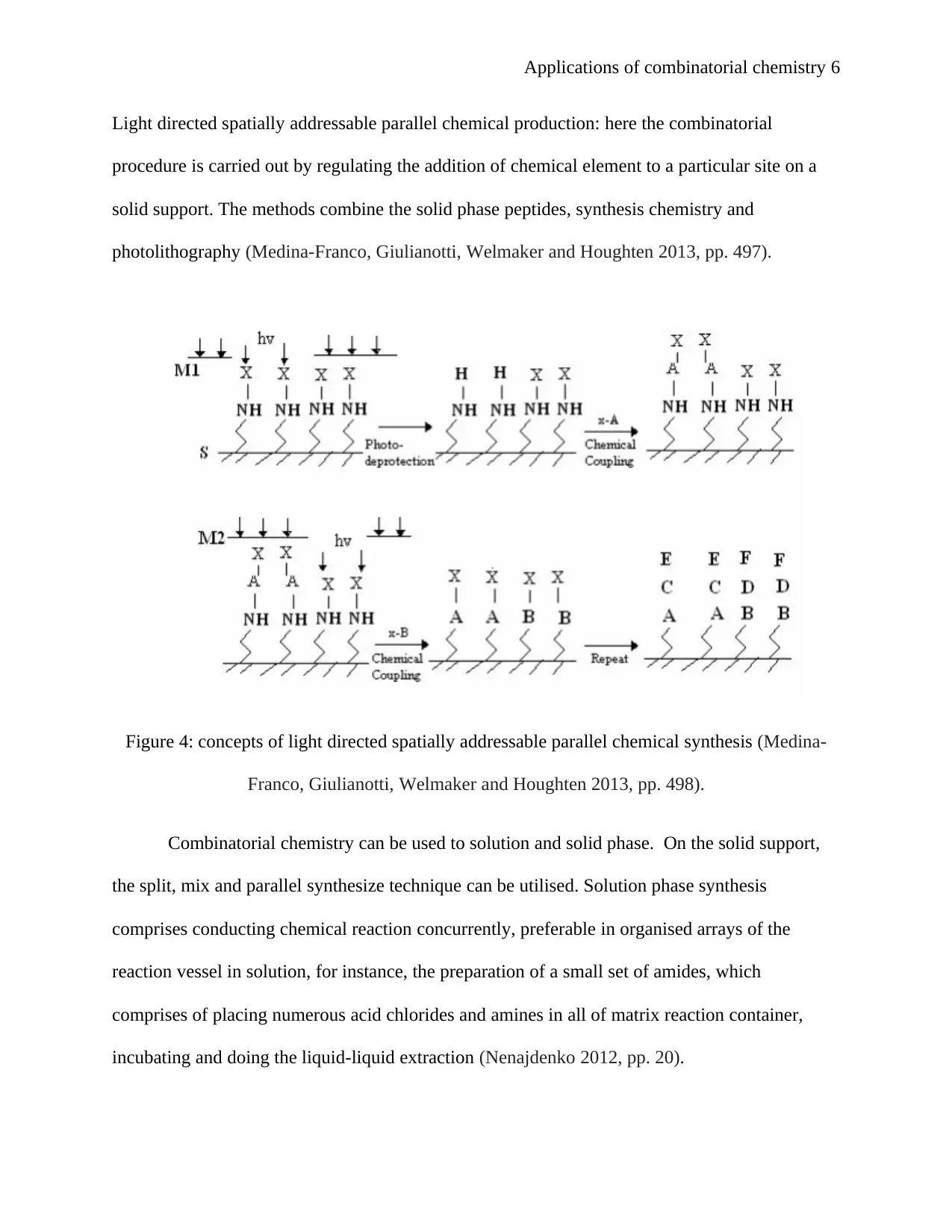
Light directed spatially addressable parallel chemical production: here the combinatorial
procedure is carried out by regulating the addition of chemical element to a particular site on a
solid support. The methods combine the solid phase peptides, synthesis chemistry and
photolithography (Medina-Franco, Giulianotti, Welmaker and Houghten 2013, pp. 497).
