Analysis of Heat Transfer in Cooling Tower - D18BT, Semester 2
VerifiedAdded on 2022/08/18
|10
|1183
|12
Report
AI Summary
This report details a heat transfer experiment conducted using a cross-flow cooling tower. The experiment aimed to investigate heat transfer mechanisms and assess the heat balance between air and water at varying water flow rates. The introduction defines cooling towers and their function in dissipating heat through water evaporation. The water circuit and air circuit components are described, including flow meters, thermocouples, and the cooling tower setup. The theoretical background, based on the heat capacity of water and heat absorbed by air, is presented along with the experimental procedure. Results are presented in tabulated data including temperature readings, flow rates, and calculated heat transfer values. The discussion compares the heat emitted by water and heat absorbed by air, acknowledging potential experimental errors. The report concludes that the experiment successfully met its objectives in understanding heat transfer principles. References to supporting literature are also provided.

Heat Transfer 1
HEAT TRANSFER
Name of Student
Instructor
Institution
Date
HEAT TRANSFER
Name of Student
Instructor
Institution
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Heat Transfer 2
Table of Contents
Aim and objective.......................................................................................................................................3
Introduction.................................................................................................................................................3
Water circuit............................................................................................................................................4
Air circuit................................................................................................................................................4
Equipment...................................................................................................................................................5
Theory.........................................................................................................................................................6
Procedure.....................................................................................................................................................6
Results.........................................................................................................................................................7
Tabulated data.........................................................................................................................................7
Calculations.............................................................................................................................................7
Discussion and Conclusion..........................................................................................................................8
References...................................................................................................................................................9
Table of Contents
Aim and objective.......................................................................................................................................3
Introduction.................................................................................................................................................3
Water circuit............................................................................................................................................4
Air circuit................................................................................................................................................4
Equipment...................................................................................................................................................5
Theory.........................................................................................................................................................6
Procedure.....................................................................................................................................................6
Results.........................................................................................................................................................7
Tabulated data.........................................................................................................................................7
Calculations.............................................................................................................................................7
Discussion and Conclusion..........................................................................................................................8
References...................................................................................................................................................9

Heat Transfer 3
Aim and objective
The primary aim of this laboratory experiment is to:
Understand the main mechanism for the transfer of heat
To understand the relationship and the significance of heat transfer and building
engineering systems
To investigate the potential to conduct a controlled laboratory experiment and obtain
reliable and robust data
To be able to collect, analyses and documents data in a professional manner
Introduction
By definition, cooling water represents heat exchangers which are primarily utilized for
dissipating of large amount of loads into the atmosphere. The cooling towers dissipate the heat in
the water through the process of evaporation of a small percentage of water which undergoes
recirculation as demonstrated in the first figure below. This eliminated heat is known as the
latent heat of vaporization (Abo et al., 2009). Cooling towers are available in two forms: the
mechanical draft towers and natural draft. This study, however, gives attention to a cross-flow
mechanical draft cooling tower shown in the diagram below (Hilton, 2014).
Aim and objective
The primary aim of this laboratory experiment is to:
Understand the main mechanism for the transfer of heat
To understand the relationship and the significance of heat transfer and building
engineering systems
To investigate the potential to conduct a controlled laboratory experiment and obtain
reliable and robust data
To be able to collect, analyses and documents data in a professional manner
Introduction
By definition, cooling water represents heat exchangers which are primarily utilized for
dissipating of large amount of loads into the atmosphere. The cooling towers dissipate the heat in
the water through the process of evaporation of a small percentage of water which undergoes
recirculation as demonstrated in the first figure below. This eliminated heat is known as the
latent heat of vaporization (Abo et al., 2009). Cooling towers are available in two forms: the
mechanical draft towers and natural draft. This study, however, gives attention to a cross-flow
mechanical draft cooling tower shown in the diagram below (Hilton, 2014).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Heat Transfer 4
Fig. 1: The Hilton Bench Top Cooling Tower H893 (Muthukumaret al, 2019)
Water circuit
From the load tank, water gets forced via the water flow meter and the control valve to
the point where the temperature is determined. As it moves, water is homogeneously dispersed
thereby forming a vast layer of water which is elongated to the air stream. Since the process is a
downward flow, cooling of water takes place majorly through the process of surface evaporation
(Shahali et al., 2016).
After the water has cooled, it falls into the basin. At this point, the water is directed past a
thermocouple and back to the load bank for re-heating and the same procedure repeats. The
surface evaporation will slowly result in a reduction into the level of water and subsequent
opening of the float operated needle valve as well as makeup tank. Assuming other
circumstances to be optimal, the degree at which the water flows from the make-up tank equals
the rate of evaporation plus any significant air-bone condensations within the air discharge
(Singla et al., 2016).