Figure 4: concepts of light directed spatially addressable parallel chemical synthesis (Medina-
Franco, Giulianotti, Welmaker and Houghten 2013, pp. 498).
Combinatorial chemistry can be used to solution and solid phase. On the solid support,
the split, mix and parallel synthesize technique can be utilised. Solution phase synthesis
comprises conducting chemical reaction concurrently, preferable in organised arrays of the
reaction vessel in solution, for instance, the preparation of a small set of amides, which
comprises of placing numerous acid chlorides and amines in all of matrix reaction container,
incubating and doing the liquid-liquid extraction (Nenajdenko 2012, pp. 20).
Light directed spatially addressable parallel chemical production: here the combinatorial
procedure is carried out by regulating the addition of chemical element to a particular site on a
solid support. The methods combine the solid phase peptides, synthesis chemistry and
photolithography (Medina-Franco, Giulianotti, Welmaker and Houghten 2013, pp. 497).
Figure 4: concepts of light directed spatially addressable parallel chemical synthesis (Medina-
Franco, Giulianotti, Welmaker and Houghten 2013, pp. 498).
Combinatorial chemistry can be used to solution and solid phase. On the solid support,
the split, mix and parallel synthesize technique can be utilised. Solution phase synthesis
comprises conducting chemical reaction concurrently, preferable in organised arrays of the
reaction vessel in solution, for instance, the preparation of a small set of amides, which