Air circuit
The cooling tower is equipped with an intake damper setting which regulates the
ingestion of air into the fan. The air then passes through the delivery chamber whereby it passes
dry and wet bulb thermocouples prior to flowing into the packed column. The moisture content
of water is reduced as the air gets into the packing’s, cooling off the water and subsequently
increasing the moisture content (Singla et al., 2016).
The air then gets into the top of the column, and as it evacuates, it goes into the droplet
arrester, that in turn traps a good number of the entrapped droplets, channelling them back to the
packing. This is then followed by a discharging of the air into the atmosphere through the air
measuring orifice, to the wet and then dry thermocouples. The whole process is observable
Fig. 1: The Hilton Bench Top Cooling Tower H893 (Muthukumaret al, 2019)
Water circuit
From the load tank, water gets forced via the water flow meter and the control valve to
the point where the temperature is determined. As it moves, water is homogeneously dispersed
thereby forming a vast layer of water which is elongated to the air stream. Since the process is a
downward flow, cooling of water takes place majorly through the process of surface evaporation
(Shahali et al., 2016).
After the water has cooled, it falls into the basin. At this point, the water is directed past a
thermocouple and back to the load bank for re-heating and the same procedure repeats. The
surface evaporation will slowly result in a reduction into the level of water and subsequent
opening of the float operated needle valve as well as makeup tank. Assuming other
circumstances to be optimal, the degree at which the water flows from the make-up tank equals
the rate of evaporation plus any significant air-bone condensations within the air discharge
(Singla et al., 2016).
Air circuit
The cooling tower is equipped with an intake damper setting which regulates the
ingestion of air into the fan. The air then passes through the delivery chamber whereby it passes
dry and wet bulb thermocouples prior to flowing into the packed column. The moisture content
of water is reduced as the air gets into the packing’s, cooling off the water and subsequently
increasing the moisture content (Singla et al., 2016).
The air then gets into the top of the column, and as it evacuates, it goes into the droplet
arrester, that in turn traps a good number of the entrapped droplets, channelling them back to the
packing. This is then followed by a discharging of the air into the atmosphere through the air
measuring orifice, to the wet and then dry thermocouples. The whole process is observable
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
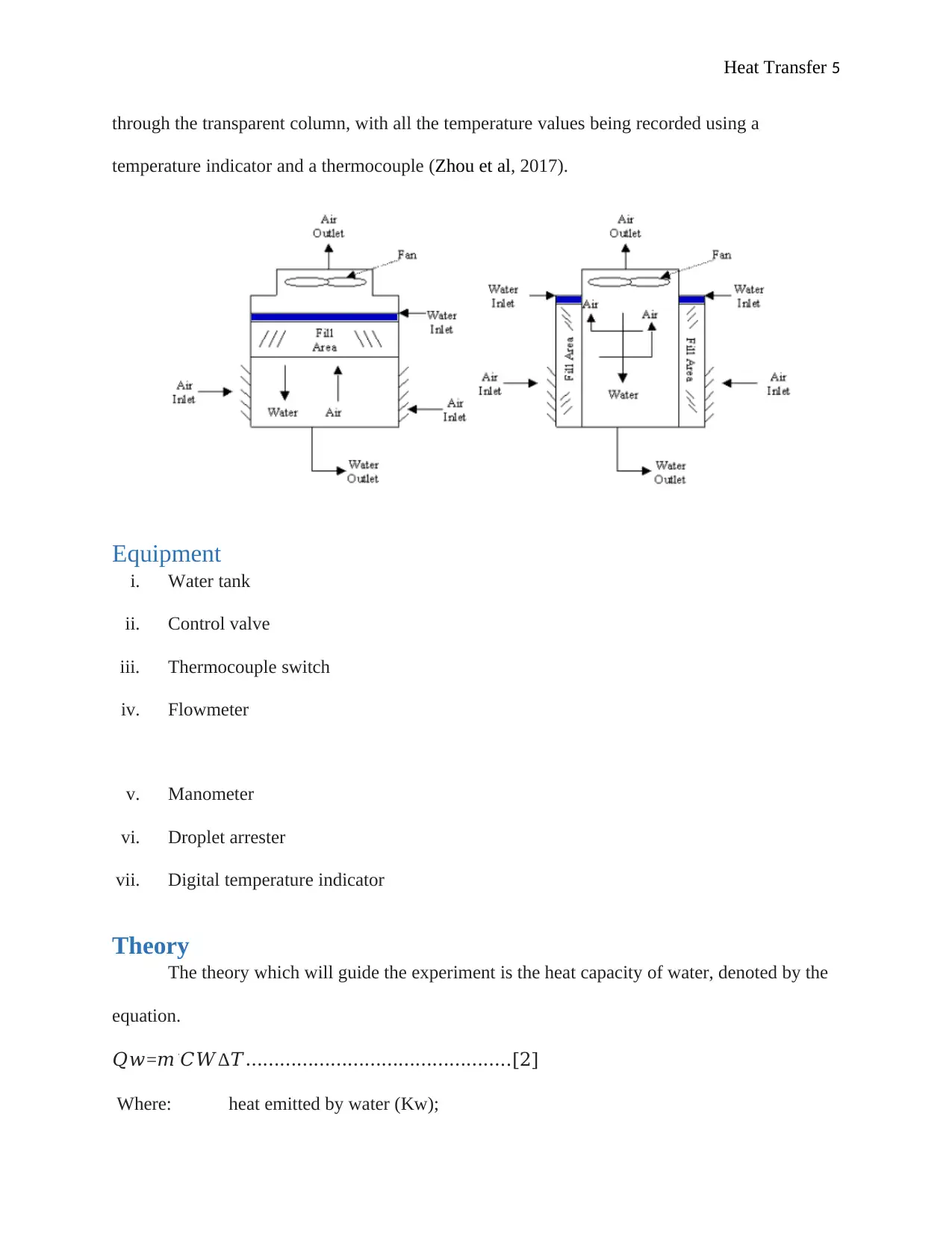
Heat Transfer 5
through the transparent column, with all the temperature values being recorded using a
temperature indicator and a thermocouple (Zhou et al, 2017).
Equipment
i. Water tank
ii. Control valve
iii. Thermocouple switch
iv. Flowmeter
v. Manometer
vi. Droplet arrester
vii. Digital temperature indicator
Theory
The theory which will guide the experiment is the heat capacity of water, denoted by the
equation.
𝑄𝑤=𝑚 ̇ 𝐶𝑊Δ𝑇………………………………………..[2]
Where: heat emitted by water (Kw);
through the transparent column, with all the temperature values being recorded using a
temperature indicator and a thermocouple (Zhou et al, 2017).
Equipment
i. Water tank
ii. Control valve
iii. Thermocouple switch
iv. Flowmeter
v. Manometer
vi. Droplet arrester
vii. Digital temperature indicator
Theory
The theory which will guide the experiment is the heat capacity of water, denoted by the
equation.
𝑄𝑤=𝑚 ̇ 𝐶𝑊Δ𝑇………………………………………..[2]
Where: heat emitted by water (Kw);

Heat Transfer 6
=specific heat (water, 𝐶𝑊=4.2 𝑘𝐽/𝑘𝑔𝐾)
𝑚 ̇ =mass flow rate (kg/s),
Δ𝑇= temperature difference between T5 and T6
Heat absorbed by the air
This shall be obtained by the below equation:
Where Procedure
Procedure
Water was added to the tank and a dent placed on the external surface to indicate the
level of water. The machine was then started, and two minutes permitted to pass, to enable a
steady reading. All temperatures were then recorded by the help of the temperature indicator.
Further, the mass flow rate of water, as well as the orifice differential, noted down. After this, the
flow rate of the water was reversed via the control valve, and the whole procedure repeated to
generate multiple values for analysis.
=specific heat (water, 𝐶𝑊=4.2 𝑘𝐽/𝑘𝑔𝐾)
𝑚 ̇ =mass flow rate (kg/s),
Δ𝑇= temperature difference between T5 and T6
Heat absorbed by the air
This shall be obtained by the below equation:
Where Procedure
Procedure
Water was added to the tank and a dent placed on the external surface to indicate the
level of water. The machine was then started, and two minutes permitted to pass, to enable a
steady reading. All temperatures were then recorded by the help of the temperature indicator.