comprises of placing numerous acid chlorides and amines in all of matrix reaction container,
incubating and doing the liquid-liquid extraction (Nenajdenko 2012, pp. 20).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
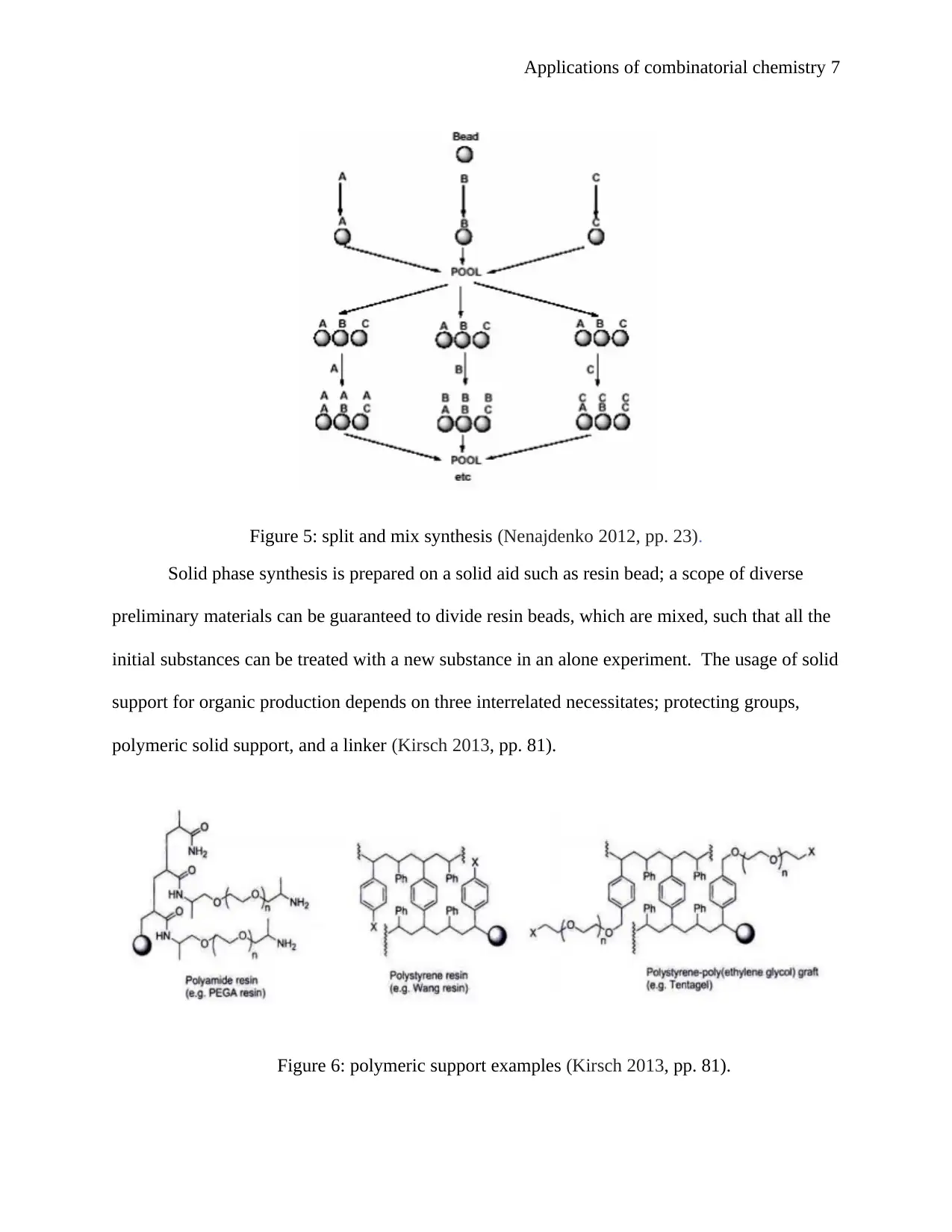
Applications of combinatorial chemistry 7
Figure 5: split and mix synthesis (Nenajdenko 2012, pp. 23).
Solid phase synthesis is prepared on a solid aid such as resin bead; a scope of diverse
preliminary materials can be guaranteed to divide resin beads, which are mixed, such that all the
initial substances can be treated with a new substance in an alone experiment. The usage of solid
support for organic production depends on three interrelated necessitates; protecting groups,
polymeric solid support, and a linker (Kirsch 2013, pp. 81).
Figure 6: polymeric support examples (Kirsch 2013, pp. 81).
Figure 5: split and mix synthesis (Nenajdenko 2012, pp. 23).
Solid phase synthesis is prepared on a solid aid such as resin bead; a scope of diverse
preliminary materials can be guaranteed to divide resin beads, which are mixed, such that all the
initial substances can be treated with a new substance in an alone experiment. The usage of solid
support for organic production depends on three interrelated necessitates; protecting groups,
polymeric solid support, and a linker (Kirsch 2013, pp. 81).
Figure 6: polymeric support examples (Kirsch 2013, pp. 81).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
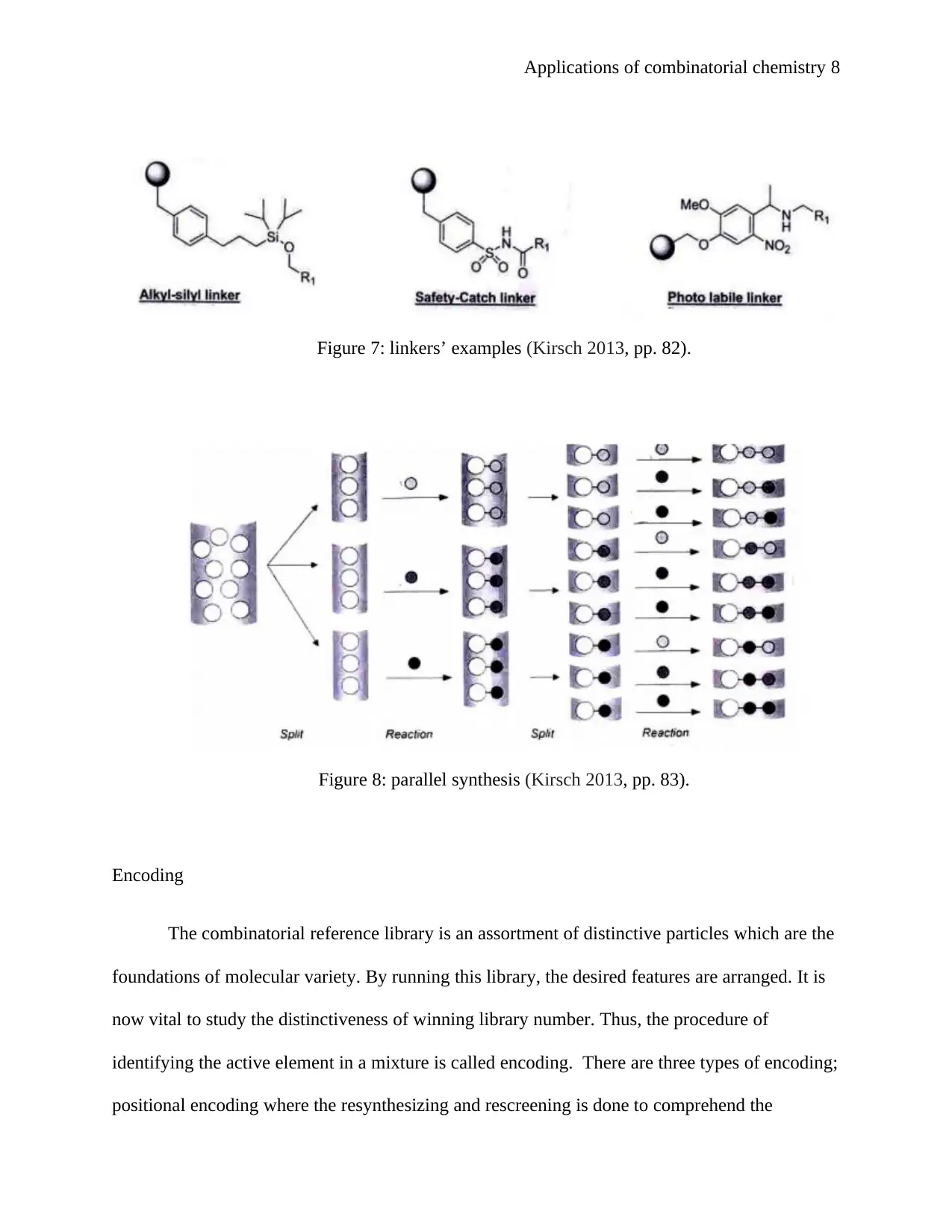
Applications of combinatorial chemistry 8
Figure 7: linkers’ examples (Kirsch 2013, pp. 82).
Figure 8: parallel synthesis (Kirsch 2013, pp. 83).
Encoding
The combinatorial reference library is an assortment of distinctive particles which are the
foundations of molecular variety. By running this library, the desired features are arranged. It is
now vital to study the distinctiveness of winning library number. Thus, the procedure of
identifying the active element in a mixture is called encoding. There are three types of encoding;
positional encoding where the resynthesizing and rescreening is done to comprehend the
Figure 7: linkers’ examples (Kirsch 2013, pp. 82).
Figure 8: parallel synthesis (Kirsch 2013, pp. 83).
Encoding
The combinatorial reference library is an assortment of distinctive particles which are the
foundations of molecular variety. By running this library, the desired features are arranged. It is
now vital to study the distinctiveness of winning library number. Thus, the procedure of
identifying the active element in a mixture is called encoding. There are three types of encoding;
positional encoding where the resynthesizing and rescreening is done to comprehend the

Applications of combinatorial chemistry 9
uniqueness of an active compound. Chemical encoding is used for the peptide libraries.
Electronic encoding method utilises a microelectronic device named as radio frequency memory
tag gauging 13*3mm enclosed in dense walled glass (West 2014, pp. 25).