Further, the mass flow rate of water, as well as the orifice differential, noted down. After this, the
flow rate of the water was reversed via the control valve, and the whole procedure repeated to
generate multiple values for analysis.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
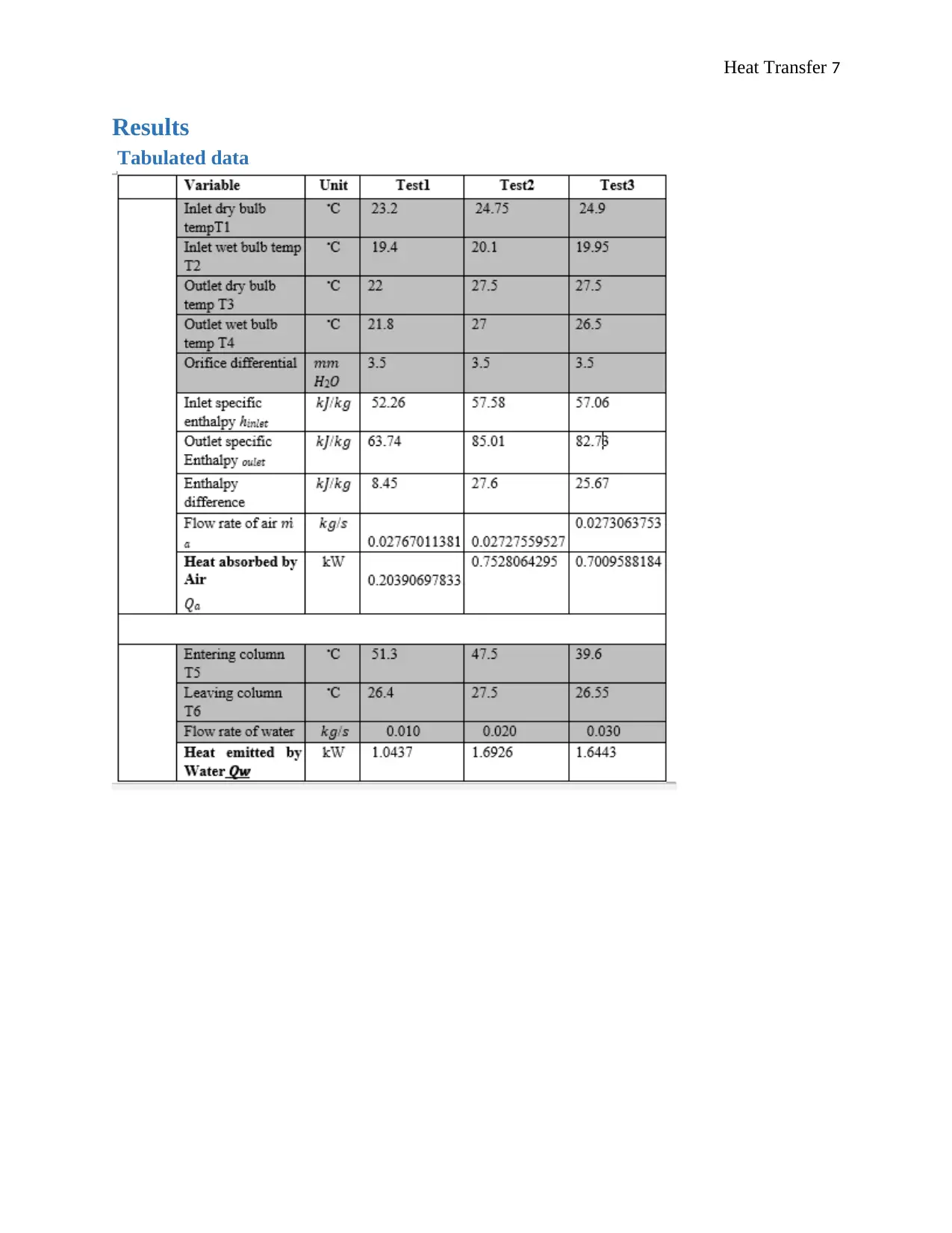
Heat Transfer 7
Results
Tabulated data
Results
Tabulated data
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Heat Transfer 8
Calculations
Calculations

Heat Transfer 9
Solving the rest gives
Heat absorbed by Air
𝑄𝑎
Heat emitted by Water 𝑄𝑤
0.7528064295 1.6926
0.7009588184 1.6443
Discussion and Conclusion
In this experiment, we have been able to determine the heat balance existing between two
fluids experimentally. From the obtained data, the heat which is emitted by the water is more or
less the same as that which is absorbed by the air. For instance, the value for the heat emitted is,
and the value for the heat absorbed by air is… nonetheless, three experiments were conducted,
and there is a slight deviation amongst the subsequent values. This slight difference is associated
with errors emanating from internal resistance of the measuring devices as well as human error
while taking the measurements. In conclusion, the assignment has been a success and has met
the objectives.
Solving the rest gives
Heat absorbed by Air
𝑄𝑎
Heat emitted by Water 𝑄𝑤
0.7528064295 1.6926
0.7009588184 1.6443
Discussion and Conclusion
In this experiment, we have been able to determine the heat balance existing between two
fluids experimentally. From the obtained data, the heat which is emitted by the water is more or
less the same as that which is absorbed by the air. For instance, the value for the heat emitted is,
and the value for the heat absorbed by air is… nonetheless, three experiments were conducted,
and there is a slight deviation amongst the subsequent values. This slight difference is associated
with errors emanating from internal resistance of the measuring devices as well as human error
while taking the measurements. In conclusion, the assignment has been a success and has met
the objectives.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Heat Transfer 10
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