Applications
The combinatorial chemistry chiefly depicts its existence in the production of peptide
libraries. The peptide takes parts in different parts in the body. By using the combinatorial
chemistry, one can make a enormous peptide, which may be dynamic. Biologically energetic
peptide hormones have a crucial part in controlling a multiple of human biological reaction, and
various low molecular mass bioactive peptides can act as antagonists. Similarly, peptide
configuration typically is initiated in molecules intended to hinder enzymes that catalyze
proteolysis, phosphorylation and other old translational protein change that may take a crucial
part in pathologies of numerous diseases conditions. Thus, the following are some of the
application of combinatorial chemistry.
I. synthesis of peptoids
Part of polypeptides reference library was established to be strong inhibitors for enzymes
like proteases and kinases important for cancer and AIDS treatment. However, these peptides
have unfavourable pharmacokinetic properties and poor bioavailability.
uniqueness of an active compound. Chemical encoding is used for the peptide libraries.
Electronic encoding method utilises a microelectronic device named as radio frequency memory
tag gauging 13*3mm enclosed in dense walled glass (West 2014, pp. 25).
Applications
The combinatorial chemistry chiefly depicts its existence in the production of peptide
libraries. The peptide takes parts in different parts in the body. By using the combinatorial
chemistry, one can make a enormous peptide, which may be dynamic. Biologically energetic
peptide hormones have a crucial part in controlling a multiple of human biological reaction, and
various low molecular mass bioactive peptides can act as antagonists. Similarly, peptide
configuration typically is initiated in molecules intended to hinder enzymes that catalyze
proteolysis, phosphorylation and other old translational protein change that may take a crucial
part in pathologies of numerous diseases conditions. Thus, the following are some of the
application of combinatorial chemistry.
I. synthesis of peptoids
Part of polypeptides reference library was established to be strong inhibitors for enzymes
like proteases and kinases important for cancer and AIDS treatment. However, these peptides
have unfavourable pharmacokinetic properties and poor bioavailability.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
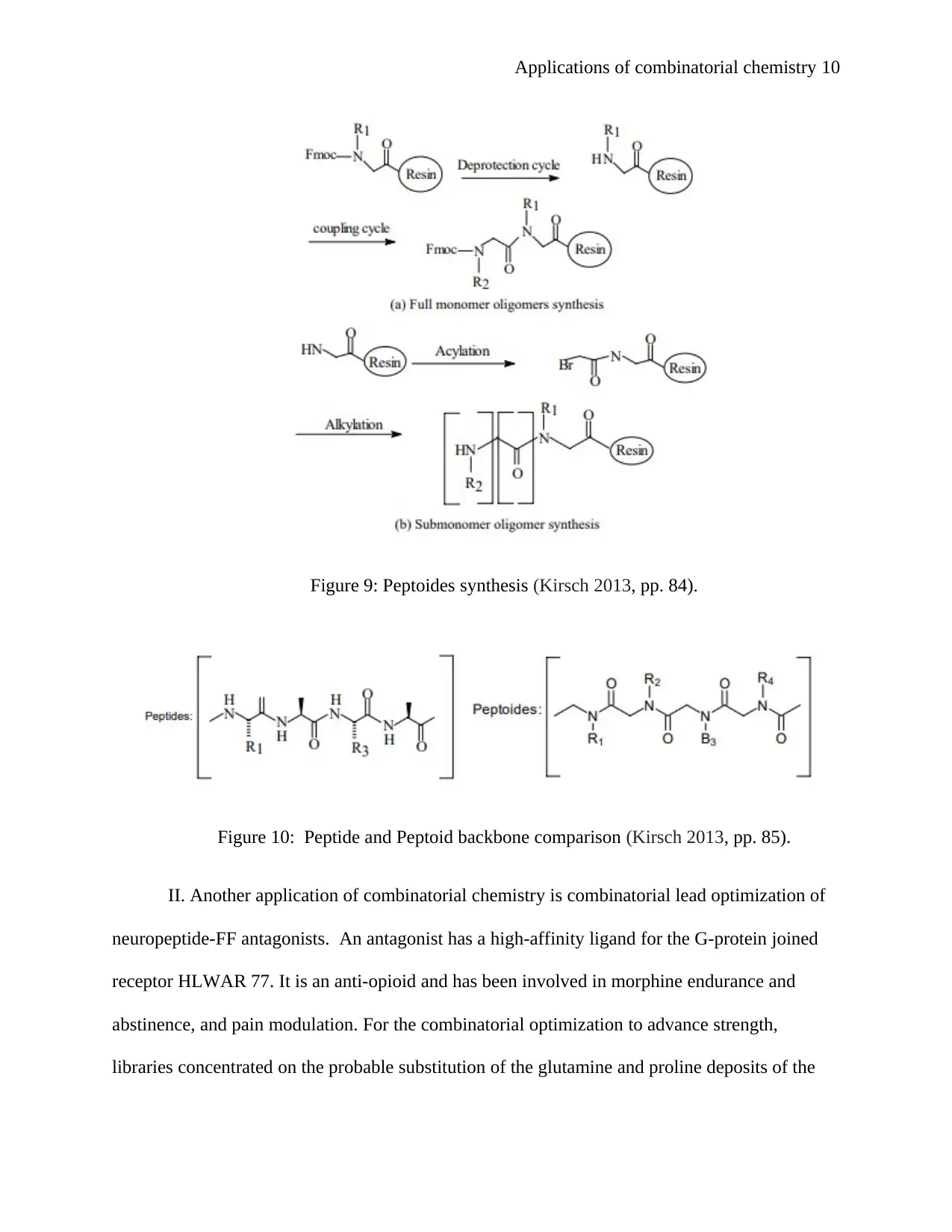
Applications of combinatorial chemistry 10
Figure 9: Peptoides synthesis (Kirsch 2013, pp. 84).
Figure 10: Peptide and Peptoid backbone comparison (Kirsch 2013, pp. 85).
II. Another application of combinatorial chemistry is combinatorial lead optimization of
neuropeptide-FF antagonists. An antagonist has a high-affinity ligand for the G-protein joined
receptor HLWAR 77. It is an anti-opioid and has been involved in morphine endurance and
abstinence, and pain modulation. For the combinatorial optimization to advance strength,
libraries concentrated on the probable substitution of the glutamine and proline deposits of the
Figure 9: Peptoides synthesis (Kirsch 2013, pp. 84).
Figure 10: Peptide and Peptoid backbone comparison (Kirsch 2013, pp. 85).
II. Another application of combinatorial chemistry is combinatorial lead optimization of
neuropeptide-FF antagonists. An antagonist has a high-affinity ligand for the G-protein joined
receptor HLWAR 77. It is an anti-opioid and has been involved in morphine endurance and
abstinence, and pain modulation. For the combinatorial optimization to advance strength,
libraries concentrated on the probable substitution of the glutamine and proline deposits of the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
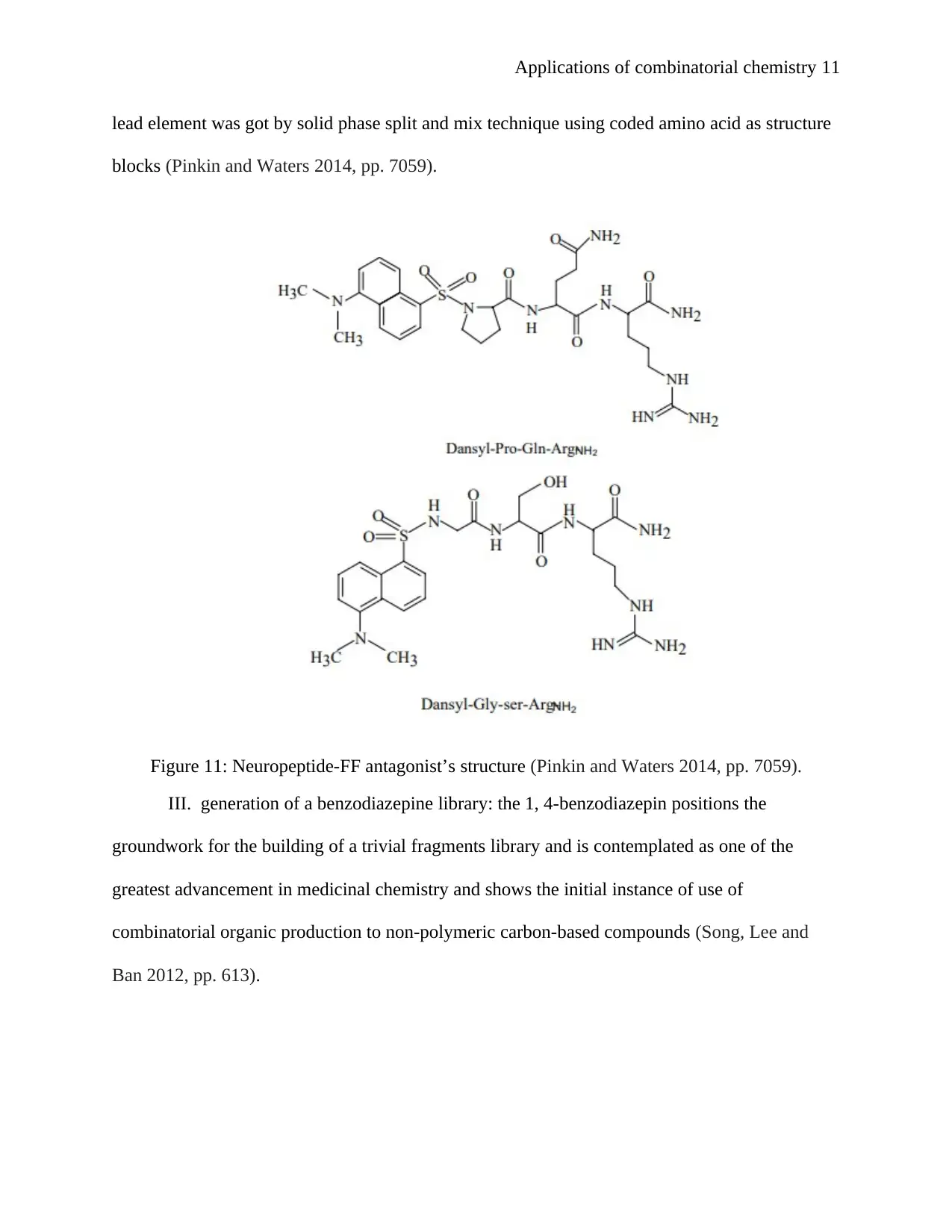
Applications of combinatorial chemistry 11
lead element was got by solid phase split and mix technique using coded amino acid as structure
blocks (Pinkin and Waters 2014, pp. 7059).
Figure 11: Neuropeptide-FF antagonist’s structure (Pinkin and Waters 2014, pp. 7059).
III. generation of a benzodiazepine library: the 1, 4-benzodiazepin positions the
groundwork for the building of a trivial fragments library and is contemplated as one of the
greatest advancement in medicinal chemistry and shows the initial instance of use of
combinatorial organic production to non-polymeric carbon-based compounds (Song, Lee and
Ban 2012, pp. 613).
lead element was got by solid phase split and mix technique using coded amino acid as structure
blocks (Pinkin and Waters 2014, pp. 7059).
Figure 11: Neuropeptide-FF antagonist’s structure (Pinkin and Waters 2014, pp. 7059).
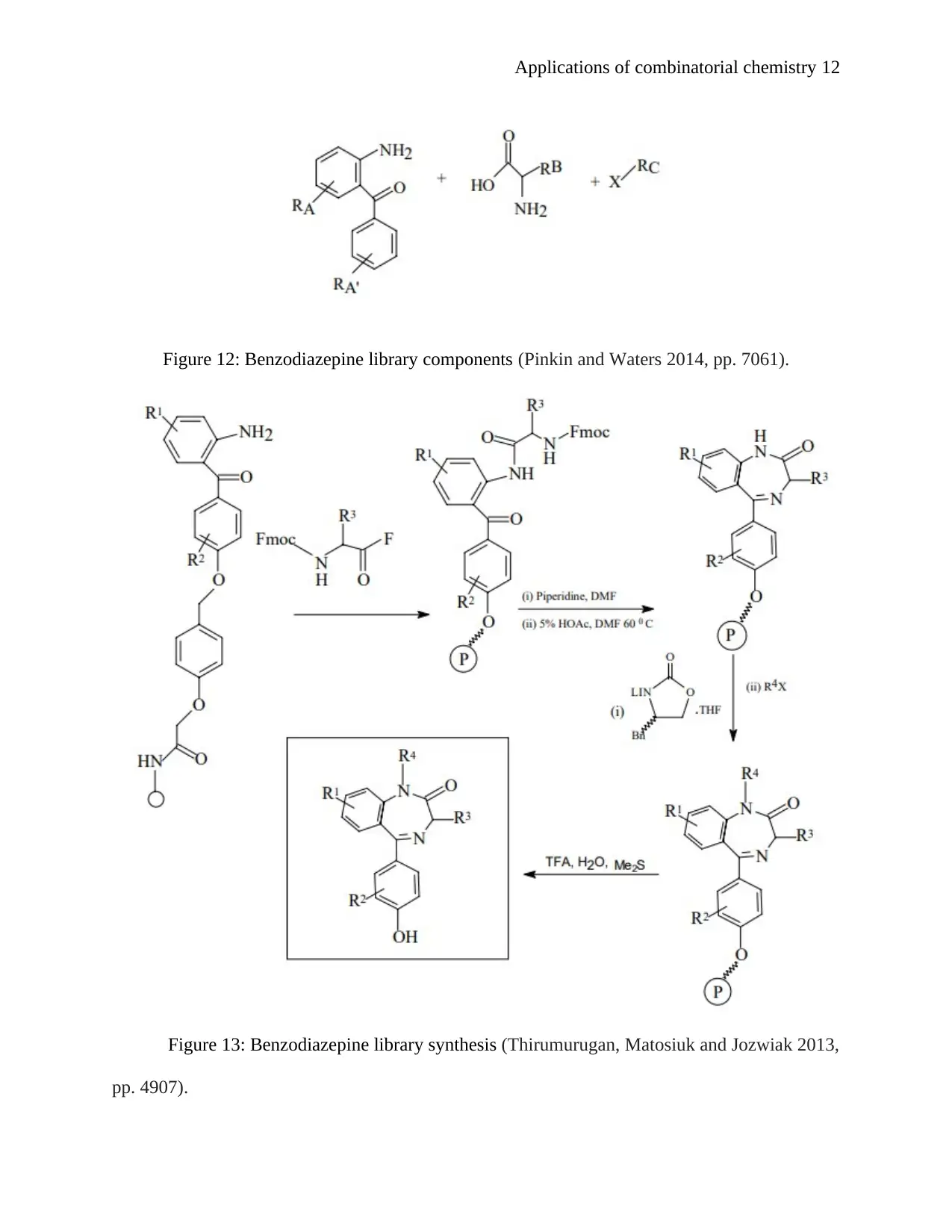
III. generation of a benzodiazepine library: the 1, 4-benzodiazepin positions the
groundwork for the building of a trivial fragments library and is contemplated as one of the
greatest advancement in medicinal chemistry and shows the initial instance of use of
combinatorial organic production to non-polymeric carbon-based compounds (Song, Lee and
Ban 2012, pp. 613).
Applications of combinatorial chemistry 12
Figure 12: Benzodiazepine library components (Pinkin and Waters 2014, pp. 7061).
Figure 13: Benzodiazepine library synthesis (Thirumurugan, Matosiuk and Jozwiak 2013,
pp. 4907).
Figure 12: Benzodiazepine library components (Pinkin and Waters 2014, pp. 7061).
Figure 13: Benzodiazepine library synthesis (Thirumurugan, Matosiuk and Jozwiak 2013,
pp. 4907).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.